肿瘤中M2型丙酮酸激酶的表达、功能及调节
2017-05-10时蒙昆综述杨晓冬孙奉昊审校
时蒙昆(综述) 杨晓冬 孙奉昊 詹 成(审校) 时 雨 王 群
(复旦大学附属中山医院胸外科 上海 200032)
肿瘤中M2型丙酮酸激酶的表达、功能及调节
时蒙昆(综述) 杨晓冬 孙奉昊 詹 成△(审校) 时 雨 王 群
(复旦大学附属中山医院胸外科 上海 200032)
高水平的糖酵解是癌细胞的重要特征之一,而其中一个重要的调节因子即M2型丙酮酸激酶(M2 type of pyruvate kinase,PKM2)。除此之外,PKM2还具有调控基因转录以及细胞周期进展、促进肿瘤形成和侵袭迁移等蛋白激酶活性。同时,PKM2受多种转录因子、癌基因蛋白和中间代谢产物等复杂因素的调控。大量研究表明,PKM2在肿瘤的发生进展过程中起着至关重要的作用,针对PKM2展开相应临床诊断和治疗研究有着良好的应用前景。
M2型丙酮酸激酶; 肿瘤代谢; 转录水平
丙酮酸激酶(pyruvate kinase,PK)催化磷酸烯醇式丙酮酸(phosphoenolpyruvate,PEP)与ADP反应生成丙酮酸和ATP,是糖酵解的关键酶之一。目前研究表明,M2型丙酮酸激酶(M2 type of pyruvate kinase,PKM2)作为PK的亚型之一,在肿瘤的形成发展中起着重要的作用。本文就PKM2在肿瘤中的表达、功能及调节进行综述。
PKM2在肿瘤中的表达 人体内存在PKM和PKLR两种PK的同工酶,而PKM2由PKM基因选择性剪接而来。通过不同的剪接方式,PKM基因有着多个不同的转录本和蛋白质亚型。目前NCBI和UCSC数据库中共记录有PKM基因的14个转录本和12种蛋白质亚型,具体信息如图1[1]:较宽处代表外显子、较窄处代表内含子。深色部分代表翻译起始密码子与终止密码子之间的序列,浅色部分代表5′UTR和3′UTR区。AA:氨基酸残基数量。
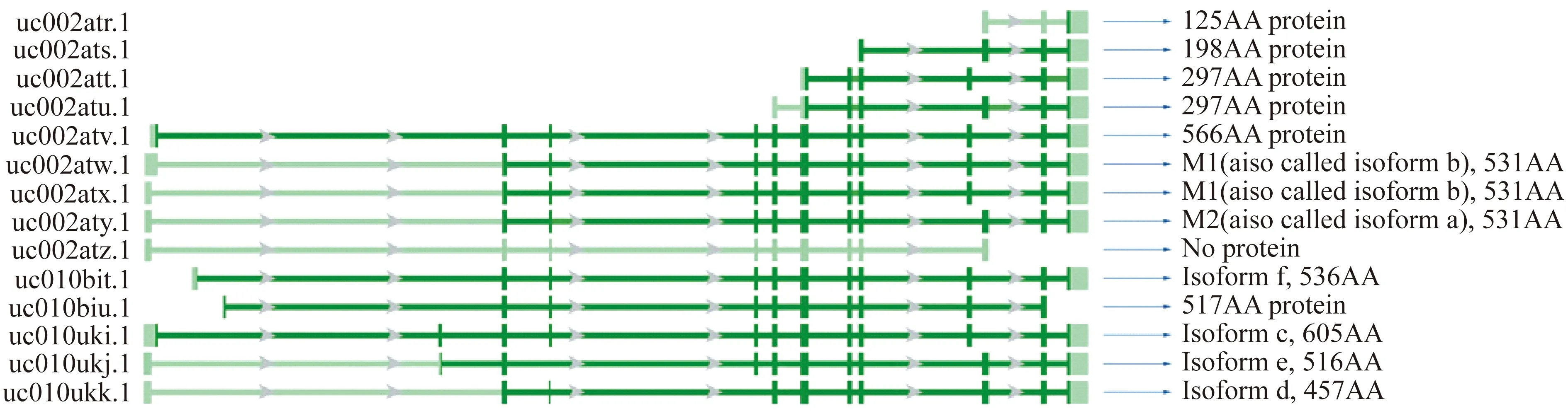
图1 PKM基因的转录本和蛋白质亚型Fig 1 Expression patterns of the transcript rariants of PKM
由图1可见PKM基因不同亚型序列间存在一定的差别,其中关于PKM1和PKM2亚型的研究较多,PKM1表达第12外显子而PKM2表达第13外显子,二者蛋白序列间仅有23个氨基酸残基存在差异,关于PKM其他蛋白亚型的研究较少,功能尚不确定。
以往的观点认为,在PKM不同蛋白质亚型中,PKM1在正常组织中占据主导地位,而PKM2在增殖旺盛的组织中高表达,例如胚胎干细胞、癌细胞等,胚胎发育的过程中PKM2逐渐被PKM1取代,随着正常组织癌变,PKM1又逐步被PKM2所取代[2]。
但近期多项研究指出,在所有正常和肿瘤组织中,PKM2均为主导亚型,并且PKM2在肿瘤组织中较正常组织细胞显著增高。Bluemlein等[3]采用质谱的方法定量检测PKM1和PKM2蛋白质水平,结果发现PKM2在多种正常组织肿瘤组织样本以及细胞系样本中均占二者总量的50%以上,在绝大部分样本中比例甚至超过95%。我们通过对TCGA数据库中25种肿瘤近8 000例样本中PKM各亚型相应的转录表达数据进行分析,在不同组织类型中PKM2占PKM各亚型总和的57.2%~89.2%,均占绝对主导地位。
传统观点认为PKM1在肿瘤组织细胞中含量明显下降,并且PKM1到PKM2的亚型转换是癌细胞中PKM2表达升高的主要原因。Bluemlein等[3]研究认为PKM1的表达在肿瘤中并无显著变化。而我们的研究发现,虽然PKM总体表达在肿瘤中显著性的上升,但PKM1相应转录本的表达在肿瘤组织细胞中不升反降,其占PKM总体的比例更是显著下降,考虑到传统研究中主要采取免疫组化、Western blot等相对定量的方法来研究PKM1和PKM2的表达特点,而这些基于抗原抗体反应的方法难以对PKM1和PKM2进行精确定量,研究受抗原抗体结合能力影响较大[1]。因此,我们认为传统研究结果中PKM1在肿瘤中的显著下降是确定存在的,并与我们的研究结果相一致。另外,我们的研究发现,PKM2上升幅度远高于PKM1下降幅度,但与PKM整体上升幅度相一致,PKM2所占比例并无明显变化,综合PKM各亚型相应转录本的表达变化,我们认为PKM2上升主要是因为PKM整体表达上升所引起的,而非PKM1亚型的转换[1]。在Desai等[4]的研究中,作者混淆了 PKM的不同亚型,因此实验结果值得进一步商榷。
PKM2在肿瘤代谢中的功能 PKM2存在四聚体和二聚体两种形式,在肿瘤细胞中主要以低活性的二聚体存在,在此种情况下,能量代谢被抑制,糖酵解中间代谢产物在细胞中积累,并参与合成代谢,从而促进癌细胞的快速增殖。因此,在PKM2 占主导作用的癌细胞内,PKM2 高活性与低活性之间的比值决定了葡萄糖是用来产生能量还是合成代谢的前体[5]。
癌细胞在代谢过程中会产生一系列活性氧簇(reactive oxygen species,ROS),ROS通过细胞氧化应激反应诱导细胞凋亡并导致其坏死。Anastasiou等[6]研究发现PKM2可参与对抗氧化应激。首先,PKM2自身结构中含有具有还原活性的半胱氨酸残基,易与ROS发生反应,降低后者在胞内的浓度;同时ROS诱导PKM2钝化使得大量葡萄糖进入磷酸戊糖途径产生还原型辅酶II,还原型辅酶II作为谷胱甘肽(GSH)还原酶的辅酶,可用于维持GSH的还原状态,对于维持细胞还原性GSH含量和功能起重要作用。
谷氨酸代谢作为糖代谢受损的代偿方式会在癌细胞糖代谢受损的情况下发挥作用,PKM2作为重要的调节因子参与此过程。Wu等[7]研究结肠癌细胞代谢的过程中发现敲除癌细胞PKM2基因后细胞内谷氨酰胺酶、运载蛋白表达增多,进一步研究发现PKM2通过miR-200a对β-catenin/c-myc信号通路有负性调控作用,PKM2减少使得β-catenin/c-myc信号通路激活,促进谷氨酸代谢以维持癌细胞增殖。
PKM2在肿瘤分子调控中发挥的作用 近年来多项研究发现,PKM2除了在细胞质内发挥作用外,还可以通过多种方式进入细胞核。PKM2在细胞质和细胞核内可以发挥蛋白激酶的作用,从PEP上转移磷酸基团磷酸化多个重要因子并参与肿瘤细胞的分子调控(表1)。
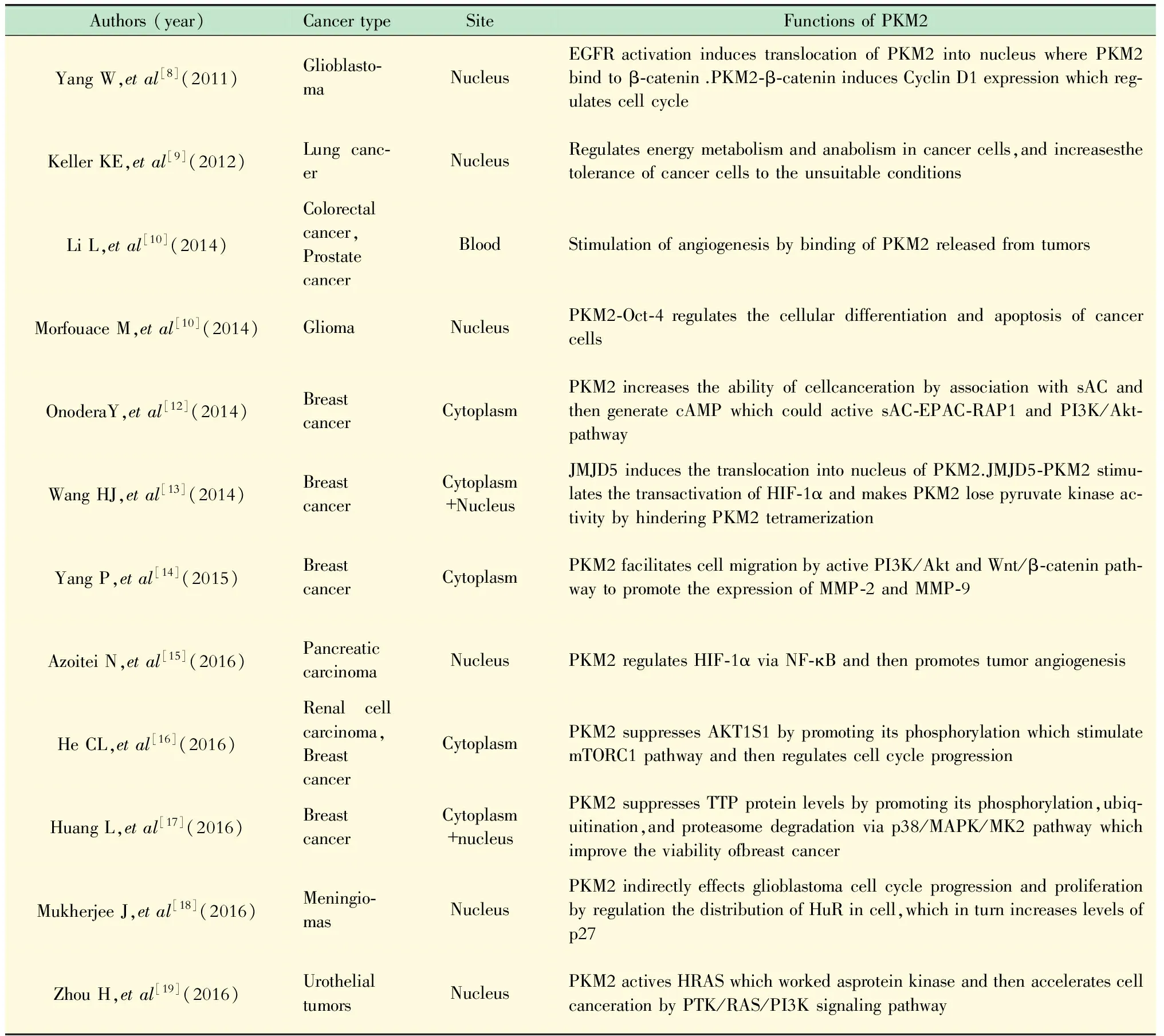
表1 PKM2在肿瘤发生中的调控作用Tab 1 The role of PKM2 during tumorigenesis
EGFR:Epidermal growth factor receptor;SAICAR:Succinylaminoimidazole carboxamide riboside;C/EBPβ:CCAAT/enhancer binding protein beta;JMJD5:Jumonji domain containing 5;AKT1S1:AKT1 substrate1;TTP:Tristetraprolin;MMP:Matrix metalloprotein.
但在一项最新的研究中,Hosios等[20]采用32P 同位素标记PEP,结果显示几乎所有蛋白质磷酸化时活性磷酸基团均来源于ATP 而不是PEP。同时Hosios 等[20]特异性地敲除PKM2 表达后,未发现存在磷酸化水平明显变化的蛋白质。这一新的研究结果认为PKM2不存在蛋白激酶功能。
PKM2转录水平的调节 DNA甲基化是PKM2转录水平上常见的调节方式。Desai等[4]利用基因探针cg24327132研究甲基化对PKM2基因表达的影响,探针在正常组织中低甲基化而在肿瘤组织中高甲基化,并且显示PKM2基因表达与cg24327132甲基化程度呈强烈的负相关,暗示低甲基化有利于PKM2基因表达。
转录因子调节为另一种重要的调节方式。由于癌细胞内基因突变或微环境变化使得许多转录因子都可以激发PKM2的高表达,例如低氧诱导分子(hypoxia induce factor 1α,HIF-1α),核因子-κB(NF-κB),Sp1(specificity protein 1),过氧化物酶体增殖物受体γ(PPARγ)等[21]。HIF-1α在许多癌细胞中高表达并调节低氧状态下的适应性反应,Luo等[22-23]研究发现HIF-1α可以促进PKM2基因的转录进一步形成正反馈调节。HIF-1α通过与PKM基因的低氧反应原件(hypoxia response element,HRE)结合促进PKM基因转录[22,24]。也有研究发现PKM2过度表达导致信号转导蛋白质和转录激活物(signal transducers and activators of transcription 3,STAT3)磷酸化而进一步激活HIF-1α转录[25]。转录因子c-Myc可以上调核抑制蛋白hnRNPA1、 hnRNPA2、 hnRNPA3,进一步促进PKM2表达[26-27]。除此之外,还有很多因素可以影响PKM2基因表达,例如mTOR信号通路可以激活HIF-1α介导的PKM基因转录和c-Myc-hnRNPs依赖的前体mRNA剪接[28]。Sp1蛋白质磷酸化之后可促进PKM2的表达[29]。人第10号染色体缺失的磷酸酶及张力蛋白同源的基因(phosphatase and tensin homolog deleted on chromosome ten,PTEN)使3、4、5-三磷酸磷脂酰肌醇去磷酸化而负性调节PKM2表达[30-31]。Yang等[32]在研究癌细胞代谢过程中发现,作为上皮细胞-间充质细胞转换过程重要调节因子Twist可激活β1-integrin/FAK/PI3K/AKT/mTOR信号通路并抑制P53信号通路上调PKM2表达水平。
PKM2翻译水平的调节 PKM2在翻译水平的调节主要由miRNA所介导,具体内容见表2。
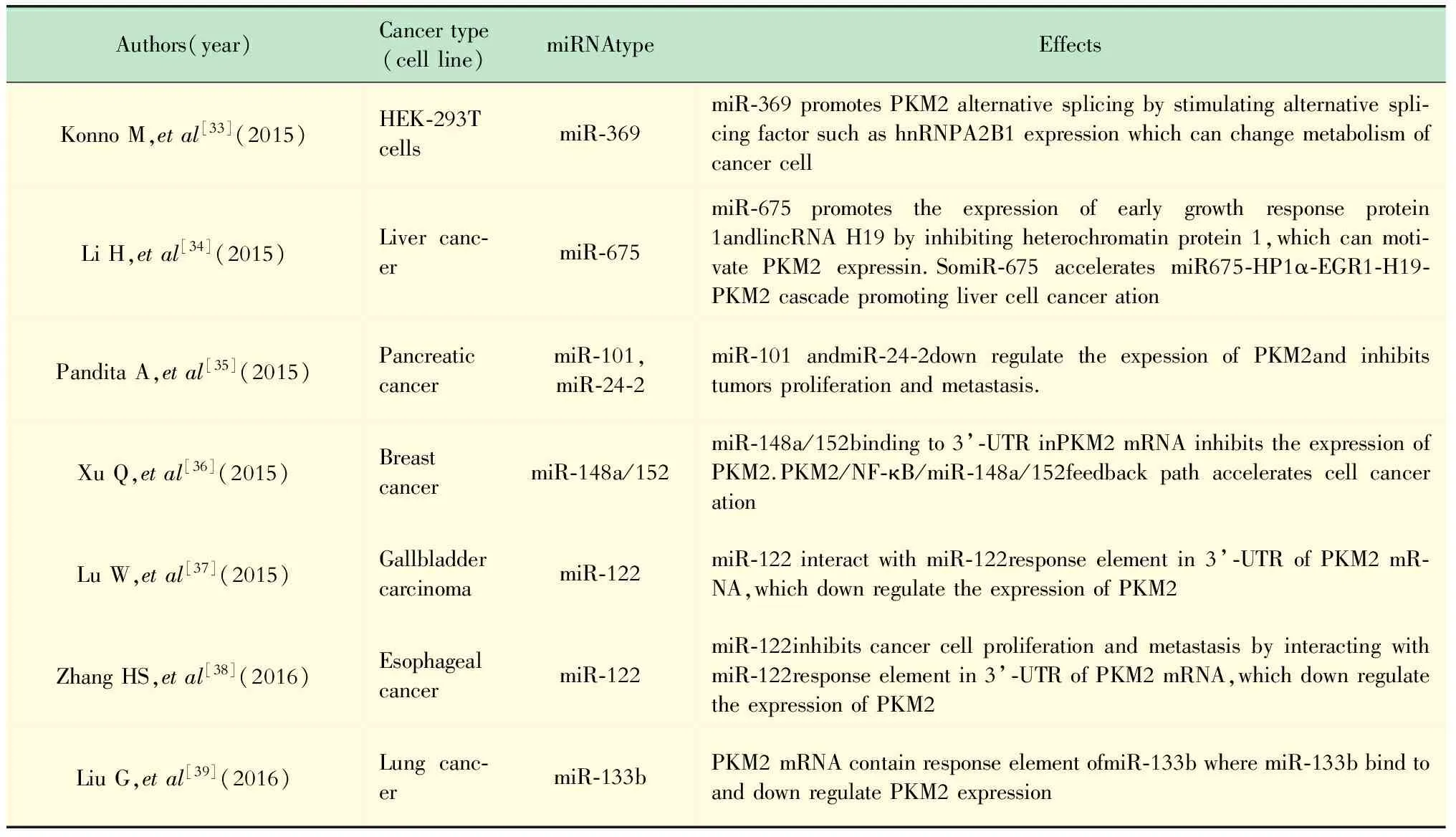
表 2 miRNA对PKM2的调节作用Tab 2 The effects of miRNA to PKM2
PKM2在蛋白质水平的调节 代谢中间产物可在蛋白质水平上对PKM2进行变构调节。PKM2存在四聚体和二聚体两种形式,分别发挥蛋白激酶和丙酮酸激酶活性,果糖1,6-双磷酸(FBP)和丝氨酸皆为PKM2的强力变构激活剂,与PKM2结合后维持其四聚体并保持高活性状态[40-42]。
除代谢中间产物对PKM2的变构调节外,更重要的调节方式为翻译后修饰。PKM2存在多种翻译后修饰,对其进行活性调节,其中磷酸化最为多见,磷酸化后常导致PKM2与其变构激活剂FBP解离致使活性降低[29]。常见的磷酸化位点为Tyr105,FGFR、BCR-ABL、FLT-3、HPV-16 E7等蛋白质皆是在此位点进行修饰[43]。除磷酸化外还存在乙酰化、羟基化等形式的翻译后修饰,以调节PKM2活性。
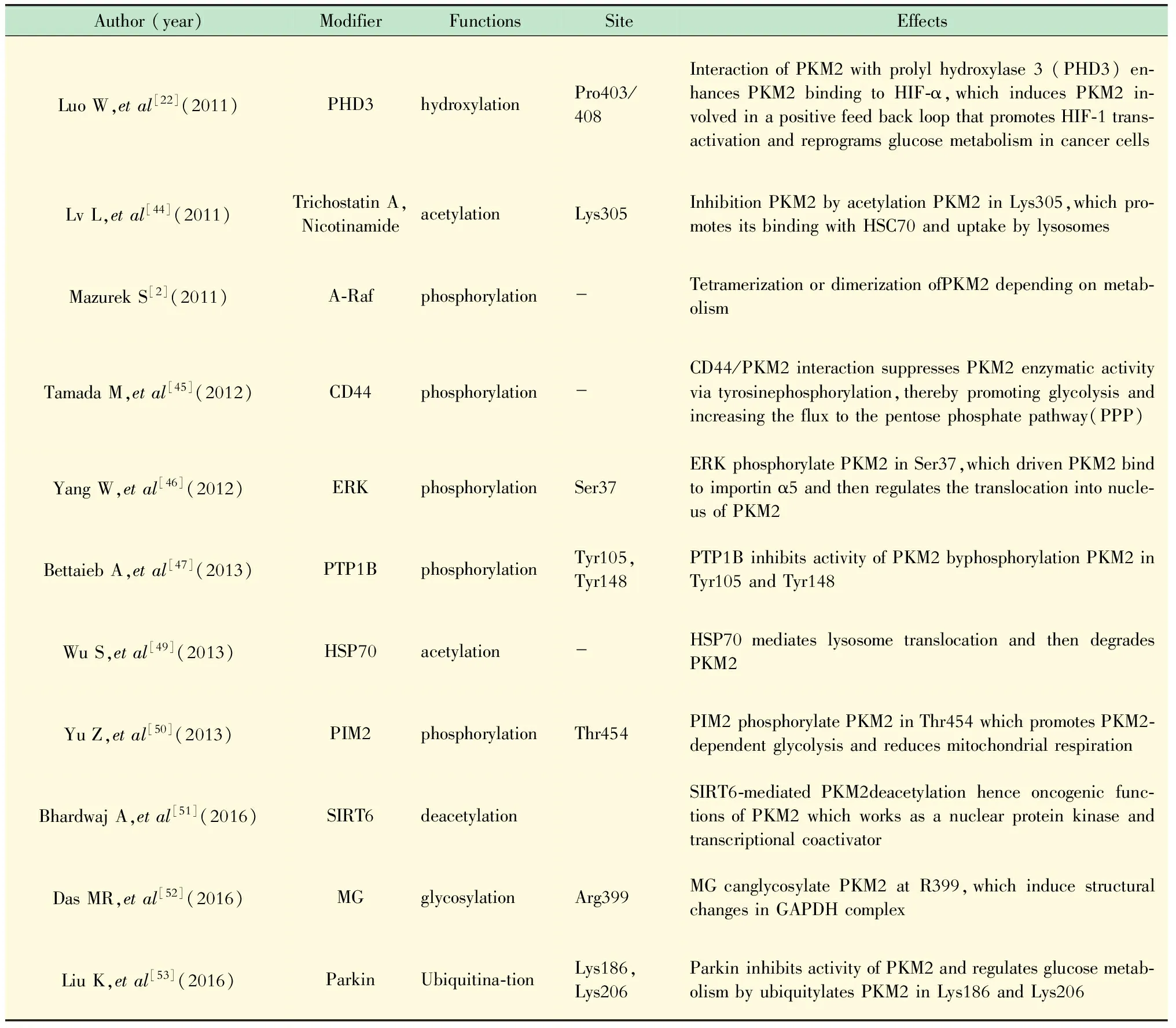
表3 PKM2翻译后修饰形式及作用Tab 3 Post-translational modification of PKM2
PHD3:Prolyl hydroxylase 3;ERK:Extracellular regulated protein kinase;PTP1B:Protein tyrosine phosphatase-1B;PCAF:P300/CBP-associated factor;HSP70:Heat shock protein 70;PIM2:Proviral integration site of murine 2;SIRT6:Sirtuins 6;MG:Methylglyoxal 0.
结语 通过对PKM2逐渐深入的研究,人们对PKM2在癌细胞中的表达、功能和调控等方面有了更深层次的理解。考虑到PKM2在肿瘤中的异常表达、在肿瘤代谢和分子调控中的显著作用以及其功能的复杂调控,我们有理由相信PKM2将在肿瘤诊断和治疗中发挥重要作用。这都使得PKM2成为肿瘤研究领域的焦点。
[1] ZHAN C,YAN L,WANG L,etal.Isoform switch of pyruvate kinase M1 indeed occurs but not to pyruvate kinase M2 in human tumorigenesis[J].PLoSOne,2015,10(3):e118663.
[2] MAZUREK S.Pyruvate kinase type M2:a key regulator of the metabolic budget system in tumor cells[J].IntJBiochemCellBiol,2011,43(7):969-980.
[3] BLUEMLEIN K,GRUNING NM,FEICHTINGER RG,etal.No evidence for a shift in pyruvate kinase PKM1 to PKM2 expression during tumorigenesis[J].Oncotarget,2011,2(5):393-400.
[4] DESAI S,DING M,WANG B,etal.Tissue-specific isoform switch and DNA hypomethylation of the pyruvate kinase PKM gene in human cancers[J].Oncotarget,2014,5(18):8202-8210.
[5] BARGER JF,PLAS DR.Balancing biosynthesis and bioenergetics:metabolic programs in oncogenesis[J].EndocrRelatCancer,2010,17(4):R287-R304.
[6] ANASTASIOU D,POULOGIANNIS G,ASARA JM,etal.Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses[J].Science,2011,334(6060):1278-1283.
[7] WU H,LI Z,YANG P,etal.PKM2 depletion induces the compensation of glutaminolysis through beta-catenin/c-Myc pathway in tumor cells[J].CellSignalling,2014,26(11):2397-2405.
[8] YANG W,XIA Y,JI H,etal.Nuclear PKM2 regulates beta-catenin transactivation upon EGFR activation[J].Nature,2011,480(7375):118-122.
[9] KELLER KE,DOCTOR ZM,DWYER ZW,etal.SAICAR induces protein kinase activity of PKM2 that is necessary for sustained proliferative signaling of cancer cells[J].MolCell,2014,53(5):700-709.
[10] LI L,ZHANG Y,QIAO J,etal.Pyruvate kinase M2 in blood circulation facilitates tumor growth by promoting angiogenesis[J].JBiolChem,2014,289(37):25812-25821.
[11] MORFOUACE M,LALIER L,OLIVER L,etal.Control of glioma cell death and differentiation by PKM2-Oct4 interaction[J].CellDeathDis,2014,5:e1036.
[12] ONODERA Y,NAM JM,BISSELL MJ.Increased sugar uptake promotes oncogenesis via EPAC/RAP1 and O-GlcNAc pathways[J].JClinInvest,2014,124(1):367-384.
[13] WANG HJ,HSIEH YJ,CHENG WC,etal.JMJD5 regulates PKM2 nuclear translocation and reprograms HIF-1alpha-mediated glucose metabolism[J].ProcNatlAcadSciUSA,2014,111(1):279-284.
[14] YANG P,LI Z,WANG Y,etal.Secreted pyruvate kinase M2 facilitates cell migration via PI3K/Akt and Wnt/beta-catenin pathway in colon cancer cells[J].BiochemBiophysResCommun,2015,459(2):327-332.
[15] AZOITEI N,BECHER A,STEINESTEL K,etal.PKM2 promotes tumor angiogenesis by regulating HIF-1alpha through NF-kappaB activation[J].MolCaner,2016,15(1):3.
[16] HE CL,BIAN YY,XUE Y,etal.Pyruvate Kinase M2 activates mTORC1 by phosphorylating AKT1S1[J].SciRep,2016,6:21524.
[17] HUANG L,YU Z,ZHANG Z,etal.Interaction with pyruvate kinase M2 destabilizes tristetraprolin by proteasome degradation and regulates cell proliferation in breast cancer[J].SciRep,2016,6:22449.
[18] MUKHERJEE J,OHBA S,SEE WL,etal.PKM2 uses control of HuR localization to regulate p27 and cell cycle progression in human glioblastoma cells[J].IntJCancer,2016,139(1):99-111.
[19] ZHOU H,WANG X,MO L,etal.Role of isoenzyme M2 of pyruvate kinase in urothelial tumorigenesis[J].Oncotarget,2016,7(17):23947-23960.
[20] HOSIOS AM,FISKE B P,GUI D Y,etal.Lack of Evidence for PKM2 Protein Kinase Activity[J].MolCell,2015,59(5):850-857.
[21] LI Z,YANG P,LI Z.The multifaceted regulation and functions of PKM2 in tumor progression[J].BiochimBiophysActa,2014,1846(2):285-296.
[22] LUO W,HU H,CHANG R,etal.Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1[J].Cell,2011,145(5):732-744.
[23] LUO W,SEMENZA GL.Pyruvate kinase M2 regulates glucose metabolism by functioning as a coactivator for hypoxia-inducible factor 1 in cancer cells[J].Oncotarget,2011,2(7):551-556.
[24] PRIGIONE A,ROHWER N,HOFFMANN S,etal.HIF1alpha modulates cell fate reprogramming through early glycolytic shift and upregulation of PDK1-3 and PKM2[J].StemCells,2014,32(2):364-376.
[25] DONG T,YAN Y,CHAI H,etal.Pyruvate kinase M2 affects liver cancer cell behavior through up-regulation of HIF-1alpha and Bcl-xL in culture[J].BiomedPharmacother,2015,69:277-284.
[26] DAVID CJ,CHEN M,ASSANAH M,etal.HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer[J].Nature,2010,463(7279):364-368.
[27] CLOWER CV,CHATTERJEE D,WANG Z,etal.The alternative splicing repressors hnRNP A1/A2 and PTB influence pyruvate kinase isoform expression and cell metabolism[J].ProcNatlAcadSciUSA,2010,107(5):1894-1899.
[28] IQBAL M A,SIDDIQUI F A,GUPTA V,etal.Insulin enhances metabolic capacities of cancer cells by dual regulation of glycolytic enzyme pyruvate kinase M2[J].MolCancer,2013,12:72.
[29] CHRISTOFK HR,VANDER HM,WU N,etal.Pyruvate kinase M2 is a phosphotyrosine-binding protein[J].Nature,2008,452(7184):181-186.
[30] CARNERO A,BLANCO-APARICIO C,RENNER O,etal.The PTEN/PI3K/AKT signalling pathway in cancer,therapeutic implications[J].CurrCancerDrugTargets,2008,8(3):187-198.
[31] NEMAZANYY I,ESPEILLAC C,PENDE M,etal.Role of PI3K,mTOR and Akt2 signalling in hepatic tumorigenesis via the control of PKM2 expression[J].BiochemSocTrans,2013,41(4):917-922.
[32] YANG L,HOU Y,YUAN J,etal.Twist promotes reprogramming of glucose metabolism in breast cancer cells through PI3K/AKT and p53 signaling pathways[J].Oncotarget,2015,6(28):25755-25769.
[33] KONNO M,KOSEKI J,KAWAMOTO K,etal.Embryonic microRNA-369 controls metabolic splicing factors and urges cellular reprograming[J].PLoSOne,2015,10(7):e132789.
[34] LI H,LI J,JIA S,etal.miR675 upregulates long noncoding RNA H19 through activating EGR1 in human liver cancer[J].Oncotarget,2015,6(31):31958-31984.
[35] PANDITA A,MANVATI S,SINGH SK,etal.Combined effect of microRNA,nutraceuticals and drug on pancreatic cancer cell lines[J].ChemBiolInteract,2015,233:56-64.
[36] XU Q,LIU L Z,YIN Y,etal.Regulatory circuit of PKM2/NF-kappaB/miR-148a/152-modulated tumor angio-genesis and cancer progression[J].Oncogene,2015,34(43):5482-5493.
[37] LU W,ZHANG Y,ZHOU L,etal.miR-122 inhibits cancer cell malignancy by targeting PKM2 in gallbladder carcinoma[J].TumourBiol,2015.DOI:10.1007/s13277-015-4308-z.
[38] ZHANG HS,ZHANG F J,LI H,etal.Tanshinone A inhibits human esophageal cancer cell growth through miR-122-mediated PKM2 down-regulation[J].ArchBiochemBiophys,2016,598:50-56.
[39] LIU G,LI YI,GAO X.Overexpression of microRNA-133b sensitizes non-small cell lung cancer cells to irradiation through the inhibition of glycolysis[J].OncolLett,2016,11(4):2903-2908.
[40] GAO X,WANG H,YANG JJ,etal.Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase[J].MolCell,2012,45(5):598-609.
[41] ASHIZAWA K,MCPHIE P,LIN KH,etal.Aninvitronovel mechanism of regulating the activity of pyruvate kinase M2 by thyroid hormone and fructose 1,6-bisphosphate[J].Biochemistry,1991,30(29):7105-7111.
[42] ASHIZAWA K,WILLINGHAM MC,LIANG CM,etal.Invivoregulation of monomer-tetramer conversion of pyruvate kinase subtype M2 by glucose is mediated via fructose 1,6-bisphosphate[J].JBiolChem,1991,266(25):16842-16846.
[43] HITOSUGI T,KANG S,VANDER H M,etal.Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth[J].SciSignal,2009,2(97):a73.
[44] LV L,LI D,ZHAO D,etal.Acetylation targets the M2 isoform of pyruvate kinase for degradation through chaperone-mediated autophagy and promotes tumor growth[J].MolCell,2011,42(6):719-730.
[45] TAMADA M,SUEMATSU M,SAYA H.Pyruvate kinase M2:multiple faces for conferring benefits on cancer cells[J].ClinCanRes, 2012,18(20):5554-5561.
[46] YANG W,ZHENG Y,XIA Y,etal.ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect[J].NatCellBiol,2012,14(12):1295-1304.
[47] BETTAIEB A,BAKKE J,NAGATA N,etal.Protein tyrosine phosphatase 1B regulates pyruvate kinase M2 tyrosine phosphorylation[J].JBiolChem2013,288(24):17360-17371.
[48] LV L,XU YP,ZHAO D,etal.Mitogenic and oncogenic stimulation of K433 acetylation promotes PKM2 protein kinase activity and nuclear localization[J].MolCell,2013,52(3):340-352.
[49] WU S,Le H.Dual roles of PKM2 in cancer metabolism[J].ActaBiochimBiophysSin(Shanghai),2013,45(1):27-35.
[50] YU Z,ZHAO X,HUANG L,etal.Proviral insertion in murine lymphomas 2 (PIM2) oncogene phosphorylates pyruvate kinase M2 (PKM2) and promotes glycolysis in cancer cells[J].JBiolChem,2013,288(49):35406-35416.
[51] BHARDWAJ A,DAS S.SIRT6 deacetylates PKM2 to suppress its nuclear localization and oncogenic functions[J].ProcNatlAcadSciUSA,2016,113(5):E538-E547.
[52] DAS MR,BAG AK,SAHA S,etal.Molecular association of glucose-6-phosphate isomerase and pyruvate kinase M2 with glyceraldehyde-3-phosphate dehy-drogenase in cancer cells[J].BMCCancer,2016,16(1):152.
[53] LIU K,LI F,HAN H,etal.Parkin regulates the activity of pyruvate kinase M2[J].JBiolChem,2016,291(19):10307-10317.
Expression,functions and regulation of PKM2 in tumor cells
SHI Meng-kun1, YANG Xiao-dong1, SUN Feng-hao1, ZHAN Cheng△, SHI Yu1, WANG Qun1
(DepartmentofThoracicSurgery,ZhongshanHospital,FudanUniversity,Shanghai200032,China)
M2 type of pyruvate kinase (PKM2) is one of the most important regulatory molecules in glycolysis,which at high level is a major feature of tumor cells.Besides,PKM2 also regulates gene transcription and cell cycle,promoting the formation,invasion and migration of tumors.Meanwhile,PKM2 can beregulated by many transcription factors,oncogene proteins,miRNA and intermediate metabolites.Extensive studies indicated that PKM2 plays an important role in the developmental tumor.In view of the significance of PKM2 in tumor cells,it holds promise for diagnosis and treatment benefits.
M2 type of pyruvate kinase; tumor metabolism; transcrptional level
国家自然科学基金(81401875,81472225);上海市自然科学基金(14ZR1406000)
R73
B
10.3969/j.issn.1672-8467.2017.02.016
2016-05-25;编辑:王蔚)
△Corresponding author E-mail:czhan10@fudan.edu.cn
* This work was supported by the National Natural Science Foundation of China (81401875,81472225) and Natural Science Foundation of Shanghai,China (14ZR1406000).
