新型含氟1,2,4-三唑[3,4-b]-1,3,4-噻二唑衍生物的合成及其除草活性
2017-06-19吴小盛陈朴青
吴小盛, 汤 君, 陈朴青, 王 涛*, 罗 劲,*
(1.江西师范大学 a. 化学化工学院 江西省化学生物学重点实验室; b. 分析测试中心,江西 南昌 330022)
·研究论文·
新型含氟1,2,4-三唑[3,4-b]-1,3,4-噻二唑衍生物的合成及其除草活性
吴小盛1a, 汤 君1a, 陈朴青1b, 王 涛1a*, 罗 劲1a,1b*
(1.江西师范大学 a. 化学化工学院 江西省化学生物学重点实验室; b. 分析测试中心,江西 南昌 330022)
以对氟苯甲酰肼为初始原料,经成盐、环化和一锅法反应制得中间体5-对氟苯基-2,4-二氢-1,2,4-三唑-3-硫酮磷亚胺(4); 4与芳基异氰酸酯发生氮杂Wittig反应,后经成环反应合成了11个新型的含氟6-芳氨基-1,2,4-三唑[3,4-b]-1,3,4-噻二唑衍生物(6a~6k),其结构经1H NMR,13C NMR, IR和HR-MS表征。对单、双子叶植物的除草活性测试结果表明:浓度为100 mg·L-1时,6c和6f对稗草和萝卜的茎和根的生长表现出优异的抑制活性,抑制率高达100%。
氮杂Wittig反应; 1,2,4-三唑[3,4-b]-1,3,4-噻二唑; 合成; 除草活性
1,2,4-三唑[3,4-b]-1,3,4-噻二唑类衍生物自1956年首次被Kanaoka[1]合成以来,因其具有抗真菌[2]、抗炎[3]、抗病毒[4-5]、镇痛剂[6]、杀虫[7]、杀菌[8]、抗结核[9-10]、抗氧化[11]、降压[12]、胆碱酯酶抑制剂[13]和抗肿瘤[14-15]等生物活性和药理活性,在过去几十年中引起了科学家广泛的兴趣。从文献报道来看,此类衍生物有关农药学的报道相对较少,其除草活性有待进一步研究。
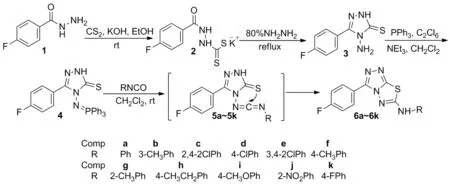
Scheme 1
氟原子是一种非常独特的元素,它不仅电负性强,而且具有很好的热稳定性和亲油性。因此将氟原子引入有机小分子中可以提高其物理化学性质以及生物活性[16],如含氟杂环化合物往往具有较好的除草[17]、杀菌[18]和杀虫[19]活性。为了进一步研究含氟杂环化合物在农药上的除草活性,本文以对氟苯甲酰肼(1)为初始原料,经成盐、环化和一锅法反应制得中间体5-对氟苯基-2,4-二氢-1,2,4-三唑-3-硫酮磷亚胺(4); 4与芳基异氰酸酯经氮杂Wittig反应,后经成环反应合成了11个新型的含氟6-芳氨基-1,2,4-三唑[3,4-b]-1,3,4-噻二唑(6a~6k, Scheme 1),其结构经1H NMR,13C NMR, IR和HR-MS确证。对单、双子叶植物的除草活性测试结果表明:用药量为100 mg·L-1时,6c和6f对稗草和萝卜的茎和根的生长表现优异的抑制活性,抑制率高达100%。
1 实验部分
1.1 仪器与试剂
XT-4型熔点仪; AVANCE 400型核磁共振仪(DMSO-d6为溶剂, TMS为内标); Nicolet 6700型傅里叶红外光谱仪(KBr压片); FinniganMAT8430型质谱仪。
1[20]、中间体对氟苯甲酰肼基二硫代甲酸盐(2)[21]和4-氨基-5-对氟苯基-2,4-二氢-1,2,4-三唑-3-硫酮(3)[21]参照文献方法合成;其余所用试剂均为分析纯,其中乙醇、三乙胺和二氯甲烷按标准方法除水。
1.2 合成
(1) 4的合成
在三口烧瓶中依次加入3 0.84 g(4 mmol), PPh31.57 g (6 mmol), C2Cl61.42 g(6 mmol)和CH2Cl220 mL,冷却至0 ℃,缓慢滴加Et3N 1.21 g (12 mmol),滴毕,于室温反应10 h。过滤,滤液蒸除溶剂得粗品,粗品经混合溶剂(CH2Cl2/石油醚=1/6,V/V)重结晶得白色固体4 1.53 g, 收率81%, m.p.201~203 ℃;1H NMRδ: 12.62(s, 1H, NH), 7.79~6.94(m, 19H, ArH)。
(2) 6的合成通法
氮气保护下,向烧瓶中依次加入4 4.0 mmol和CH2Cl240 mL,待固体溶解后,快速加入取代苯基异氰酸酯4.6 mmol,加毕,待4反应完全(TLC检测)。抽滤,滤饼用(3×100 mL)工业酒精洗涤,经混合溶剂(DMSO/EtOH=1/8,V/V)重结晶得化合物6。
6a: 淡黄色固体,收率74%, m.p.>300 ℃;1H NMRδ: 10.81(s, 1H, NH), 8.29~8.26(m, 2H, ArH), 7.61~7.59(d,J=7.6 Hz, 2H, ArH), 7.50~7.43(t,J=8.8 Hz, 4H, ArH), 7.16~7.12(t,J=7.6 Hz, 1H, ArH);13C NMRδ: 116.0(d,JCF=23.0 Hz), 120.3(d,JCF=8.0 Hz), 125.5, 129.1, 129.8, 135.5, 144.8, 150.3, 156.9(d,JCF=238.0 Hz), 160.9, 162.5; IRν: 3 410(NH), 3 016, 1 608, 1 237(N—N=C), 689(C—S—C) cm-1;HR-MS(ESI)m/z: Calcd for C15H10N5SF{[M+H]+} 312.064 1, found 312.063 8。
6b: 黄色固体,收率68%, m.p.279~281 ℃;1H NMRδ: 10.78(s, 1H, NH), 8.29~8.26 (m, 2H, ArH), 7.49~7.45(t,J=8.8 Hz, 2H, ArH), 7.40~7.38(d,J=8.8 Hz, 1H, ArH), 7.34~7.30(t,J=7.6 Hz, 1H, ArH), 6.96~6.94(d,J=7.2 Hz, 1H, ArH), 2.36(s, 3H, CH3);13C NMRδ: 17.83, 115.5, 116.2(d,JCF=23.0 Hz), 119.0, 122.7, 124.1, 127.9(d,JCF=9.0 Hz), 129.2, 138.7, 139.0, 144.0, 150.3, 160.9, 162.8(d,JCF=246.0 Hz); IRν: 3 432(NH), 3 012, 1 577, 1 231(N—N=C), 688(C—S—C) cm-1; HR-MS(ESI)m/z: Calcd for C16H12N5SF{[M+H]+} 326.079 7, found 326.079 2。
6c: 黄色固体,收率70%, m.p.>300 ℃;1H NMRδ: 11.35(s, 1H, NH), 8.18(s, 1H, ArH), 7.95(s, 1H, ArH), 7.63~7.60(d,J=6.8 Hz, 2H, ArH), 7.42~7.40(d,J=8.0 Hz, 2H, ArH);13C NMRδ: 116.0(d,JCF=23.0 Hz), 119.2, 125.7, 126.3, 126.6, 127.8(d,JCF=9.0 Hz), 129.2, 131.2, 134.4, 151.1, 152.8, 162.5(d,JCF=245.0 Hz), 164.5; IRν: 3 419(NH), 3 031, 1 580, 1 230(N—N =C), 684(C—S—C) cm-1; HR-MS(ESI)m/z: Calcd for C15H8N5SFCl2{[M+H]+} 379.986 1, found 379.985 5。
6d: 黄色固体,收率75%, m.p.>300 ℃;1H NMRδ: 10.94(s, 1H, NH), 8.26~8.22(d,J=8.8 Hz, 2H, ArH), 7.63~7.61(d,J=8.0 Hz, 2H, ArH), 7.51~7.44(m, 4H, ArH);13C NMRδ: 116.2(d,JCF=22.0 Hz), 120.0, 122.5, 126.8, 128.0(d,JCF=8.0 Hz), 129.3, 138.1, 144.1, 150.3, 152.7, 161.8(d,JCF=228.0 Hz), 163.9; IRν: 3 433(NH), 3 044, 1 579, 1 236(N—N=C), 678(C—S—C) cm-1;HR-MS(ESI)m/z: Calcd for C15H9N5SFCl{[M+H]+} 346.025 1, found 346.024 8。
6e: 黄色固体,收率68%, m.p.>300 ℃;1H NMRδ: 11.26(s, 1H, NH), 8.22(s, 1H, ArH), 7.93(s, 1H, ArH), 7.65~7.63(d,J=6.8 Hz, 2H, ArH), 7.45~7.43(d,J=8.0 Hz, 2H, ArH);13C NMRδ: 116.1(d,JCF=22.0 Hz), 116.2, 118.3, 119.5, 122.5, 124.6, 127.8(d,JCF=8.0 Hz), 129.0, 131.1, 131.4, 138.9, 144.1, 150.2, 160.4, 163.1(d,JCF=181.0 Hz); IRν: 3 419(NH), 3 027, 1 583, 1 229(N—N=C), 682(C—S—C) cm-1; HR-MS(ESI)m/z: Calcd for C15H8N5SFCl2{[M+H]+} 379.986 1, found 379.985 5。
“简单来说,就是一个前体mRNA分子可以经过不同的加工方式,形成不同的成熟mRNA产物。”该论文第一作者、中国农业大学玉米改良中心陈秋月博士解释,这就像用剪刀去剪绳子,再把剪下来的绳段重新打结一样,剪哪里、剪几刀、留下哪些绳段、以什么顺序打结,这些都会产生不同的新绳。
6f: 淡黄色固体,收率62%, m.p.>300 ℃;1H NMRδ: 10.86(s, 1H, NH), 8.28~8.27(d,J=5.6 Hz, 2H, ArH), 7.51~7.43(m, 4H, ArH), 7.25~7.20(m, 2H, ArH), 2.31(s, 3H, CH3);13C NMRδ: 21.2, 115.5, 116.2(d,JCF=20.0 Hz), 118.9, 122.7, 124.1, 127.9(d,JCF=9.0 Hz), 129.2, 138.7, 139.0, 144.0, 150.3, 160.9, 162.8(d,JCF=246.0 Hz); IRν: 3 418(NH), 3 009, 1 570, 1 236(N—N=C), 678(C—S—C) cm-1; HR-MS(ESI)m/z: Calcd for C16H12N5SF{[M+H]+} 326.079 7, found 326.079 3。
6g: 黄色固体,收率66%, m.p.274~275 ℃;1H NMRδ: 10.04(s, 1H, NH), 8.22~8.19(m,J=5.6 Hz, 2H, ArH), 7.79~7.77(d,J=7.6 Hz, 1H, ArH), 7.45~7.40(t,J=8.8 Hz, 2H, ArH), 7.33~7.29(t,J=7.2 Hz, 2H, ArH), 7.17~7.16(t,J=6.8 Hz, 1H, ArH), 2.32(s, 3H, CH3);13C NMRδ: 18.52, 116.1(d,JCF=22.0 Hz), 116.2, 122.6(d,JCF=4.0 Hz), 122.9, 125.6, 126.9, 127.8(d,JCF=8.0 Hz), 130.8, 131.0, 137.0, 143.8, 150.7, 162.5(d,JCF=198.0 Hz), 164.0; IRν: 3 428(NH), 3 034, 1 565, 1 238(N—N=C), 682(C—S—C) cm-1; HR-MS(ESI)m/z: Calcd for C16H12N5SF{[M+H]+} 326.079 7, found 326.079 3。
6h: 黄色固体,收率60%, m.p.297~298 ℃;1H NMRδ: 10.61(s, 1H, NH), 8.28~8.24(m,J=5.6 Hz, 4H, ArH), 7.51~7.43(m, 4H, ArH), 7.01~6.99(d,J=8.8 Hz, 2H, ArH), 4.04~4.02(m, 2H, OCH2CH3), 1.35~1.32(m, 3H, OCH2CH3);13C NMRδ: 14.6, 63.2, 115.1, 116.2(d,JCF=22.0 Hz), 120.4, 122.6(d,JCF=4.0 Hz), 127.9(d,JCF=9.0 Hz), 132.1, 144.0, 150.2, 154.8, 161.3, 162.8(d,JCF=246.0 Hz); IRν: 3 441(NH), 3 036, 1 234(N—N=C), 678(C—S—C) cm-1;HR-MS(ESI)m/z: Calcd for C17H14N5OSF{[M+H]+} 356.098 1, found 356. 097 7。
6i: 淡黄色固体,收率65%, m.p.>300 ℃;1H NMRδ: 10.65(s, 1H, NH), 8.41(s, 1H, ArH), 8.28~8.27(d,J=5.2 Hz, 2H, ArH), 7.63~7.44(m, 2H, ArH), 7.35~7.32(d,J=8.8 Hz, 2H, ArH), 7.04~7.01(d,J=8.8 Hz, 1H, ArH), 6.86~6.84(d,J=8.8 Hz, 2H, ArH), 3.71(s, 3H, OCH3);13C NMRδ: 115.3, 116.3(d,JCF=23.0 Hz), 120.4, 122.6(d,JCF=4.0 Hz), 127.5(d,JCF=9.0 Hz), 132.1, 143.8, 150.4, 154.9, 161.5, 162.8(d,JCF=246.0 Hz); IRν: 3 440(NH), 3 029, 1 583, 1 230(N—N=C), 679(C—S—C) cm-1; HR-MS(ESI)m/z: Calcd for C16H12N5OSF{[M+H]+} 342.074 7, found 342.075 4。
6j: 黄色固体,收率75%, m.p.>300 ℃;1H NMRδ: 11.08(s, 1H, NH), 8.22(s, 2H, ArH), 7.93(s, 1H, ArH), 7.59~7.40(d,J=6.8Hz, 2H, ArH), 7.30~6.89(m, 3H, ArH);13C NMRδ: 111.5, 116.1(d,JCF=22.0 Hz), 119.7, 123.5, 125.3, 127.8(d,JCF=9.0 Hz), 129.0, 137.3, 140.5, 144.7, 150.4, 161.4, 162.9(d,JCF=245.0 Hz); IRν: 3 428(NH), 3 031, 1 580, 1 239(N—N=C), 694(C—S—C) cm-1; HR-MS(ESI)m/z: Calcd for C15H9N6O2SF{[M+H]+} 357.049 2, found 357.048 8。
6k: 淡黄色固体,收率72%, m.p.278~279 ℃;1H NMRδ: 11.28(s, 1H, NH), 8.28~8.25(t,J=6.4 Hz, 1H, ArH), 7.68~7.65(t,J=6.4 Hz, 2H, ArH), 7.49~7.45(t,J=8.8 Hz, 2H, ArH), 7.37~7.27(m, 3H, ArH);13C NMRδ: 116.0(d,JCF=22.0 Hz), 116.4(d,JCF=22.0 Hz), 119.9, 121.5(d,JCF=9.0 Hz), 126.8, 127.9(d,JCF=8.0 Hz), 138.2, 144.2, 150.4, 152.4, 157.4(d,JCF=218.0 Hz), 161.7(d,JCF=245 Hz); IRν: 3 437(NH), 3 018, 1 567, 1 234(N—N=C), 687(C—S—C) cm-1; HR-MS(ESI)m/z: Calcd for C15H9N5SF2{[M+H]+} 330.054 7, found 330.055 2。
2 结果与讨论
2.1 合成
4对水敏感,极容易吸水分解,需保存于干燥器中。芳基异氰酸酯也容易吸水变质,因此在合成6时,二氯甲烷等溶剂要除水干燥,且整个反应需氮气保护。
由6的1H NMR可以看出,由于受不同取代基的影响,NH活泼氢的吸收峰位于δ10.04~11.28,均为单宽峰,苯环上的氢吸收峰位于δ7.12~8.26,δ2.32处的单峰为苯环相连的甲基吸收峰,δ3.71处的单峰是甲氧基的吸收峰,乙氧基中的亚甲基的吸收峰在δ4.02~4.04,其甲基吸收峰在δ1.32~1.35;由6的IR图谱可见,N—H的吸收峰在3 436 cm-1处,N=N—C的振动吸收峰在1 228 cm-1处,C—S—C的振动吸收峰在680 cm-1处;HR-MS测试分子量和理论分子量误差也很小(<0.000 7)。
2.3 除草活性
供试植物为稗草(代表单子叶植物)和萝卜(代表双子叶植物),依据文献[22]方法测试了6在浓度为10 mg·L-1和100 mg·L-1时对它们的茎长和根长的抑制率,其结果见表1。表1中的测试数据结果表明:当浓度为100 mg·L-1时,当浓度为100 mg·L-1时,6a~6k对稗草的茎和根的抑制率非常优异,有9个达到A级,尤其是6c、 6e、 6f和6g抑制率均为100%。当浓度为10 mg·L-1时,6a~6k对稗草的茎和根的抑制率都在C级以下,说明随着浓度从100 mg·L-1降为10 mg·L-1,对稗草的茎和根的抑制率也大幅度降低。

表1 6对单、双子叶植物(稗草、萝卜)的抑制活性aTable 1 The inhibition percentage of 6 to barnyard grass and radish
a负抑制率表示促进植物生长,活性标准:A级:≥90%; B级:≥70%; C级:≥50%; D级:<50%。
6a~6k对萝卜的茎和根的抑制率有9个达到A级,其中6a、 6c和6f抑制率达到了100%。当浓度为10 mg·L-1时,对萝卜的茎和根的抑制率只有2个C级,多数化合物如6a和6c为负值,说明具有一定的促进作用,随着浓度的降低对萝卜的茎和根的抑制率也减小。
总之随着浓度降低,6a~6k对稗草和萝卜的茎和根的抑制率也下降。当浓度为100 mg·L-1时,6c和6f对稗草和萝卜的除草活性非常好,抑制率都是100%。随着Ar基团的变化,对稗草和萝卜茎和根的抑制率并不是很有规律的变化,在随后的工作中还需适当引入更多的吸电子和供电子基团,进一步研究其除草活性。
3 结论
本文以对氟苯甲酰肼为初始原料,设计合成了11种新型的含氟6-芳氨基-1,2,4-三唑[3,4-b]-1,3,4-噻二唑(6a~6k)。对单、双子叶植物的除草活性测试结果表明:浓度为100 mg·L-1时, 6c和6f对稗草、萝卜的茎和根的抑制率非常优异,均达到了100%。该研究结果为寻找合成含氟杂环化合物农药提供了一种较好的思路。
[1] 史海健,王忠义,史好新,等. 2,5-二取代2,3-二氢-1,2,4-均三唑并[3,4-b]-1,3,4-噻二唑的合成及生物活性[J].应用化学,1999,16(5):53-56.
[2] Karabasanagouda T, Adhikari A V, Shetty N S. Synthesis and antimicrobial activities of some novel 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles and 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazines carrying thioalkyl and sulphonyl phenoxy moieties[J].Eur J Med Chem,2007,42(4):521-529.
[3] Chawla G, Kumar U, Bawa S,etal. Synthesis and evaluation of anti-inflammatory,analgesic and ulcerogenic activities of 1,3,4-oxadiazole and 1,2,4-triazolo[3,4-b]-1,3,4- thiadiazole derivatives[J].J Enzyme Inhib Med Chem,2012,27(5):658-665.
[4] Kritsanida M, Mouroutsou A, Marakos P,etal. Synthesis and antiviral activity evaluation of some new 6-substituted 3-(1-adamantyl)-1,2,4-triazolo[3,4-b][1,3,4]thiadiazoles[J].Farmaco,2002,57(13):253-257.
[5] 魏学,郑玉国,薛伟,等. 新型1,2,4-三唑并[3,4-b]-1,3,4-噻二唑类衍生物合成及其抗病毒活性[J].合成化学,2010,18(5):595-598.
[6] Hussein M A, Shaker R M, Ameen M A,etal. Synthesis,anti-inflammatory,analgesic,and antibacterial activities of some triazole,triazolothiadiazole,and triazolothiadiazine derivatives[J].Arch Pharmacal Res, 2011,34(8):1239-1250.
[7] Khawass S M, Khalil M A, Hazzaa A A,etal. Synthesis of some 1,2,4-triazolo[3,4-b][1,3,4]thiadiazoles as potential anthelmintics[J].Farmaco,1989,44(7):703-709.
[8] Holla B S, Shivananda M K, Akberali P M,etal. Studies on arylfuran derivatives.Part VI:Synthesis,characterization and antibacterial activities of some 6-(5-aryl-2-furyl)-1,2,4- triazolo[3,4-b]-1,3,4- thiadiazoles and 6-(5-nitro-2-furyl)-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazoles[J].Farmaco,1996,51(12):785-792.
[9] Kumar G V S Y, Prasad R, Mallikarjuna P B,etal. Synthesis and pharmacological evaluation of clubbed isopropylthiazole derived triazolothiadiazoles,triazolothiadiazines and mannich bases as potential antimicrobial and antitubercular agents[J].Eur J Med Chem,2010,45(11):5120-5129.
[10] Bonde C G, Peepliwal A, Gaikwad N J. Synthesis and antimycobacterial activity of azetidine-,quinazoline-,and triazolo-thiadiazole-containing pyrazines[J].Arch Pharm Chem Life Sci,2010,343(4):228-236.
[11] Hanif M, Saleem M, Hussain M T,etal. Synthesis,urease inhibition, antioxidant and antibacterial studies of some 4-amino-5-aryl-3H-1,2,4-triazole-3-thiones and their 3,6-disubstituted 1,2,4-triazolo[3,4-b]1,3,4- thiadiazole derivatives[J].J Braz Chem Soc,2012,23(5):854-860.
[12] 张自义,李明,赵岚,等. 3-烷基/芳基-6-(3′-吡啶基) 均三哇并[ 3,4-b]1,3,4-噻二唑类化合物的合成[J].有机化学,1993,13(4):397-402.
[13] Khan I, Bakht S M, Ibrar A S.etal. Exploration of a library of triazolothiadiazole and triazolothiadiazine compounds as a highly potent and selective family of cholinesterase and monoamine oxidase inhibitors:Design,synthesis,X-ray diffraction analysis and molecular docking studies[J].RSC Adv,2015,5:21249-21267.
[14] Rashid M, Husain A, Siddiqui A A,etal. Benzimidazole clubbed with triazolo- thiadiazoles and triazolo-thiadiazines:New anticancer agents[J].Eur J Med Chem,2013,62:785-798.
[15] Ibrahim D A. Synthesis and biological evaluation of 3,6-disubstituted [1,2,4]triazolo[3,4-b][1,3,4]thiadiazole derivatives as anovel class of potential antitumor agents[J].Eur J Med Chem,2009,44(7):2776-2781.
[16] Welch J T. Tetrahedron report number 221:Advances in the preparation of biologically active organofluorine compounds[J].Tetrahedron,1987,43(14):3123-3125.
[17] Li G Y, Qian X H, Cui J N,etal. Synthesis and herbicidal activities of fluorine-containing 3-pyridylmethyl-2- phenyliminothiazolidine derivatives[J].J Fluorine Chem,2006,127(2):182-186.
[18] Ren Q Y, Cui Z P, Lia Z,etal. A facile synthesis and fungicidal activities of novel fluorine-containing pyrido[4,3-d]pyrimidin-4(3H)-ones [J].J Fluorine Chem,2006,128(11):1369-1375.
[19] Zheng X M, Li Z, Wang Y L,etal. Syntheses and insecticidal activities of novel 2,5-disubstituted 1,3,4-oxadiazoles[J].J Fluorine Chem,2003,123(2):163-169.
[20] 蔡兴象,李红光,林奇,等. 含氟苯甲酰肼的合成方法改进[C].兰州:全国第十四届大环化学暨第六届超分子化学学术研讨会,2008.
[21] Reid J R, Heindel N D. Improved syntheses of 5-substituted-4-amino-3-mereapto-(4H)-1,2,4-triazoles[J].J Hetercycl Chem,1976,13(4):925-926.
[22] 王涛. 靶标酶抑制剂的设计、合成与性质研究[D].武汉:华中师范大学,2004.
Synthesis and Herbicidal Activities of Novel Fluorine-Containing 1,2,4-Triazolo[3,4-b]-1,3,4-thiadiazole Derivatives
WU Xiao-sheng1a, TANG Jun1a, CHEN Pu-qing1b, WANG Tao1a*, LUO Jin1a,1b*
(a. Jiangxi Province Key Laboratory of Chemical Biology, College of Chemistry and Chemical Engineering;b. Analytical & Testing Center, 1. Jiangxi Normal University, Nanchang 330022, China)
5-(4-Fluorophenyl)-2,4-2H- 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole iminophosphorane (4) was prepared by salt forming, cyclization and one pot reaction, using benzoyl hydraziono as starting material. Eleven novel fluorine-containing 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole derivatives(6a~6k) were successfully synthesized by the aza-Wittig reaction with 4 with arylisocyanates, and then annulation reaction. The structures were characterized by1H NMR,13C NMR, IR and HR-MS. The results of preliminary bioassay demonstrated that 6c and 6f showed 100% inhibitory activities on barnyard grass and radish at the dosage of 100 mg·L-1.
aza-Wittig reaction; 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazole; synthesis; herbicidal activity
2017-03-01
国家自然科学基金资助项目(21262018, 21562026); 江西省自然科学基金资助项目(20161BAB203085); 江西省自然科学青年基金资助项目(20161BAB213072)
吴小盛(1963-),男,汉族,江西抚州人,硕士研究生,主要从事新型杂环化合物的合成与除草活性的研究。 E-mail: 1340125160@qq.com
罗劲,实验师, Tel. 0791-88122739, E-mail: jinluo@jxnu.edu.cn; 王涛,教授, E-mail: wangtao@jxnu.edu.cn
O626.2
A
10.15952/j.cnki.cjsc.1005-1511.2017.06.17041
