FOXO transcription factors in non-alcoholic fatty liver disease☆
2017-05-07CharlieDong
X.Charlie Dong
Department of Biochemistry and Molecular Biology,Center for Diabetes and Metabolic Diseases,Indiana University School of Medicine,Indianapolis,IN,USA
1.Introduction
Forkhead box O(FOXO)transcription factors belong to the O subfamily of the forkhead box protein family.1There is a singleFOXOgene inCaenorhabditis elegans(DAF-16)andDrosophila(dFOXO),and fourFOXOgenes(FOXO1/3/4/6)in mammals.FOXO proteins are highly conserved,especially the forkhead box and transactivation domains and the AKT serine/threonine protein kinase phosphorylation sites(Fig.S1).Mammals and other animals,such asCaenorhabditis elegansandDrosophila,share similar insulin/insulin-like growth factor(IGF)1 signaling cascades(Fig.1).Insulin/IGF1 activate insulin receptor/IGF1 receptor,which subsequently activate insulin receptor substrates through tyrosine phosphorylation.The activated insulin receptor substrates stimulate phosphoinositide 3-kinase,which converts phosphatidylinositol-4,5-bisphosphate [PI(4,5)P2] to phosphatidylinositol-3,4,5-trisphosphate[PI(3,4,5)P3].This stimulates 3-phosphoinositidedependent protein kinase 1 and mechanistic target of rapamycin complex 2,which activate AKT atThr308 and Ser473,respectively.2-4FOXOs are the immediate downstream effectors of AKT(Fig.2).
FOXO transcriptional activity can be regulated by various posttranslational modifications,though is predominantly regulated by phosphorylation and acetylation.5AKT kinases play a critical role in FOXO inactivation by phosphorylating a few conserved serine/threonine sites of each FOXO(FOXO1-Thr24/Ser256/Ser319,FOXO3-Thr32/Ser253/Ser315,FOXO4-Thr32/Ser197/Ser262,FOXO6-Thr26/Ser184).6In addition to AKT,there are a number of other kinases thatcan phosphorylateFOXOs,includingadenosinemonophosphate(AMP)-activated protein kinase(AMPK),c-Jun N-terminal kinase(JNK),extracellular signal-regulated kinase(ERK),p38 mitogen-activated protein kinase,mammalian sterile 20-like kinase 1,and protein kinase R-like endoplasmic reticulum kinase.7In addition to phosphorylation,FOXOs can be acetylated by p300/cyclic AMP response element-binding(CREB)binding protein(CBP)acetyltransferases and deacetylated by sirtuin(SIRT)1 and histone deacetylase 3.8-17
FOXOs have pleiotropic functions in animal systems,with effects on cell survival,anti-oxidative stress,autophagy,and metabolism(Fig.3).In this short review,I will summarize our current understanding of liver FOXOs and their role in NAFLD development.
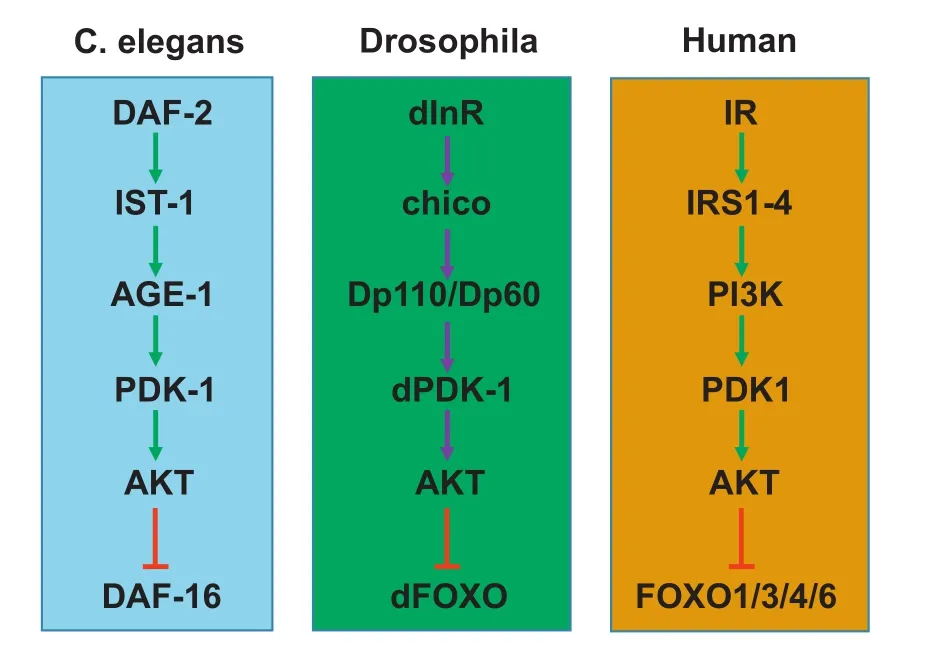
Fig.1.The insulin/insulin-like signaling pathways are evolutionally conserved.The FOXO transcription factors are regulated by the insulin/insulin-like signaling pathways that are well conserved in C.elegans,Drosophila,and mammals.Stimulation by insulin or insulin-like growth factors(IGFs),activates the insulin/IGFs receptors,and subsequently the signaling cascade of IRS→PI3K→PDK1→AKT.As a result,FOXOs are phosphorylated and inhibited by AKT.Abbreviations:FOXO,forkhead box O;IR,insulin receptor;IRS,insulin receptor substrate;PI3K,phosphoinositide 3-kinase;PDK1,3-phosphoinositide-dependent protein kinase 1;AKT,RAC-alpha serine/threonineprotein kinase.
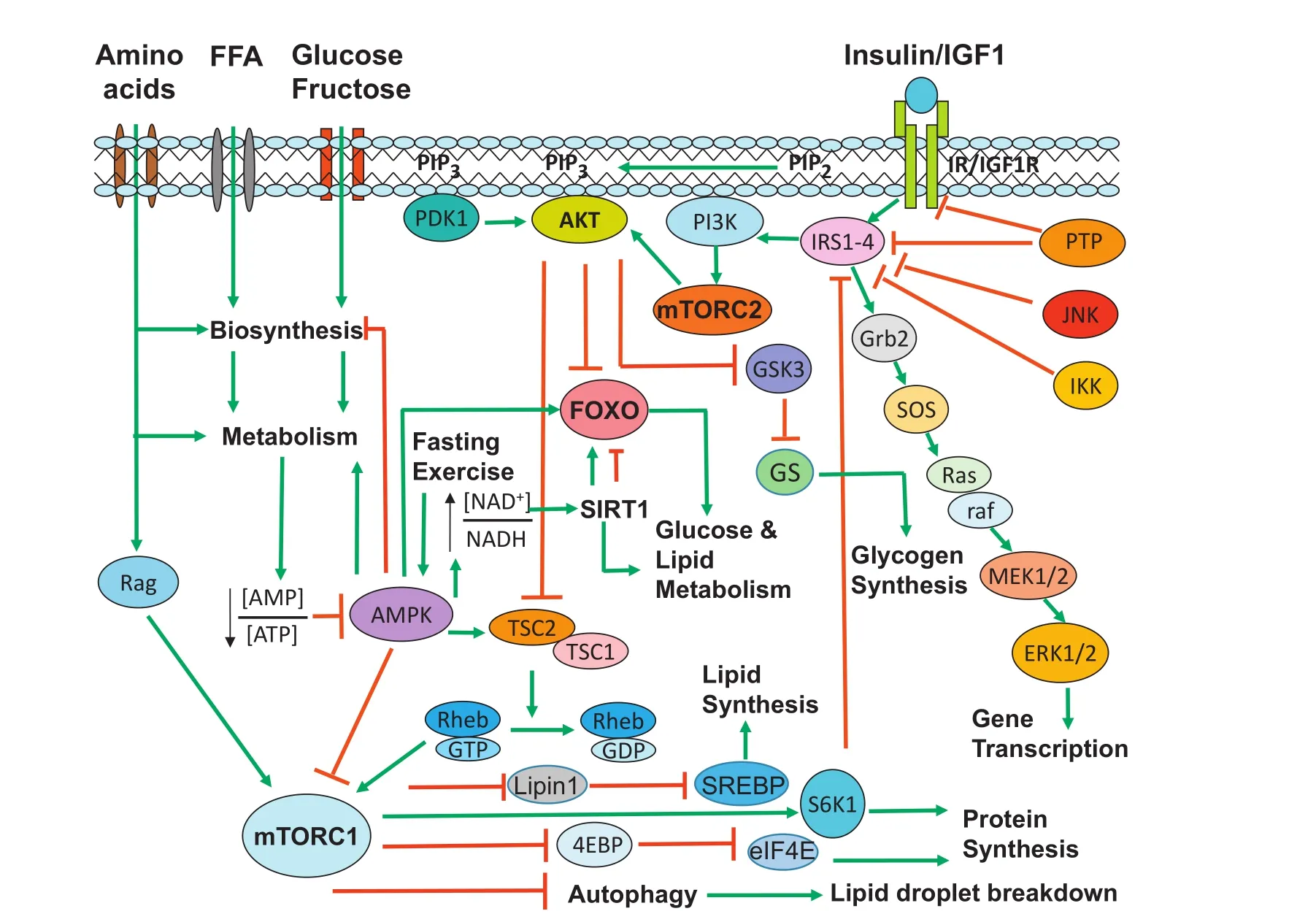
Fig.2.Insulin signaling and nutrient sensing pathways in hepatocytes.Major signaling cascades in the insulin and amino acid signaling pathways are outlined in this simpli fied diagram.Insulin and nutrient signaling is normally integrated to maintain metabolic homeostasis.Insulin plays a critical role in glucose,lipid,and protein metabolism.Upon insulin stimulation,the insulin signaling cascade(IR→IRS→PI3K→PDK1/mTORC2→AKT)is activated.As a major kinase in the downstream of the insulin signaling,AKT controls hepatic glucose and lipid homeostasis.AKT activates glycogen synthesis by inhibiting GSK3 through phosphorylation.Meanwhile,AKT inhibits the FOXO transcriptional activity for hepatic gluconeogenesis through phosphorylation and nuclear exclusion of FOXO.AKT also promotes lipid and protein synthesis through activation of mTORC1.In addition to insulin,amino acids also activate mTORC1 to promote protein synthesis and inhibit autophagy.mTORC1 stimulates lipogenesis through activation of SREBPs.FOXO is also modulated via deacetylation by SIRT1,an NAD+-dependent deacetylase.The energy sensor AMPK regulates metabolic homeostasis through activation of FOXO and inhibition of mTORC1.Abbreviations:IR,insulin receptor;IRS,insulin receptor substrate;PI3K,phosphoinositide 3-kinase;PDK1,3-phosphoinositide-dependent protein kinase 1;AKT,RAC-alpha serine/threonine-protein kinase;mTORC2,mammalian target of rapamycin complex 2;FOXO,forkhead box O;SIRT1,sirtuin 1;AMPK,AMP-activated protein kinase.
2.FOXOs in glucose and lipid metabolism
The interplay between FOXO transcription factors and insulin and nutrient signaling pathways indicates that FOXOs play an important role in both glucose and lipid metabolism(Fig.2).18-40The role of FOXOs in the regulation of genes that are critically involved in glucose,triglyceride,and cholesterol metabolism is summarized below.
2.1.FOXOs in hepatic glucose metabolism
FOXOs have been shown to play a critical role in hepatic glucose homeostasis.Knockout of eitherFoxO1alone orFoxO1/3/4altogether specifically in mouse liver leads to lower blood glucose levels under both fasting and non-fasting conditions.21,25,26,35,36,40FoxO6whole body knockout mice also exhibit lower levels of fasting and non-fasting blood glucose.18In response to starvation,FOXOs transcriptionally activate the hepatic gluconeogenic program by inducing a number of genes including phosphoenolpyruvate carboxykinase 1,glucose-6-phosphatase catalytic subunit,and pyruvate dehydrogenase kinase 4.24,26,35,36,38,40,41Meanwhile,FOXOs also inhibit glycolysis,likely through suppression of glucokinase and pyruvate kinase gene expression(Fig.3).24,26,35,36,38,41By doing so,FOXOs help maintain normal blood glucose levels during starvation.However,under insulin resistant or diabetic conditions,with the tight control of insulin signaling lacking,FOXOs continuously activate hepatic gluconeogenesis and thereby promote hyperglycemia.26,41

Fig.3.FOXOs have pleiotropic functions.Major FOXO functions are highlighted here to indicate the involvement of FOXOs in multiple cellular processes including cell cycle control,cell differentiation,glucose and lipid metabolism,energy homeostasis,autophagy,ROS detoxi fication,ER stress,DNA repair,and immune response.Numerous genes have been identi fied as FOXO targets.Owing to the limited space,only a small number of the FOXO-regulated genes for each biological process are listed here.Abbreviations:FOXO,forkhead box O;ROS,reactive oxygen species;ER,endoplasmic reticulum.
2.2.FOXOs in hepatic triglyceride metabolism
FOXOs play a critical role in triglyceride homeostasis by regulatingde novolipogenesis,fatty acid oxidation,import of free fatty acids from the blood circulation,and export of triglyceride-rich very low density lipoproteins to the blood circulation(Fig.3).In the regulation ofde novolipogenesis,FOXOs suppress the lipogenic master regulator sterol regulatory element binding protein(SREBP)1at the transcriptional level.As a result,a number of genes involved in fatty acid biosynthesis are also modulated by FOXOs,including acetyl-CoA carboxylase alpha,fatty acid synthase,adenosine triphosphate citrate lyase,malic enzyme 1,mitochondrial glycerol-3-phosphate acyltransferase,and stearoyl-CoA desaturase 1.20,21,23,29,31,36-38Moreover,FOXOs activate lipolysis and fatty acid oxidation genes including adipose triacylglycerol lipase,hormonesensitive lipase,lipoprotein lipase,and carnitine palmitoyltransferase 1.21,31,37,38,42Interestingly,FOXO1 also suppresses expression of the G0/G1 switch-2 gene that encodes an inhibitor of adipose triacylglycerol lipase.37FOXO1 has been shown to upregulate fatty acid transporters such as CD36 molecule.43In addition,FOXOs promote lipid droplet breakdown through activation of lipophagy,an autophagy process that degrades lipid droplets for energy production.A number of autophagy-related genes including autophagy related 5(ATG5),ATG12,ATG14,beclin 1,phosphatidylinositol 3-kinase catalytic subunit type 3(PIK3C3),and sestrin 3 are regulated by FOXOs.A role of autophagy in the promotion of lipid metabolism in the liver has been suggested by numerous studies34,44-49;however,the underlying mechanism remains largely unclear.
2.3.FOXOs in hepatic cholesterol metabolism
FOXOs also regulate a number of genes involved in cholesterol biosynthesis and metabolism(Fig.3).SREBP-2,the master regulator of cholesterol biosynthesis,is a direct target of FOXOs,especially FOXO3.32HepaticFoxO1/3/4triple knockouts show increased expression of theSREBP-2gene.32As expected,a number of SREBP-2 target genes including 3-hydroxy-3-methylglutaryl-CoA reductase and 3-hydroxy-3-methylglutaryl-CoA synthase 1 are also suppressed by FOXOs.21,23,32,36,38In addition to cholesterol biosynthesis,FOXO1 regulates cholesterol conversion to bile acids by modulating bile acid biosynthetic genes including cytochrome P450 family 7 subfamily A polypeptide 1(CYP7A1),CYP7B1,andCYP8B1,although there are inconsistent findings with regard to the role of FOXO1 in theCYP7A1gene regulation.FOXO1 also upregulates the genes encoding biliary cholesterol transporters-ATP binding cassette subfamily G member 5 and member 8.50-56
In addition,FOXOs regulate low-density lipoprotein(LDL)-cholesterol homeostasis.Normally,LDL-cholesterol is degraded through a LDL receptor(LDLR)-mediated clearance process;however,when the level of proprotein convertase subtilisin/kexin type 9(PCSK9)is elevated,the interaction between PCSK9 and LDLR leads to the degradation of LDLR and causes an increase in LDL-cholesterol.57Interestingly,thePCSK9gene is suppressed by FOXO3 and SIRT6.When FOXO3 or SIRT6 is de ficient in the liver,circulating LDL-cholesterol levels are elevated.58
3.FOXOs in non-alcoholic steatohepatitis
As FOXOs play a critical role in glucose and lipid homeostasis,it is not surprising that dysregulation of hepatic FOXOs may lead to metabolic disorders.Studies ofFoxOgene knockouts and overexpression in mice have provided strong evidence regarding the role of FOXOs in hepatic steatosis.On a regular diet,deletion ofFoxO1/3orFoxO1/3/4genes in mouse liver leads to mild or moderate hepatic steatosis,respectively.29,31,36Overexpression of a constitutively activeFOXO1transgene reduces hepatic triglyceride content.37,38When challenged by high-fat diets,FoxO1/3/4liverspecific knockout mice develop very severe hepatic steatosis,especially on a high-fat plus cholesterol diet.29
FOXOs have been shown to modulate in flammation through regulation of a number of genes including interleukin 1 beta,tolllike receptor 4,C-C motif chemokine ligand 2,C-C motif chemokine receptor 2,and adhesion G protein-coupled receptor E1(also namedEMR1 or F4/80)(Fig.3).Overexpression of constitutively activeFOXO1mutant in macrophages mediated by a LysM-Cre induces the expression of the C-C motif chemokine receptor 2 gene and increases the number of proin flammatory M1-type macrophages in mouse adipose tissue(though whether similar changes occur in hepatic macrophages or Kupffer cells is unclear).59Mice that are de ficient inFoxO1/3/4specifically in hepatocytes are susceptible to high-fat plus cholesterol diet-induced in flammation and liver injury.29It has been reported that FOXO1 expression and activity is elevated inpatients with steatohepatitis.60More studies are needed to clarify the role of FOXOs in human non-alcoholic steatohepatitis.
4.FOXOs in fibrosis
Human NAFLD is a progressive liver disease that begins with simple steatosis,transitions to hepatic in flammation,and later develops fibrosis as extracellular matrix proteins such as collagen gradually accumulate in the liver.Hepatic stellate cells(HSCs)are believed to play a crucial role in the development of liver fibrosis.61FOXO1hasbeen shownto inhibitproliferationandtransdifferentiation of HSCs,partly through the regulation of cyclindependent kinase inhibitor 1B and superoxide dismutase 2.62After a bile duct ligation,FoxO1+/-mice are more predisposed to hepatic fibrosis than wild-type mice.62Using the immortalized human HSC cell line LX-2,it has been shown that FOXO1 and FOXO3 are also involved in the tumor necrosis factor-related apoptosisinducing ligand-mediated apoptosis of HSCs.63In addition to their effect on HSCs,FOXO1/3/4 in hepatocytes play a protective role in diet-induced liver fibrosis.When hepaticFoxO1/3/4genes are deleted in mice,expression of fibrogenic genes including type I collagen alpha 1 and tissue inhibitor of metalloproteinase 1 is greatly elevated after the knockout mice are challenged with either a high-fat or high-fat plus cholesterol diet.29
5.Conclusions
As FOXOs have been implicated in longevity in different organisms,5,64-67their salutary functions in the liver,including maintaining glucose,triglyceride,and cholesterol homeostasis,and modulating in flammation and fibrosis,may contribute to the prolonged lifespan and protection against NAFLD(Fig.4).Importantly,FOXO activity needs to be controlled according to dynamic environmental cues,as over-or under-activation may lead to undesirable consequences.For example,under insulin resistant conditions,FOXOs are constitutively active,resulting in elevated hepatic glucose output and M1-type macrophage activation.21,30,40,59,60,68-71Additional studies are needed to fully understand the role of FOXOs in normal hepatic function and NAFLD development.
Con flict of interest
The author declares that he has no con flict of interest.

Fig.4.A working model depicting the involvement of FOXOs in the pathogenesis of NASH.A simplistic view of the role of FOXOs in the development of NASH from the perspective of three major cell types in the liver-hepatocytes,Kupffer cells,and hepatic stellate cells(HSCs).Crosstalk between these and other cell types is not illustrated here.In hepatocytes,FOXOs suppress the development of steatosis by promoting lipophagy and fatty acid oxidation and inhibiting triglyceride and cholesterol biosynthesis.In immune cells including Kupffer cells and circulating macrophages,the role of FOXOs is unclear as both pro-and anti-in flammation activities of FOXOs have been reported in the literature.Additional studies are needed to clarify the role of FOXOs in hepatic immune cells.In HSCs,FOXO1 has been shown to suppress HSC proliferation and transdifferentiation,thus inhibiting hepatic fibrosis.Abbreviations:FOXO,forkhead box O;NASH,non-alcoholic steatohepatitis.
This work was supported in part by the USA National Institutes of Health(NIH)grants including DK091592 and DK107682 from the National Institute of Diabetes and Digestive and Kidney Diseases and AA024550 from the National Institute on Alcohol Abuse and Alcoholism,by the Showalter Scholar award from Indiana University School of Medicine and Showalter Trust,and by Indiana Clinical and Translational Sciences Institute grant ULITR001108 from the NIH National Center for Advancing Translational Sciences,Clinical and Translational Sciences Award.
Appendix A.Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.livres.2017.11.004.
1.Carlsson P,Mahlapuu M.Forkhead transcription factors:key players in development and metabolism.Dev Biol.2002;250:1-23.
2.Pajvani UB,Accili D.The new biology of diabetes.Diabetologia.2015;58:2459-2468.
3.Webb AE,Brunet A.FOXO transcription factors:key regulators of cellular quality control.Trends Biochem Sci.2014;39:159-169.
4.Arden KC.FOXO animal models reveal a variety of diverse roles for FOXO transcription factors.Oncogene.2008;27:2345-2350.
5.Calnan DR,Brunet A.The FoxO code.Oncogene.2008;27:2276-2288.
6.Manning BD,Toker A.AKT/PKB signaling:navigating the network.Cell.2017;169:381-405.
7.Klotz LO,S'anchez-Ramos C,Prieto-Arroyo I,Urb'anek P,Steinbrenner H,Monsalve M.Redox regulation of FoxO transcription factors.Redox Biol.2015;6:51-72.
8.Daitoku H,Sakamaki J,Fukamizu A.Regulation of FoxO transcription factors by acetylation and protein-protein interactions.Biochim Biophys Acta.2011;1813:1954-1960.
9.Matsuzaki H,Daitoku H,Hatta M,Aoyama H,Yoshimochi K,Fukamizu A.Acetylation of Foxo1 alters its DNA-binding ability and sensitivity to phosphorylation.Proc Natl Acad Sci U S A.2005;102:11278-11283.
10.Pramanik KC,Fofaria NM,Gupta P,Srivastava SK.CBP-mediated FOXO-1 acetylation inhibits pancreatic tumor growth by targeting SirT.Mol Cancer Ther.2014;13:687-698.
11.Mihaylova MM,Vasquez DS,Ravnskjaer K,et al.Class IIa histone deacetylases are hormone-activated regulators of FOXO and mammalian glucose homeostasis.Cell.2011;145:607-621.
12.Perrot V,Rechler MM.The coactivator p300 directly acetylates the forkhead transcription factor Foxo1 and stimulates Foxo1-induced transcription.Mol Endocrinol.2005;19:2283-2298.
13.Banks AS,Kim-Muller JY,Mastracci TL,et al.Dissociation of the glucose and lipid regulatory functions of FoxO1 by targeted knockin of acetylationdefective alleles in mice.Cell Metab.2011;14:587-597.
14.Frescas D,Valenti L,Accili D.Nuclear trapping of the forkhead transcription factor FoxO1 via Sirt-dependent deacetylation promotes expression of glucogenetic genes.J Biol Chem.2005;280:20589-20595.
15.Daitoku H,Hatta M,Matsuzaki H,et al.Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity.Proc Natl Acad Sci U S A.2004;101:10042-10047.
16.Jing E,Gesta S,Kahn CR.SIRT2 regulates adipocyte differentiation through FoxO1 acetylation/deacetylation.Cell Metab.2007;6:105-114.
17.Wang F,Tong Q.SIRT2 suppresses adipocyte differentiation by deacetylating FOXO1 and enhancing FOXO1's repressive interaction with PPARgamma.Mol Biol Cell.2009;20:801-808.
18.Calabuig-Navarro V,Yamauchi J,Lee S,et al.Forkhead box O6(FoxO6)depletion attenuates hepatic gluconeogenesis and protects against fat-induced glucose disorder in mice.J Biol Chem.2015;290:15581-15594.
19.Cheng Z,Guo S,Copps K,et al.Foxo1 integrates insulin signaling with mitochondrial function in the liver.Nat Med.2009;15:1307-1311.
20.Deng X,Zhang W,O-Sullivan I,et al.FoxO1 inhibits sterol regulatory elementbinding protein-1c(SREBP-1c)gene expression via transcription factors Sp1 and SREBP-1c.J Biol Chem.2012;287:20132-20143.
21.Dong XC,Copps KD,Guo S,et al.Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation.Cell Metab.2008;8:65-76.
22.Gross DN,Wan M,Birnbaum MJ.The role of FOXO in the regulation of metabolism.Curr Diab Rep.2009;9:208-214.
23.Haeusler RA,Han S,Accili D.Hepatic FoxO1 ablation exacerbates lipid abnormalities during hyperglycemia.J Biol Chem.2010;285:26861-26868.
24.Haeusler RA,Hartil K,Vaitheesvaran B,et al.Integrated control of hepatic lipogenesis versus glucose production requires FoxO transcription factors.Nat Commun.2014;5:5190.
25.Haeusler RA,Kaestner KH,Accili D.FoxOs function synergistically to promote glucose production.J Biol Chem.2010;285:35245-35248.
26.O-Sullivan I,Zhang W,Wasserman DH,et al.FoxO1 integrates direct and indirect effects of insulin on hepatic glucose production and glucose utilization.Nat Commun.2015;6:7079.
27.Imae M,Fu Z,Yoshida A,Noguchi T,Kato H.Nutritional and hormonal factors control the gene expression of FoxOs,the mammalian homologues of DAF-16.J Mol Endocrinol.2003;30:253-262.
28.Lee S,Dong HH.FoxO integration of insulin signaling with glucose and lipid metabolism.J Endocrinol.2017;233:R67-R79.
29.Pan X,Zhang Y,Kim HG,Liangpunsakul S,Dong XC.FOXO transcription factors protect against the diet-induced fatty liver disease.Sci Rep.2017;7:44597.
30.Qu S,Altomonte J,Perdomo G,et al.Aberrant Forkhead box O1 function is associatedwith impaired hepaticmetabolism.Endocrinology.2006;147:5641-5652.
31.Tao R,Wei D,Gao H,Liu Y,DePinho RA,Dong XC.Hepatic FoxOs regulate lipid metabolism via modulation of expression of the nicotinamide phosphoribosyltransferase gene.J Biol Chem.2011;286:14681-14690.
32.Tao R,Xiong X,DePinho RA,Deng CX,Dong XC.Hepatic SREBP-2 and cholesterol biosynthesis are regulated by FoxO3 and Sirt6.J Lipid Res.2013;54:2745-2753.
33.Tikhanovich I,Cox J,Weinman SA.Forkhead box class O transcription factors in liver function and disease.J Gastroenterol Hepatol.2013;28(Suppl 1):125-131.
34.Xiong X,Tao R,DePinho RA,Dong XC.The autophagy-related gene 14(Atg14)is regulated by forkhead box O transcription factors and circadian rhythms and plays a critical role in hepatic autophagy and lipid metabolism.J Biol Chem.2012;287:39107-39114.
35.Xiong X,Tao R,DePinho RA,Dong XC.Deletion of hepatic FoxO1/3/4 genes in mice significantly impacts on glucose metabolism through downregulation of gluconeogenesis and upregulation of glycolysis.PLos One.2013;8:e74340.
36.Zhang K,Li L,Qi Y,et al.Hepatic suppression of Foxo1 and Foxo3 causes hypoglycemia and hyperlipidemia in mice.Endocrinology.2012;153:631-646.
37.Zhang W,Bu SY,Mashek MT,et al.Integrated regulation of hepatic lipid and glucose metabolism by adipose triacylglycerol lipase and FoxO proteins.Cell Rep.2016;15:349-359.
38.Zhang W,Patil S,Chauhan B,et al.FoxO1 regulates multiple metabolic pathways in the liver:effects on gluconeogenic,glycolytic,and lipogenic gene expression.J Biol Chem.2006;281:10105-10117.
39.Zhu J,Mounzih K,Chehab EF,Mitro N,Saez E,Chehab FF.Effects of FoxO4 overexpression on cholesterol biosynthesis,triacylglycerol accumulation,and glucose uptake.J Lipid Res.2010;51:1312-1324.
40.Matsumoto M,Pocai A,Rossetti L,Depinho RA,Accili D.Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver.Cell Metab.2007;6:208-216.
41.Dong X,Park S,Lin X,Copps K,Yi X,White MF.Irs1 and Irs2 signaling is essential for hepatic glucose homeostasis and systemic growth.J Clin Invest.2006;116:101-114.
42.Kamei Y,Mizukami J,Miura S,et al.A forkhead transcription factor FKHR upregulates lipoprotein lipase expression in skeletal muscle.FEBS Lett.2003;536:232-236.
43.Bastie CC,Nahl'e Z,McLoughlin T,et al.FoxO1 stimulates fatty acid uptake and oxidation in muscle cells through CD36-dependent and-independent mechanisms.J Biol Chem.2005;280:14222-14229.
44.Jaber N,Dou Z,Chen JS,et al.Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function.Proc Natl Acad Sci U S A.2012;109:2003-2008.
45.Kang X,Petyaykina K,Tao R,Xiong X,Dong XC,Liangpunsakul S.The inhibitory effect of ethanol on Sestrin3 in the pathogenesis of ethanol-induced liver injury.Am J Physiol Gastrointest Liver Physiol.2014;307:G58-G65.
46.Sinha RA,Singh BK,Zhou J,et al.Loss of ULK1 increases RPS6KB1-NCOR1 repression of NR1H/LXR-mediated Scd1 transcription and augments lipotoxicity in hepatic cells.Autophagy.2017;13:169-186.
47.Lin CW,Zhang H,Li M,et al.Pharmacological promotion of autophagy alleviates steatosis and injury in alcoholic and non-alcoholic fatty liver conditions in mice.J Hepatol.2013;58:993-999.
48.Schneider JL,Suh Y,Cuervo AM.De ficient chaperone-mediated autophagy in liver leads to metabolic dysregulation.Cell Metab.2014;20:417-432.
49.Singh R,Kaushik S,Wang Y,et al.Autophagy regulates lipid metabolism.Nature.2009;458:1131-1135.
50.Biddinger SB,Haas JT,Yu BB,et al.Hepatic insulin resistance directly promotes formation of cholesterol gallstones.Nat Med.2008;14:778-782.
51.Li T,Ma H,Park YJ,et al.Forkhead box transcription factor O1 inhibits cholesterol 7alpha-hydroxylase in human hepatocytes and in high fat diet-fed mice.Biochim Biophys Acta.2009;1791:991-996.
52.Haeusler RA,Pratt-Hyatt M,Welch CL,Klaassen CD,Accili D.Impaired generation of 12-hydroxylated bile acids links hepatic insulin signaling with dyslipidemia.Cell Metab.2012;15:65-74.
53.Li T,Kong X,Owsley E,Ellis E,Strom S,Chiang JY.Insulin regulation of cholesterol 7alpha-hydroxylase expression in human hepatocytes:roles of forkhead box O1 and sterol regulatory element-binding protein 1c.J Biol Chem.2006;281:28745-28754.
54.Park WH,Pak YK.Insulin-dependent suppression of cholesterol 7α-hydroxylase is a possible link between glucose and cholesterol metabolisms.Exp Mol Med.2011;43:571-579.
55.Dansen TB,Kops GJ,Denis S,et al.Regulation of sterol carrier protein gene expression by the forkhead transcription factor FOXO3a.J Lipid Res.2004;45:81-88.
56.Li T,Ma H,Chiang JY.TGFbeta1,TNFalpha,and insulin signaling crosstalk in regulation of the rat cholesterol 7alpha-hydroxylase gene expression.J Lipid Res.2008;49:1981-1989.
57.Cohen JC,Hobbs HH.Genetics.Simple genetics for a complex disease.Science.2013;340:689-690.
58.Tao R,Xiong X,DePinho RA,Deng CX,Dong XC.FoxO3 transcription factor and Sirt6 deacetylase regulate low density lipoprotein(LDL)-cholesterol homeostasis via control of the proprotein convertase subtilisin/kexin type 9(Pcsk9)gene expression.J Biol Chem.2013;288:29252-29259.
59.Kawano Y,Nakae J,Watanabe N,et al.Loss of Pdk1-Foxo1 signaling in myeloid cells predisposes to adipose tissue in flammation and insulin resistance.Diabetes.2012;61:1935-1948.
60.Valenti L,Rametta R,Dongiovanni P,et al.Increased expression and activity of the transcription factor FOXO1 in nonalcoholic steatohepatitis.Diabetes.2008;57:1355-1362.
61.Tsuchida T,Friedman SL.Mechanisms of hepatic stellate cell activation.Nat Rev Gastroenterol Hepatol.2017;14:397-411.
62.Adachi M,Osawa Y,Uchinami H,Kitamura T,Accili D,Brenner DA.The forkhead transcription factor FoxO1 regulates proliferation and transdifferentiation of hepatic stellate cells.Gastroenterology.2007;132:1434-1446.
63.Park SJ,Sohn HY,Yoon J,Park SI.Down-regulation of FoxO-dependent c-FLIP expression mediates TRAIL-induced apoptosis in activated hepatic stellate cells.Cell Signal.2009;21:1495-1503.
64.Morris BJ,Willcox DC,Donlon TA,Willcox BJ.FOXO3:a major gene for human longevity-a mini-review.Gerontology.2015;61:515-525.
65.Schaible R,Sussman M.FOXO in aging:did evolutionary diversi fication of FOXO function distract it from prolonging life?Bioessays.2013;35:1101-1110.
66.Mathew R,Pal Bhadra M,Bhadra U.Insulin/insulin-like growth factor-1 signalling(IIS)based regulation of lifespan across species.Biogerontology.2017;18:35-53.
67.Martins R,Lithgow GJ,Link W.Long live FOXO:unraveling the role of FOXO proteins in aging and longevity.Aging Cell.2016;15:196-207.
68.Cook JR,Matsumoto M,Banks AS,Kitamura T,Tsuchiya K,Accili D.A mutant allele encoding DNA binding-de ficient FoxO1 differentially regulates hepatic glucose and lipid metabolism.Diabetes.2015;64:1951-1965.
69.Su D,Coudriet GM,Hyun Kim D,et al.FoxO1 links insulin resistance to proin flammatory cytokine IL-1beta production in macrophages.Diabetes.2009;58:2624-2633.
70.Fan W,Morinaga H,Kim JJ,et al.FoxO1 regulates Tlr4 in flammatory pathway signalling in macrophages.EMBO J.2010;29:4223-4236.
71.Miao H,Zhang Y,Lu Z,Liu Q,Gan L.FOXO1 involvement in insulin resistancerelated pro-in flammatory cytokine production in hepatocytes.Inflamm Res.2012;61:349-358.
杂志排行
Liver Research的其它文章
- Interleukin-22 in the pathogenesis and potential treatment of liver diseases☆
- Recent development and gene therapy for glycogen storage disease type Ia☆
- Long non-coding RNA in liver metabolism and disease:Current status☆
- Interaction between stress responses and circadian metabolism in metabolic disease☆
- Decoding the role of extracellular vesicles in liver diseases☆
- Guide for Authors
