Decoding the role of extracellular vesicles in liver diseases☆
2017-05-07FengyanDengNancyMageeYuxiaZhang
Fengyan Deng,Nancy Magee,Yuxia Zhang
Department of Pharmacology,Toxicology&Therapeutics,University of Kansas Medical Center,Kansas City,KS,USA
1.Introduction
The release of membrane-surrounded extracellular vesicles(EVs)was described more than 40 years ago.1,2EVs were originally thought to bud directly from the cell membrane;however,it has become clear that EVs are a heterogeneous population of small vesicular structures released by cells.3Based on their size and mechanisms of formation and release,EVs are categorized into three main classes:exosomes,microvesicles(MVs),and apoptotic bodies.4Exosomes are the smallest EVs(30-15 nm)derived from the endosome system and are formed in multivesicular bodies(MVBs).3Compared to exosomes,MVsarelargerin size(50-1000 nm in diameter)and originate from the cell surface where they are released by direct budding from the plasma membrane.3Apoptotic bodies are the largest EV subtype(1-5μm)and are released from apoptotic cells via outward blebbing and fragmentation of the cell membrane.4Common characteristics among EV subtypes,as well as the use of inconsistent terminology and isolation methods,has made clear distinctions between exosomes and MVs dif ficult.Therefore,in this review we use the term EVs to indicate both exosomes and MVs.
Cells use EVs as a means of intercellular communication.EV cargos contain selectively enriched proteins,lipids,and ribonucleic acids(RNAs)that are transferred from donor cells to recipient cells to regulate cellular activities,such as gene expression,proliferation,differentiation,and metabolism.5,6Emerging evidence highlights that EVs,especially exosomes,play critical roles in various physiological as well as pathological processes,including tissue regeneration,neurodegenerative diseases,cancerprogression and metastasis,immune modulation,and angiogenesis.7-9Most cell types in the liver can produce EVs,which has attracted great interest in the field of liver pathology.10-12This review provides an overview of the biogenesis and secretion of EVs and summarizes the latest findings on the roles of EVs in liver physiology and pathology.We also discuss their utilization in biomarker discovery and in therapeutic applications.
2.Biogenesis,release,and puri fication of EVs
Exosomes and MVs can be differentiated based on their size and mechanism of biogenesis.Exosome biogenesis starts within the endosomal system.Invagination of the plasma membrane forms the early endosome,which matures into the late endosome that then produces intraluminal vesicles(ILVs)in multivesicular bodies(MVBs)(Fig.1).13During this process,lipids,proteins,and RNAs are sorted into these ILVs.13,14After maturation,MVBs may either be directed to lysosomes,where their cargos are degraded,or transportedtothe plasma membrane with which theyfuse torelease the ILVs into the extracellular environment(referred to as exosome release)(Fig.1).In contrast to exosomes,understanding of MV generation in cells is lacking.It is commonly accepted that MVs are released as plasma membrane-derived vesicles via direct budding from the cell surface,a step similar to the final stage of cytokinesis.15Additionally,stress responses and an increase in intracellular Ca2+concentration can induce MV release from cells.16Meanwhile,activation of pre-apoptotic caspase 3 is involved in MV release from lipotoxic hepatocytes.17Because of our limited knowledge of MV biogenesis,this review focuses on exosome biogenesis and secretion.
2.1.Exosome biogenesis
Accumulating evidence has demonstrated that two mechanisms are involved in cargo selection,clustering,and formation of ILVs within MVBs.These are a mechanism controlled by endosomal sorting complex required for transport(ESCRT)machinery and an ESCRT-independent mechanism.18,19The ESCRT consists of four multi-subunit protein complexes(ESCRT 0,I,II,and III)along with ESCRT-associated proteins.ESCRT-0 recognizes and binds ubiquitinated proteins,ESCRT-I and ESCRT-II initiate bud formation,ESCRT-III drives the pinching off of ILVs to become exosomes,and the ESCRT-associated proteins,such as vacuolar protein sorting 4(Vps4)ATPase and apoptosis-linked gene 2-interacting protein X(ALIX),allow dissociation and recycling of ESCRT complexes(see EVpedia:http://www.evpedia.info,and Visiclepedia:http://www.microvesicles.org).20,21
The critical sorting signal for endocytosis and MVB formation is initiated by ubiquitination of plasma membrane proteins.22,23During this process,the ESCRT-0 complex proteins,including hepatocyte growth factor-regulated tyrosine kinase substrate(Hrs)and signal-transducing adaptor molecule(STAM),cooperatively recognize,bind,and sequesterubiquitinated proteins.22,24,25Consequently,depletion of Hrs or STAM1 decreases exosome generation in some cells.19,26ESCRT-0 also acts as a key adaptor for the recruitment of ESCRT-I to endosomes.Bacheet al.27reported that Hrs binds to the ESCRT-I subunit,tumor susceptibility gene 101(Tsg101),and the depletion of Hrs subsequentlyleads toa reduction in membrane-associated ESCRT-I subunits,resulting in reduced MVB formation.Meanwhile,depletion of Tsg101 also leads to a reduction in exosome generation.28,29The recruitment of ESCRT-I to endosomes,in turn,recruits ESCRT-II and ESCRT-III to endosomes.23,30-32ALIX,an ESCRT-III binding protein,promotes ILV formation and cargo packaging.Colomboet al.19described that in primary dendritic cells,silencing ALIX decreases the secretion of EVs.Vps4,another ESCRT-III binding protein,promotes the scission of endosomal membranes and dissociation ofthe ESCRT-III complex from ILVs in the final steps of ILV formation.33Many studies have used ESCRT inhibition as a tool to investigate the biogenesis and secretion of exosomes.For example,a study using an RNA interference library targeting 23 components of the ESCRT machinery and their associated proteins in major histocompatibility complex class II(MHC II)-expressing HeLa-CIITA cells demonstrated that silencing ESCRT-0 or ESCRT-I can reduce exosome secretion and alter the size or composition of exosomes.19In contrast,knock down of ESCRT-III increases exosome secretion without altering their features.19
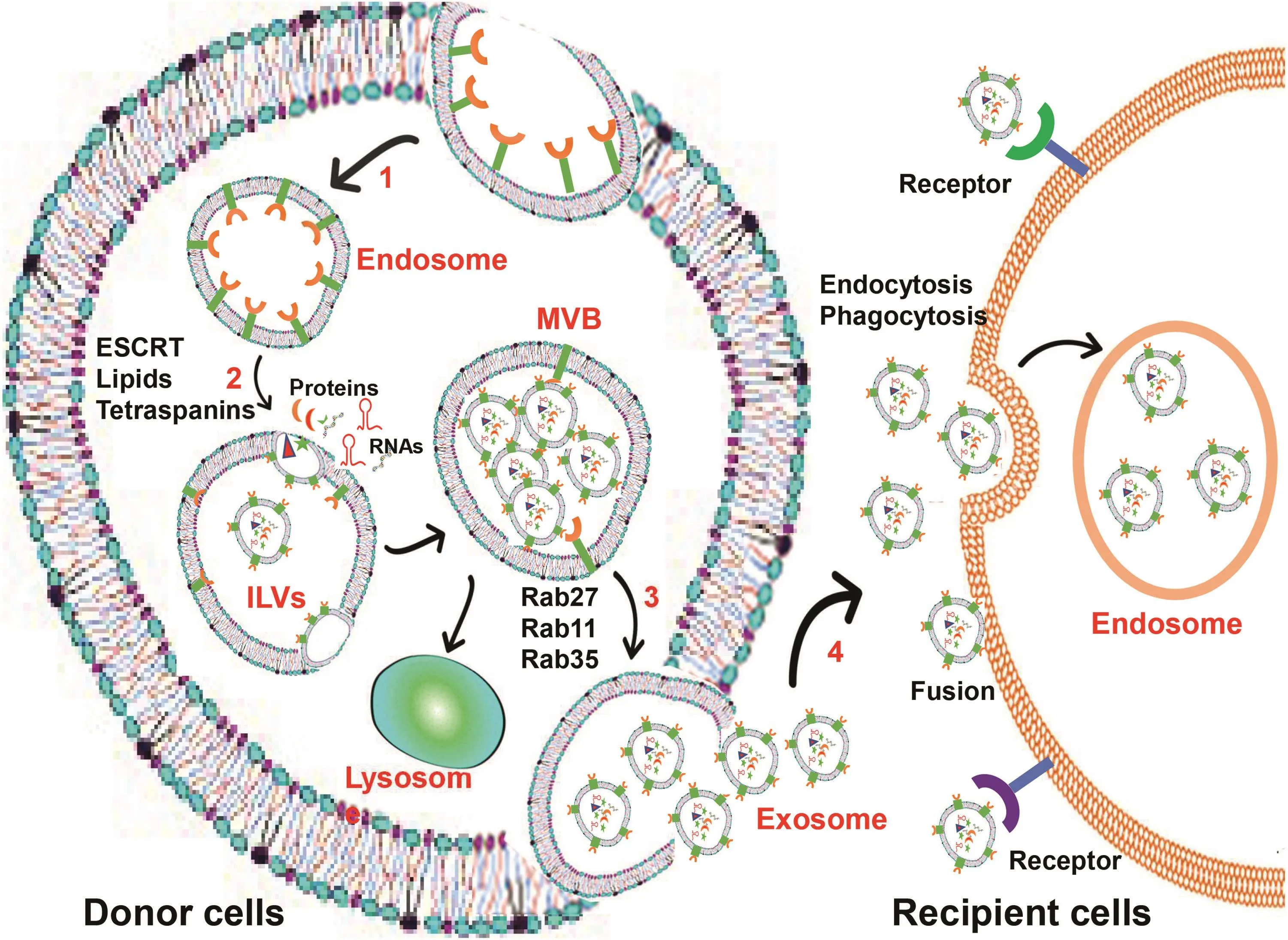
Fig.1.Exosome biogenesis and secretion.Schematic representation of exosome biogenesis and release from donor cells,and the uptake of exosomes by recipient cells.(1)Early endosomes are formed via endocytosis at the plasma membrane.(2)Early endosomes mature into late endosomes and then form ILVs,which are deposited in MVBs.(3)Several molecules are involved in the biogenesis of ILVs,including the endosomal-sorting complex required for transport(ESCRT)machinery,lipids,and tetraspanins.Mature MVBs can either be directed to lysosomes for degradation,or transported to the plasma membrane where they fuse with the membrane for exosome release.(4)Several RAB proteins,such as RAB27,RAB11,and RAB35,are involved in the transport of MVBs to the plasma membrane.The exosome internalization of recipient cells occurs mainly by receptor-mediated endocytosis,phagocytosis or fusion of exosomes with plasma membrane.Abbreviations:ILVs,intraluminal vesicles;MVBs,multivesicular bodies;RAB,Ras-related protin Rab.
Recently,ESCRT-independentmechanismsassociatedwith lipids,tetraspanins,or heat shock proteins were identi fied in ILV formation and exosome biogenesis.Exosome membranes are enriched in ceramides,cholesterol,and sphingomyelin.34,35Pharmacological or genetic inhibition of neutralsphingomyelinase leads to impaired ceramide biogenesis and decreased exosome secretion.36In contrast,drug or genetic mutation-induced cholesterol accumulation in MVBs increases exosome secretion.37Additionally,studies have demonstrated that tetraspanins,such as CD63,CD81,and tetraspanin 8(TSPAN8),are enriched in exosomes and are involved in cargo sorting into ILVs in an ESCRT-independent and ceramide-independent manner.38-40Moreover,the binding of transferrin receptor tothe chaperone heat shock protein cognate 70(HSC70)and ALIX can directly enhance the special cargo sorting of transferrin receptor into exosomes during reticulocyte maturation.41
Although both ESCRT-dependentand ESCRT-independent mechanisms have been described during the process of exosome biogenesis,it is still unclear whether these mechanisms coexist simultaneously to regulate formation of a single MVB type,or whether they indicate the existence of heterogeneous MVB populations that are regulated by various mechanisms during exosome biogenesis within cells.
2.2.Exosome secretion
The RAB family of small guanosine triphosphatases(GTPases)controls intracellular vesicular trafficking.42Several Rab GTPases,including RAB11,RAB35,RAB27A and RAB27B,play important roles in exosome release.43-45These RAB proteins help MVBs dock to the plasma membrane,which is required for the final fusion of two membranes allowing exosome release from MVBs.RAB11 has been linked to exosome release in K562 cells.46RAB35 is a target of TBC1 domain family member 10 A-C(TBC1D10 A-C),which regulates exosome secretion,and the inhibition of RAB35 results in intracellular accumulation of endosomal vesicles and impairs exosome secretion.44A study using a library of shRNAs to specifically knock down RAB proteins demonstrated that depletion of RAB5A,RAB9A,RAB2B,RAB27A,or RAB27B efficiently decreases exosome release from Hela-CIITA cells.45Additionally,this study showed that RAB27A silencing leads to an increase in the size of MVBs;whereas,RAB27B silencing results in MVB redistribution to the perinuclear region.45Certain other proteins,such as RAB7,diacylglycerol kinase alpha(DGKα),and vesicleassociated membraneprotein 7(VAMP7),also mediate exosome release.29,47,48
Exosome uptake of recipient cells is mediated predominately by endocytosis and phagocytosis.Additionally,uptake of exosomes can be mediated by the fusion of exosomes with the plasma membrane or receptors on recipient cells that recognize exosome membrane-bound proteins(Fig.1).49
2.3.Technologies for purifying EVs
Isolation of EVs largely relies on differences in the size,density,and expression of surface markers of the various EV subtypes.EV isolation can be achieved by a variety of methods,including differentialultracentrifugation,sucrose gradientcentrifugation,immunoaf finity separation,micro filtration,polymer-based precipitation using commercial kits(e.g.,ExoQuick),and micro fluidic technology.50
Differential ultracentrifugation is the most common method for EV puri fication.An initial low speed centrifugation at 2000×gfor 20 min is required to remove cells and cell debris,followed by a centrifugation at 10,000×gfor 1 h to separate large MVs and apoptotic bodies.Exosomes and smaller MVs are subsequently separated by ultracentrifugation at 100,000×gfor 1 h.51This method has been described as the gold standard;however,such stepped centrifugation procedures cannot separate small vesicles from large protein oligomers or RNA-protein complexes.A sucrose gradient centrifugation step has also been used as an extra step after ultracentrifugation,providing a clean separation of EVs from aggregatesofproteinsorRNA-protein complexes.Recently,immunoaf finity techniques using specific antibodies and integrated with af finity chromatography have been described for EV puri fication.These techniques were developed based on the differential expression of protein markers on the surface of different vesicles(e.g.,CD63 or TSG101 on exosomes,phosphatidyl serine on MVs,and annexin-V on apoptotic bodies)that can provide very specific selection of an EV sub-population.However,af finity chromatography requires special equipment and is time consuming.This barrier to scalability is likely to hinder its wide application.52
Recently,a micro fluidic technology that integrates immunoisolationand proteinanalysiswas described forone-stepisolation of EVs from bio fluids.The bio fluid is pushed through a micro fluidic device(e.g.,a microchip)coated with antibody that allows the specific selection of EVs.Heet al.53reported that microchip analysis of EVs enables selective isolation of EV sub-populations and quantitative detection of EV biomarkers from 30μL of plasma sample within~100 min,which markedly improves detection sensitivity compared to conventional EV isolation methods.In addition,the advantages of simplicity and general applicability of micro fluidic technology enables it to be readily scaled up for highthroughput screening of cancers and non-cancerous diseases.However,many challenges remain in the pro filing of surface phenotypes associated with cancers and developing reliable microchips for specific EV selection.
After puri fication,EVs can be con firmed either by direct visualization using electron microscopy or by assessment of surface marker expression by western blotting, fluorescence staining,or flow cytometry.Nanoparticle tracking analysis is a standard method to determine the size distribution of EVs.
3.EVs in liver physiology
Most cell types in the liver,such as hepatocytes,cholangiocytes,hepatic stellate cells(HSCs),sinusoidal endothelial cells(SECs),Kupffer cells,natural killer(NK)T cells,and adult liver stem cells,are exosome-secreting cells as well as targets for exosomes derived from other cells.10-12Exosomes are released continuously in basal conditions,and can be induced under various stress conditions.7,9,17,54A proteomic analysis of exosomes derived from primary rat hepatocytes identi fied 251 proteins in exosomes,109 of which can be found in exosomes from other cell types.10Additionally,hepatocyte-specific proteins involved in the metabolism of lipoproteins,endogenous compounds and xenobiotics,are also found in hepatocyte-derived exosomes,supporting a potential role of hepatocyte-derived exosomes in liver physiology.10
Exosomes released by one cell type can alter the function of recipient cells.Nojimaet al.7demonstrated that hepatocytederived exosomes induce hepatocyte proliferationin vitro,as well as liver regeneration in mouse models of liver ischemia/reperfusion injury or partial hepatectomy by transferring neutral ceramidase and sphingosine kinase 2(SK2),leading to increased synthesis of sphingosine-1-phosphate (S1P)in recipienthepatocytes.In another study,biliary exosomes were taken up by cholangiocytes and in fluenced cholangiocyteproliferation.55Recently,Wanget al.56demonstrated that biliary exosomes induce liver T-cell proliferation and macrophage activatation.Furthermore,SEC-derived exosomes are enriched in sphingosine kinase 1(SK1),which triggers S1P-dependent migration of HSCs.57Taken together,these findings indicate that exosomes derived fromvarious types of cells in the liver play critical roles in cell-to-cell communication that contributes to the maintenance of normal liver function.
4.EVs in liver diseases
Studies from the past few years have implicated aberrant biogenesis of EVs in the pathogenesis of various liver diseases(Fig.2).Here,we summarize recent findings regarding the roles of EVs in liver cancer,non-alcoholic fatty liver disease(NAFLD),alcoholic liver disease(ALD),and viral hepatitis.
4.1.EVs in liver cancer
Recent studies have highlighted EVs as crucial regulators involved in all aspects of cancer pathobiology,including tumorigenesis,angiogenesis,immunobiology,and metastasis.58-60The roles of EVs in hepatocellular carcinoma(HCC)formation and tumor growth have been extensively investigated.HCC cells appear to secret more EVs compared with non-cancer cells.61Additionally,HCC cell-derived EV cargos are different from those of equivalent healthy cells,and can be transmitted into healthy recipient cells.62Transforming growth factorβactivated kinase-1(TAK1),an essentialregulator that controls cellular homeostasis and tumorigenesis in the liver,is suppressed by EVs derived from the HCC cell line,Hep3B.62Concomitantly,the downstream signaling associated with TAK1 is modulated in recipient cells.62In another study,Weiet al.63found that HCC cell-derived EVs not only transport miRNAs into recipient cells,but also promote the growth and invasion of the parental HCC cells themselves.Together,these findings indicate that EVs play crucial roles in HCC growth.
In addition to modulating the local environment,emerging evidence shows that tumor cells exert tissue-specific effects on distant sites via secreted exosomes.For example,EVs derived from pancreatic ductal adenocarcinoma cells can induce pre-metastatic niche formation in the liver and facilitate cancer metastasis to the liver,which is associated with an increase in macrophage migration inhibitory factor(MIF)in exosomes.58,59Consistently,MIF depletion prevents pre-metastatic niche formation in the liver.59Recently,epidermal growth factor receptor(EGFR)-containing exosomes derived from gastric cancer cells have been shown to promote liver-specific metastasis through the suppression of miR-26a/b and subsequent activation of hepatocyte growth factor in the liver.64
Studies have also revealed anti-tumor function of EVs mediated by special EV cargos.Luginiet al.60reported that exosomes derived from human NK cells contain killer proteins such as Fas ligand and perforin molecules,which induce cytotoxic activity in tumor cell lines.This finding highlights the contribution of EVs to the tumorrelated immune response and indicates a potential application for using EVs in cancer immunotherapy.In another study,Fonsatoet al.12found that human adult liver stem cell(HLSC)-derived exosomes contain anti-tumor microRNAs(miR-451 and miR-31)that inhibit ectopic tumor development in severe combined immune de ficiency(SCID)mice.Recently,Weiet al.63reported that vacuolar protein sorting 4 homolog A(Vps4A),a key regulator of exosome biogenesis,is frequently down-regulated in HCC tissues,and that exosomes derived from cells stably expressing Vps4A decrease cell growth,migration,and invasion ability of HCC cells.Subsequent small RNA sequencing indicated that Vps4A facilitates the secretion and uptake of anti-tumor miRNAs into HCC cells,leading to the inactivation of the ephosphatidylinositol-3-kinase(PI3K)/AKT pathway in HCC cells.63Together,these observations suggest that EV cargos are important regulators of cancer formation,growth,and metastasis.
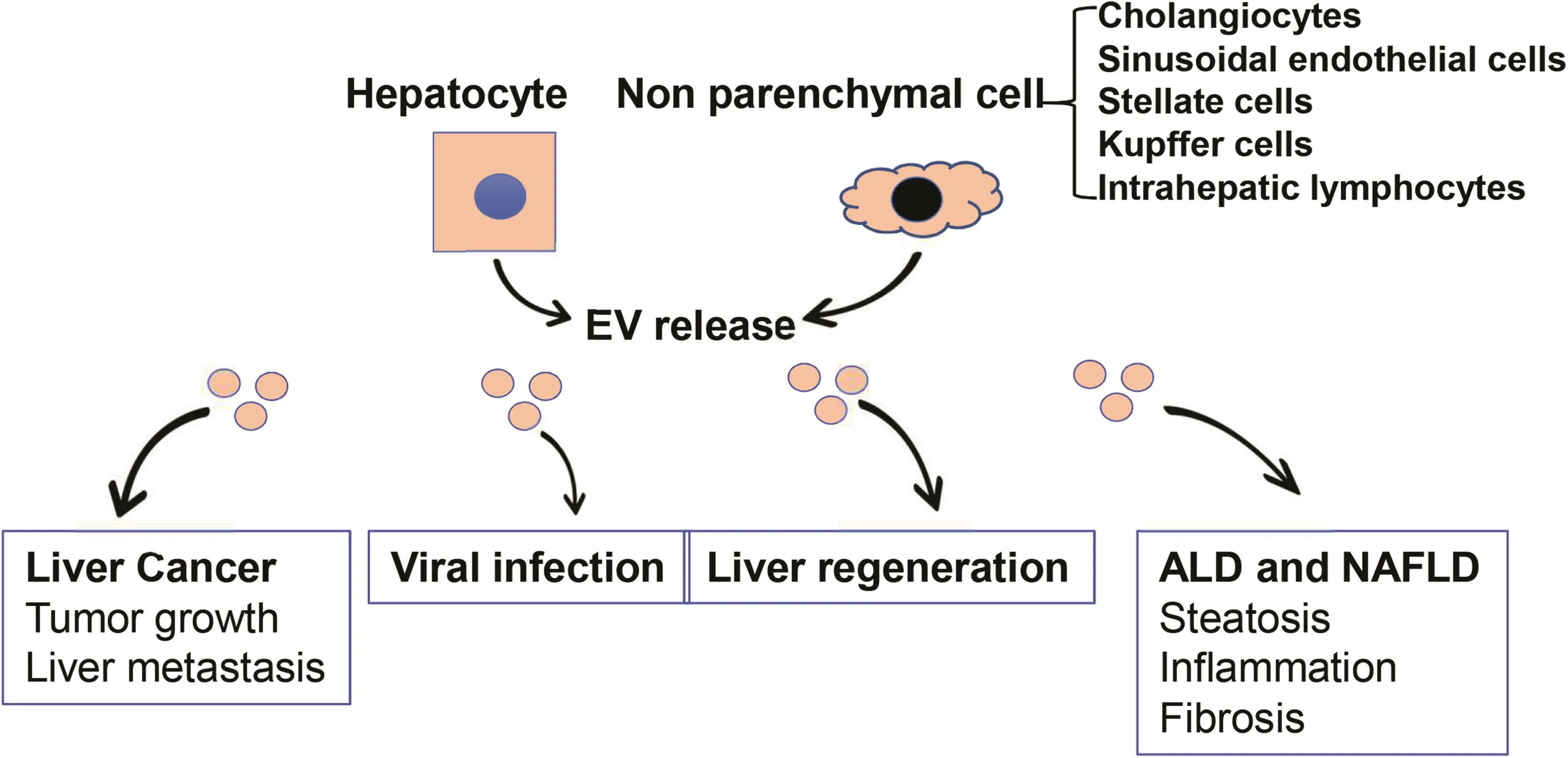
Fig.2.Roles of EVs in liver diseases.Both hepatocytes and non-parenchymal cells in the liver are EV-secreting cells as well as recipient cells for EVs derived from other cells.EVs from cancer cells promote tumor growth and create a premetastatic niche for tumor metastasis in the liver.Hepatocyte-derived EVs promote viral hepatitis and liver regeneration.EVs from hepatic endothelial cells inhibit viral progression,whereas EVs from stem cells reduce liver injury.EVs from stressed hepatocytes drive progression of alcoholic and nonalcoholic steatohepatitis through activation of macrophages and subsequent promotion of liver fibrosis.Abbreviation:EVs,extracellular vesicles.
4.2.EVs in NAFLD and ALD
NAFLD affects 25.24%(95%CI:22.10-28.65)of the general population and is rapidly becoming a major health concern worldwide.65The mechanisms underlying the pathogenesis and progression of NAFLD are incompletely understood;however,the most accepted hypothesis is that stressed or damaged cells release signals to in fluence steatosis,in flammation,and fibrosis.The involvement of EVs in stress signal transduction between cells has been suggested in the pathogenesis of NAFLD.The release of exosomes from hepatocytes was increased in mice under diet-induced steatohepatitis,and the exosome number in plasma was correlated with disease severity.66Heinrichet al.67also found that exosome numbers were significantly increased in rats fed a high-fat diet.In addition,plasma-derived exosomes from rats fed a high-fat diet significantly stimulated the production of reactive oxygen species(ROS)and induced expression of vascular cell adhesion molecule 1(VCAM1)in primary rat endothelial cells,indicating a proin flammatory response.67Recently,Ibrahimet al.68showed that mixed lineage kinase mediates the release of chemokine CXCL10-containing EVs from fat-laden hepatocytes,leading to macrophage recruitment and activation.In another study,Hirsovaet al.17reported that lysophosphatidyl choline(LPC),an intracellular metabolite of saturated palmitic acid,induces EV release from hepatocytes,which are then taken up by macrophages,resulting in a pro-in flammatory macrophage phenotype.In addition,the LPC-induced EV release is death receptor 5(DR5),caspase and Rhoassociated coiled-coilcontainingprotein kinase1 (ROCK1)-dependent.17Furthermore,knockdown of ROCK1 by shRNA or inhibition of ROCK1 by the specific inhibitor,fasudil,significantly decreases LPC-induced EV release and the progression of NAFLD.17These findings indicate the importance of EV release from fat-laden hepatocytes in driving in flammation and fibrosis during the progression of NAFLD.Based on these findings,further investigations on the pathogenesis and progression of human NAFLD are awaited.
Roles of EVs in the pathogenesis of ALD have also been extensively studied.Patients with ALD display increased blood levels of exosomes.69EV numbers in serum and the levels of some proin flammatory miRNAs in EVs(miR-192,miR-122,miR-30a,miR-744,miR-1246,miR-30b and miR-130a)were significantly increased in alcohol-fed mice.69Vermaet al.70found that exposure of hepatocytes to alcohol stimulated CD40 ligand(CD40L)-containing EV release,leading to macrophage activation.In supportof a pro-in flammatory role of CD40L in ALD,CD40-de ficient mice(CD40-/-)are protected from alcohol-induced liver injury and macrophage in filtration.70Additionally,this study showed that patients with ALD have increased blood levels of EVs enriched with CD40L.Together,these observations highlight EVs as important players in hepatocyte-to-macrophage communication during the pathogenesis of ALD.Recent studies also suggest the involvement of long noncoding RNAs(lncRNAs)in the pathogenesis of ALD.Yanget al.71showed that lncRNAs,AK054921 and AK128652,are the two most abundantly expressed lncRNAs in normal human liver and that theirlevels weresignificantlyelevated during ALD progression,indicating these two lncRNAs as novel candidates for ALD biomarkers.Whether these lncRNAs are released as EV cargos is still questionable.
4.3.EVs in viral hepatitis
The functional involvement of EVs in hepatitis C virus(HCV)infection has been documented in several studies.EVs from HCV-infected cells can transmit infection to naïve human hepatoma Huh7.5.1 cells and establish productive infection.72Exosome release inhibitors(GW4869 or spiroepoxide)strongly reduce the rate of HCV RNA transfer,suggesting that exosomes are dominant mediators in transferring HCV RNA between hepatocytes.73In addition,Dreuxet al.74showed that exosomes mediate the transfer of viral RNA from HCV-permissive cells to non-permissive plasmacytoid dendritic cells,which subsequently activates innate immunity.Furthermore,Devhareet al.75demonstrated that exosomes from HCV-infected hepatocytes activate human HSCs,which is crucial for fibrogenic activation in HCV patients.Additionally,this study showed that miR-19a was highly enriched in EVs isolated from HCV-infected hepatocytes and in sera of chronic HCV patients with fibrosis.Uptake of miR-19a into HSCs results in HSC activation through the depletion of suppressor of cytokine signaling(SOCS)3 and subsequentlyactivatesasignalingcascadeinvolving signal transducer and activator of transcription 3(STAT3),transforming growth factor β1(TGF-β1),and SMAD family member 3(SMAD3).75
The involvement of EVs in HBV infection is evidenced by signi ficant changes in EV contents in HBV-infected Huh7 cells.76Moreover,exosomes isolated from sera of chronic hepatitis B patients contain HBV viral components and can induce active infection in naïve human hepatocytes.77Recently,Yanget al.77found that exosomes shuttle HBV viral components into NK cells,leading to NK cell dysfunction through the downregulation of retinoic acid inducible gene I(RIGI)and inactivation of nuclear factorκB(NF-κB)and p38 mitogen-activated protein kinase pathways.
5.EVs as potential biomarkers for liver diseases
A growing body of evidence shows that EV cargos(both proteins and nucleic acids)can act as novel biomarkers for the diagnosis and therapeutic monitoring of many different types of liver disease.Among EV cargos,nucleic acids,especially microRNAs,have attracted great research interest.Some EV microRNAs have been reported as potential biomarkers of HCC(Table 1).MiR-21 is specifically enriched in serum exosomes compared to exosomedepleted supernatants or whole serum.78Meanwhile,serum exosomal miR-21 levels are significantly higher in patients with HCC than in patients with viral hepatitis or healthy volunteers.78Furthermore,high levels of exosomal miR-21 are correlated with advanced tumor stage.78These findings indicate that exosomal miR-21 in serum may serve as a promising biomarker for HCC diagnosis.Another study has described that levels of exosomal miR-718 are significantly decreased in sera from patients with HCC recurrence after liver transplantation compared with those without recurrence.61Additionally,the decrease in miR-718 levels is associated with malignant progression of HCC.61Hence,miR-718 may serve as a biomarker for predicting HCC recurrence.Sohnet al.79found that levels of miR-18a,miR-221,miR-222 and miR-224 were significantly higher in patients with HCC compared with levels in patients with chronic hepatitis B or liver cirrhosis,while levels of miR-101,miR-106b,miR-122 and miR-195 were lower in patients with HCC than in patients with chronic hepatitis B.Other exosomal microRNAs,such as miR-939,miR-595,and miR-519d,have also been identi fied as HCC biomarker candidates.80
EV-associated microRNAs as potential biomarkers for ALD are summarized in Table 1.Several studies have pointed toward miR-122 as a promising biomarker for diagnosis of alcohol-induced liver injury.54,69,81Circulating miR-122 is predominantly found in the exosome-rich fraction,and is correlated with increases in sera levels of the liver injury marker,alanine aminotransferase(ALT),in response to alcohol.81Consistently,alcohol consumption dramatically increases miR-122 levels in circulating exosomes.54Meanwhile,miR-122 enriched exosomes are taken up by macrophages,leading to sensitization of the pro-in flammatory response to lipopolysaccharides(LPS).54Furthermore,pro-in flammatory effects of exosomes can be prevented by exosome-mediated delivery of a miR-122 inhibitor.54Recently,another two microRNAs,miR-192 and miR-30a,were identi fied in EVs isolated from the sera of alcohol-fed mice and ALD patients,indicating that,in addition to miR-122,these two EV-associated microRNAs are also novel candidate biomarkers for ALD.69
Inaddition to HCC andALD,EV-associated microRNAs mayserve as potential biomarkers for other liver diseases.82-87For example,serum exosomal miR-122,miR-134,miR-424-3p,miR629-5p,and miR-199a are candidate biomarkers for HCV.82-84and serum exosomal miR-214 is a potential biomarker for liver fibrosis.85Human biliary EVs contain abundant miR species,of which miR-191,miR-486-3p,and miR-1274b can be used to diagnose cholangiocarcinoma(CCA).86Recently,levels of exosomal miR-34a,miR-34b,and miR-34c were found to be significantly decreased in patients with hepatoblastoma.87The authors suggested an exosomal miR-34s panel as a diagnostic and prognostic biomarker for patients with hepatoblastoma.87
EV-associated proteins as potential biomarkers for liver diseases are displayed in Table 2.Serum exosomal soluble CD81 is increased in patients with chronic hepatitis C,and its level is associated with the level of ALT and severe liver fibrosis.88The increase in plasma exosomal soluble receptor-type protein tyrosine phosphatases gamma(sPTPRG)is associated with hepatocyte damage,indicating that EVs containing sPTPRG is a candidate biomarker forhepatocyte injury.89In a proteomic study searching for liver injury biomarkers in urine,several exosomal proteins,including CD26,CD81,CD10,and solute carrier family 3 member 1(SLC3A1),were specifically increased in urine-derived EVs after acute liver injury,demonstrating that detection of these exosomal proteins in urine may indicate liver injury.90Recently,a study screening for noninvasive biomarkers for diagnosis of primary sclerosing cholangitis(PSC),CCA,and HCC,identi fied several proteins that are specifically enriched in serum-derived EVs,including aminopeptidase N(AMPN),gamma-glutamyltransferase 1(GGT1),polymeric immunoglobulin receptor(PIGR),vanin 1(VNN1),immunoglobulin heavy constant alpha 1(IGHA1),C-reactive protein(CRP),alpha-1-acid glycoprotein 1 (A1AG1),and alectin-3-binding protein(LG3BP).91
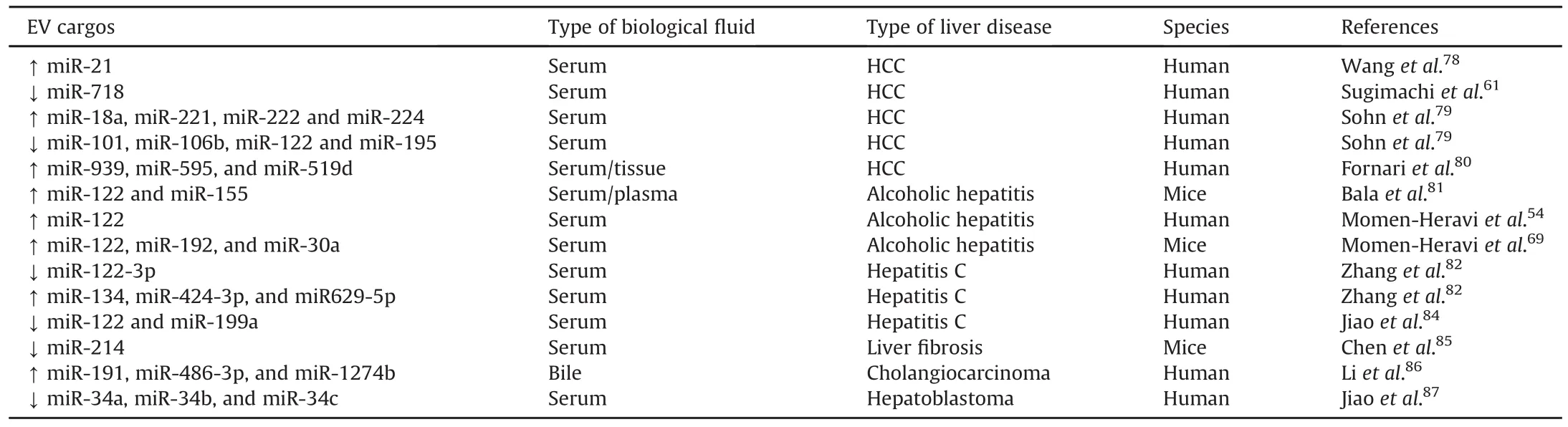
Table 1EV-associated microRNAs as potential biomarkers for liver diseases.
6.EVs as novel therapeutics for liver diseases
A large body of literature has documented the involvement EVs in promoting tissue and organ damage repair.Mesenchymal stem cell(MSC)-derived-EVs are rapidly emerging as one of the most ef ficient therapeutic agents for treating liver diseases.EVs from MSCs activate cell proliferation and promote liver regeneration,alleviate liver fibrosis,suppress liver in flammatory response,increase antitumor activities,and increase the sensitivity of cancer cells to chemotherapeutic agents.8,92-95Liet al.8found that exosomes derived from human umbilical cord MSCs can alleviate carbon tetrachloride(CCl4)-induced liver fibrosis by inhibiting the epithelial-to-mesenchymal transition.Recently,Nojimaet al.7discovered that hepatocyte-derived exosomes can induce cell proliferation and liver regeneration in mouse models of liver ischemia/reperfusion injury or partial hepatectomy by transferring neutral ceramidase and sphingosine kinase 2(SK2).Moreover,EVs isolated fromhuman menstrual blood-derived stem cells have antiapoptotic capacityand can improve liver function and survival ratesin a fulminant hepatic failure mouse model.96These findings demonstrate that the application of EVs might open new perspectives for hepatic regenerative therapies.
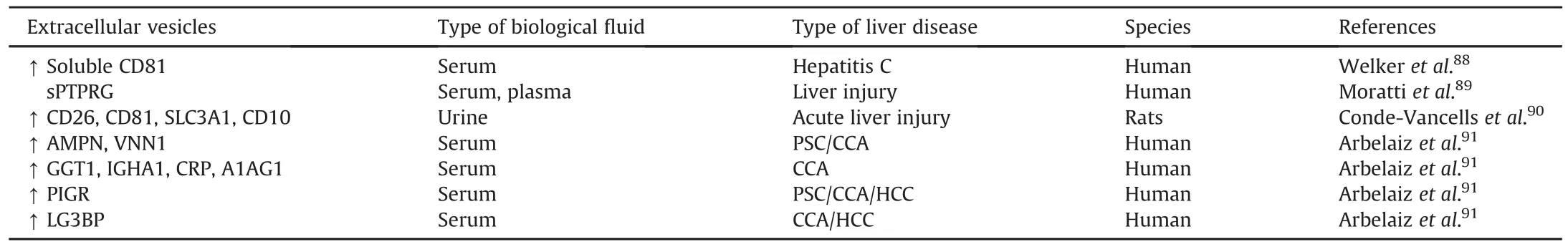
Table 2EV-associated proteins as potential biomarkers for liver diseases.
EVs are bioavailable,biocompatible and resistant to RNases and proteases;therefore,they have attracted great interest for their potential as natural carriers for drugs,proteins,microRNAs,silencing RNA(siRNA)and other molecules.97-99In 2011,Alvarez-Ervitiet al.100reported the first exosome-based exogenous siRNA delivery for gene silencing.In this study,self-derived dendritic cells were used for exosome production to reduce immunogenicity.Intravenous injection of engineered neuron-targeting exosomes that contain siRNA targeting beta-site APP cleaving enzyme 1(BACE1)(a therapeutic target in Alzheimer's disease)resulted in a specific knockdown of BACE1 in the brain.100miR-155 is an exosomal microRNA that regulates in flammation in alcoholic hepatitis.81,101Recently,B cell-derived exosomes,which harbor very low levels of endogenous miR-155,have been engineered as ef ficient vehicles to deliver a miR-155 mimic or inhibitors into hepatocytes and macrophages.102,103In vitroculture of RAW macrophage cells with exosomes containing miR-155 inhibitor significantly blocked LPS-induced production of tumor necrosis factor α (TNFα),and partially prevented LPS repression of SOCS 1.103In addition,in vivogene delivery of engineered exosomes containing exo-miR-155 mimic to liver macrophages and hepatocytes was successfully achieved in miR-155 KO mice.102,103This study also reported that exosome-based gene delivery causes very minimal cytotoxicity.103Together,these studies open new avenues for treating liver diseases with exosome based delivery of nucleic acids.
Because of the important roles of EVs in liver diseases,EV removal from the circulation could be an attractive therapeutic option.104Two approaches to remove circulating EVs have been reported,including the deletion of genes required for EV biogenesis and release,and the use of an af finity plasmapheresis platform known as the Aethlon adaptive dialysis-like af finity platform technology(ADAPT™)system to remove EVs from the entire circulatory system.104However,no complete EV blockade has been reported to date.
7.Conclusions and future prospects
EVs and EV cargos(e.g.,proteins,lipids,and RNAs)contribute significantly to cell-to-cell communication in liver physiology and pathophysiology.Hence,EVs have attracted great interest for their potential as disease biomarkers and novel therapeutic modalities in liver and other diseases.To date,numerous EV-associated micro-RNAs and proteins have been identi fied as prospective biomarkers for various liver diseases,and different strategies for the therapeutic application of EVs are currently being evaluated.However,despite the signi ficant advances that have been made,many questions still remain unanswered.For example,characterization of the function of EV-cargos in liver pathology requires further investigation.Also,despite the appeal of using EVs in therapeutic intervention,there are still challenges remaining before actualization.For example,comprehensive blockade of EV secretion is an attractive strategy because of the detrimental effects that EVs have in liver diseases.However,blocking total EV secretion increases the risk of developing other disorders,due to the multifarious roles of EVs.Overall,further functional analyses of EV cargos may facilitate their use in the diagnosis and therapy of liver diseases.
Con flict of interest
The authors declare that they have no con flict of interest.
This work was supported by the USA National Institutes of Health grants NCI K22CA184146,P20 GM103549,P20GM103418,P30GM118247,and T32ES007079.
1.Crawford N.The presence of contractile proteins in platelet microparticles isolated from human and animal platelet-free plasma.Br J Haematol.1971;21:53-69.
2.Anderson HC.Vesicles associated with calci fication in the matrix of epiphyseal cartilage.J Cell Biol.1969;41:59-72.
3.Abels ER,Breake field XO.Introduction to extracellular vesicles:biogenesis,RNA cargo selection,content,release,and uptake.Cell Mol Neurobiol.2016;36:301-312.
4.Kalra H,Simpson RJ,Ji H,et al.Vesiclepedia:a compendium for extracellular vesicleswith continuouscommunity annotation.PLoSBiol.2012;10.e1001450.
5.Vlassov AV,Magdaleno S,Setterquist R,Conrad R.Exosomes:current knowledge of their composition,biological functions,and diagnostic and therapeutic potentials.Biochim Biophys Acta.2012;1820:940-948.
6.Mathivanan S,Fahner CJ,Reid GE,Simpson RJ.ExoCarta 2012:database of exosomal proteins,RNA and lipids.Nucleic Acids Res.2012;40:D1241-D1244.
7.Nojima H,Freeman CM,Schuster RM,et al.Hepatocyte exosomes mediate liverrepairand regeneration viasphingosine-1-phosphate.JHepatol.2016;64:60-68.
8.Li T,Yan Y,Wang B,et al.Exosomes derived from human umbilical cord mesenchymal stem cells alleviate liver fibrosis.Stem Cells Dev.2013;22:845-854.
9.Qu Z,Wu J,Wu J,Luo D,Jiang C,Ding Y.Exosomes derived from HCC cells induce sorafenib resistance in hepatocellular carcinoma both in vivo and in vitro.J Exp Clin Cancer Res.2016;35:159.
10.Conde-Vancells J,Rodriguez-Suarez E,Embade N,et al.Characterization and comprehensive proteome pro filing of exosomes secreted by hepatocytes.J Proteome Res.2008;7:5157-5166.
11.Pan Q,Ramakrishnaiah V,Henry S,et al.Hepatic cell-to-cell transmission of small silencing RNA can extend the therapeutic reach of RNA interference(RNAi).Gut.2012;61:1330-1339.
12.Fonsato V,Collino F,Herrera MB,et al.Human liver stem cell-derived microvesicles inhibit hepatoma growth in SCID mice by delivering antitumor microRNAs.Stem Cells.2012;30:1985-1998.
13.Huotari J,Helenius A.Endosome maturation.EMBO J.2011;30:3481-3500.
14.Grant BD,Donaldson JG.Pathways and mechanisms of endocytic recycling.Nat Rev Mol Cell Biol.2009;10:597-608.
15.Muralidharan-Chari V,Clancy JW,Sedgwick A,D'Souza-Schorey C.Microvesicles:mediators of extracellular communication during cancer progression.J Cell Sci.2010;123:1603-1611.
16.Hirsova P,Ibrahim SH,Verma VK,et al.Extracellular vesicles in liver pathobiology:small particles with big impact.Hepatology.2016;64:2219-2233.
17.Hirsova P,Ibrahim SH,Krishnan A,et al.Lipid-induced signaling causes release of in flammatory extracellular vesicles from hepatocytes.Gastroenterology.2016;150:956-967.
18.Wollert T,Hurley JH.Molecular mechanism of multivesicular body biogenesis by ESCRT complexes.Nature.2010;464:864-869.
19.Colombo M,Moita C,van Niel G,et al.Analysis of ESCRT functions in exosome biogenesis,composition and secretion highlights the heterogeneity of extracellular vesicles.J Cell Sci.2013;126:5553-5565.
20.Henne WM,Buchkovich NJ,Emr SD.The ESCRT pathway.Dev Cell.2011;21:77-91.
21.Henne WM,Stenmark H,Emr SD.Molecular mechanisms of the membrane sculpting ESCRT pathway.Cold Spring Harb Perspect Biol.2013;5.pii:a016766.
22.Mayers JR,Wang L,Pramanik J,et al.Regulation of ubiquitin-dependent cargo sorting by multiple endocytic adaptors at the plasma membrane.Proc Natl Acad Sci U S A.2013;110:11857-11862.
23.Katzmann DJ,Babst M,Emr SD.Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex,ESCRT-I.Cell.2001;106:145-155.
24.Mayers JR,Fyfe I,Schuh AL,Chapman ER,Edwardson JM,Audhya A.ESCRT-0 assembles as a heterotetrameric complex on membranes and binds multiple ubiquitinylated cargoes simultaneously.J Biol Chem.2011;286:9636-9645.
25.Raiborg C,Stenmark H.The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins.Nature.2009;458:445-452.
26.Tamai K,Tanaka N,Nakano T,et al.Exosome secretion of dendritic cells is regulated by Hrs,an ESCRT-0 protein.Biochem Biophys Res Commun.2010;399:384-390.
27.Bache KG,Brech A,Mehlum A,Stenmark H.Hrs regulates multivesicular body formation via ESCRT recruitment to endosomes.J Cell Biol.2003;162:435-442.
28.Abrami L,Brandi L,Moayeri M,et al.Hijacking multivesicular bodies enables long-term and exosome-mediated long-distance action of anthrax toxin.Cell Rep.2013;5:986-996.
29.Baietti MF,Zhang Z,Mortier E,et al.Syndecan-syntenin-ALIX regulates the biogenesis of exosomes.Nat Cell Biol.2012;14:677-685.
30.Babst M,Katzmann DJ,Snyder WB,Wendland B,Emr SD.Endosome-associated complex,ESCRT-II,recruits transport machinery for protein sorting at the multivesicular body.Dev Cell.2002;3:283-289.
31.Babst M,Katzmann DJ,Estepa-Sabal EJ,Meerloo T,Emr SD.Escrt-III:an endosome-associated heterooligomeric protein complex required for mvb sorting.Dev Cell.2002;3:271-282.
32.Wollert T,Wunder C,Lippincott-Schwartz J,Hurley JH.Membrane scission by the ESCRT-III complex.Nature.2009;458:172-177.
33.Davies BA,Azmi IF,Katzmann DJ.Regulation of Vps4 ATPase activity by ESCRT-III.Biochem Soc Trans.2009;37:143-145.
34.Laulagnier K,Motta C,Hamdi S,et al.Mast cell-and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization.Biochem J.2004;380:161-171.
35.M¨obius W,van Donselaar E,Ohno-Iwashita Y,et al.Recycling compartments and the internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway.Traffic.2003;4:222-231.
36.Trajkovic K,Hsu C,Chiantia S,et al.Ceramide triggers budding of exosome vesicles into multivesicular endosomes.Science.2008;319:1244-1247.
37.Strauss K,Goebel C,Runz H,et al.Exosome secretion ameliorates lysosomal storage of cholesterol in Niemann-Pick type C disease.J Biol Chem.2010;285:26279-26288.
38.van Niel G,Charrin S,Simoes S,et al.The tetraspanin CD63 regulates ESCRT-independent and-dependent endosomal sorting during melanogenesis.Dev Cell.2011;21:708-721.
39.Perez-Hernandez D,Guti'errez-V'azquez C,Jorge I,et al.The intracellular interactome of tetraspanin-enriched microdomains reveals their function as sorting machineries toward exosomes.J Biol Chem.2013;288:11649-11661.
40.Nazarenko I,Rana S,Baumann A,et al.Cell surface tetraspanin Tspan8 contributes to molecular pathways of exosome-induced endothelial cell activation.Cancer Res.2010;70:1668-1678.
41.G'eminard C,De Gassart A,Blanc L,Vidal M.Degradation of AP2 during reticulocyte maturation enhances binding of hsc70 and Alix to a common site on TFR for sorting into exosomes.Traffic.2004;5:181-193.
42.Stenmark H.Rab GTPases as coordinators of vesicle traf fic.Nat Rev Mol Cell Biol.2009;10:513-525.
43.Savina A,Furl'an M,Vidal M,Colombo MI.Exosome release is regulated by a calcium-dependentmechanism inK562cells.JBiolChem.2003;278:20083-20090.
44.Hsu C,Morohashi Y,Yoshimura S,et al.Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C.J Cell Biol.2010;189:223-232.
45.Ostrowski M,Carmo NB,Krumeich S,et al.Rab27a and Rab27b control different steps of the exosome secretion pathway.Nat Cell Biol.2010;12:19-30.
46.Savina A,Fader CM,Damiani MT,Colombo MI.Rab11 promotes docking and fusion of multivesicular bodies in a calcium-dependent manner.Traffic.2005;6:131-143.
47.Alonso R,Mazzeo C,M'erida I,Izquierdo M.A new role of diacylglycerol kinase alpha on the secretion of lethal exosomes bearing Fas ligand during activation-induced cell death of T lymphocytes.Biochimie.2007;89:213-221.
48.Fader CM,S'anchez DG,Mestre MB,Colombo MI.TI-VAMP/VAMP7 and VAMP3/cellubrevin:two v-SNARE proteins involved in specific steps of the autophagy/multivesicular body pathways.Biochim Biophys Acta.2009;1793:1901-1916.
49.Mulcahy LA,Pink RC,Carter DR.Routes and mechanisms of extracellular vesicle uptake.J Extracell Vesicles.2014;3:24641.
50.Huang-Doran I,Zhang CY,Vidal-Puig A.Extracellular vesicles:novel mediators of cell communication in metabolic disease.Trends Endocrinol Metab.2017;28:3-18.
51.Witwer KW,Buz'as EI,Bemis LT,et al.Standardization of sample collection,isolation and analysis methods in extracellular vesicle research.J Extracell Vesicles.2013;2:20360.
52.Coumans FAW,Brisson AR,Buzas EI,et al.Methodological guidelines to study extracellular vesicles.Circ Res.2017;120:1632-1648.
53.He M,Crow J,Roth M,Zeng Y,Godwin AK.Integrated immunoisolation and protein analysis of circulating exosomes using micro fluidic technology.Lab Chip.2014;14:3773-3780.
54.Momen-Heravi F,Bala S,Kodys K,Szabo G.Exosomes derived from alcoholtreated hepatocytes horizontally transfer liver specific miRNA-122 and sensitize monocytes to LPS.Sci Rep.2015;5:9991.
55.Masyuk AI,Huang BQ,Ward CJ,et al.Biliary exosomes in fluence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia.Am J Physiol Gastrointest Liver Physiol.2010;299:G990-G999.
56.Wang Y,Wang G,Wang Z,Zhang H,Zhang L,Cheng Z.Chicken biliary exosomes enhance CD4(+)T proliferation and inhibit ALV-J replication in liver.Biochem Cell Biol.2014;92:145-151.
57.Wang R,Ding Q,Yaqoob U,et al.Exosome adherence and internalization by hepatic stellate cells triggers sphingosine 1-phosphate-dependent migration.J Biol Chem.2015;290:30684-30696.
58.Yu Z,Zhao S,Ren L,et al.Pancreatic cancer-derived exosomes promote tumor metastasis and liver pre-metastatic niche formation.Oncotarget.2017;8:63461-63483.
59.Costa-Silva B,Aiello NM,Ocean AJ,et al.Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver.Nat Cell Biol.2015;17:816-826.
60.Lugini L,Cecchetti S,Huber V,et al.Immune surveillance properties of human NK cell-derived exosomes.J Immunol.2012;189:2833-2842.
61.Sugimachi K,Matsumura T,Hirata H,et al.Identi fication of a bona fide microRNA biomarker in serum exosomes that predicts hepatocellular carcinoma recurrence after liver transplantation.Br J Cancer.2015;112:532-538.
62.Kogure T,Lin WL,Yan IK,Braconi C,Patel T.Intercellular nanovesiclemediated microRNA transfer:a mechanism of environmental modulation of hepatocellular cancer cell growth.Hepatology.2011;54:1237-1248.
63.Wei JX,Lv LH,Wan YL,et al.Vps4A functions as a tumor suppressor by regulating the secretion and uptake of exosomal microRNAs in human hepatoma cells.Hepatology.2015;61:1284-1294.
64.Zhang H,Deng T,Liu R,et al.Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis.Nat Commun.2017;8:15016.
65.Younossi ZM,Koenig AB,Abdelatif D,Fazel Y,Henry L,Wymer M.Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence,incidence,and outcomes.Hepatology.2016;64:73-84.
66.Povero D,Eguchi A,Niesman IR,et al.Lipid-induced toxicity stimulates hepatocytes to release angiogenic microparticles that require Vanin-1 for uptake by endothelial cells.Sci Signal.2013;6.ra88.
67.Heinrich LF,Andersen DK,Cleasby ME,Lawson C.Long-term high fat feeding of rats results in increased numbers of circulating microvesicles with proin flammatory effects on endothelial cells.Br J Nutr.2015;113:1704-1711.
68.Ibrahim SH,Hirsova P,Tomita K,et al.Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes.Hepatology.2016;63:731-744.
69.Momen-Heravi F,Saha B,Kodys K,Catalano D,Satishchandran A,Szabo G.Increased number of circulating exosomes and their microRNA cargos are potential novel biomarkers in alcoholic hepatitis.J Transl Med.2015;13:261.
70.Verma VK,Li H,Wang R,et al.Alcohol stimulates macrophage activation through caspase-dependent hepatocyte derived release of CD40L containing extracellular vesicles.J Hepatol.2016;64:651-660.
71.Yang Z,Ross RA,Zhao S,Tu W,Liangpunsakul S,Wang L.LncRNA AK054921 and AK128652 are potential serum biomarkers and predictors of patient survival with alcoholic cirrhosis.Hepatol Commun.2017;1:513-523.
72.Ramakrishnaiah V,Thumann C,Fofana I,et al.Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells.Proc Natl Acad Sci U S A.2013;110:13109-13113.
73.Longatti A,Boyd B,Chisari FV.Virion-independent transfer of replicationcompetent hepatitis C virus RNA between permissive cells.J Virol.2015;89:2956-2961.
74.Dreux M,Garaigorta U,Boyd B,et al.Short-range exosomal transfer of viral RNA from infected cells to plasmacytoid dendritic cells triggers innate immunity.Cell Host Microbe.2012;12:558-570.
75.Devhare PB,Sasaki R,Shrivastava S,Di Bisceglie AM,Ray R,Ray RB.Exosomemediated intercellular communication between hepatitis C virus-infected hepatocytes and hepatic stellate cells.J Virol.2017;91.pii:e02225-e02216.
76.Zhao X,Wu Y,Duan J,et al.Quantitative proteomic analysis of exosome protein content changes induced by hepatitis B virus in Huh-7 cells using SILAC labeling and LC-MS/MS.J Proteome Res.2014;13:5391-5402.
77.Yang Y,Han Q,Hou Z,Zhang C,Tian Z,Zhang J.Exosomes mediate hepatitis B virus(HBV)transmission and NK-cell dysfunction.Cell Mol Immunol.2017;14:465-475.
78.Wang H,Hou L,Li A,Duan Y,Gao H,Song X.Expression of serum exosomal microRNA-21 in human hepatocellular carcinoma.Biomed Res Int.2014;2014:864894.
79.Sohn W,Kim J,Kang SH,et al.Serum exosomal microRNAs as novel biomarkers for hepatocellular carcinoma.Exp Mol Med.2015;47:e184.
80.Fornari F,Ferracin M,TrerˋeD,et al.Circulating microRNAs,miR-939,miR-595,miR-519d and miR-494,Identify cirrhotic patients with HCC.PLoS One.2015;10:e0141448.
81.Bala S,Petrasek J,Mundkur S,et al.Circulating microRNAs in exosomes indicate hepatocyte injury and in flammation in alcoholic,drug-induced,and in flammatory liver diseases.Hepatology.2012;56:1946-1957.
82.Zhang S,Ouyang X,Jiang X,et al.Dysregulated serum microRNA expression pro file and potential biomarkers in hepatitis C virus-infected patients.Int J Med Sci.2015;12:590-598.
83.Bukong TN,Momen-Heravi F,Kodys K,Bala S,Szabo G.Exosomes from hepatitis C infected patients transmit HCV infection and contain replication competent viral RNA in complex with Ago2-miR122-HSP90.PLoS Pathog.2014;10:e1004424.
84.Jiao X,Fan Z,Chen H,et al.Serum and exosomal miR-122 and miR-199a as a biomarker to predict therapeutic ef ficacy of hepatitis C patients.J Med Virol.2017;89:1597-1605.
85.Chen L,Chen R,Kemper S,Charrier A,Brigstock DR.Suppression of fibrogenic signaling in hepatic stellate cells by Twist1-dependent microRNA-214 expression:role of exosomes in horizontal transfer of Twist1.Am J Physiol Gastrointest Liver Physiol.2015;309:G491-G499.
86.Li L,Masica D,Ishida M,et al.Human bile contains microRNA-laden extracellular vesicles that can be used for cholangiocarcinoma diagnosis.Hepatology.2014;60:896-907.
87.Jiao C,Jiao X,Zhu A,Ge J,Xu X.Exosomal miR-34s panel as potential novel diagnostic and prognostic biomarker in patients with hepatoblastoma.J Pediatr Surg.2017;52:618-624.
88.Welker MW,Reichert D,Susser S,et al.Soluble serum CD81 is elevated in patients with chronic hepatitis C and correlates with alanine aminotransferase serum activity.PLoS One.2012;7:e30796.
89.Moratti E,Vezzalini M,Tomasello L,Giavarina D,Sorio C.Identi fication of protein tyrosine phosphatase receptor gamma extracellular domain(sPTPRG)as a natural soluble protein in plasma.PLoS One.2015;10:e0119110.
90.Conde-Vancells J,Rodriguez-Suarez E,Gonzalez E,et al.Candidate biomarkers in exosome-like vesicles puri fied from rat and mouse urine samples.Proteomics Clin Appl.2010;4:416-425.
91.Arbelaiz A,Azkargorta M,Krawczyk M,et al.Serum extracellular vesicles contain protein biomarkers for primary sclerosing cholangitis and cholangiocarcinoma.Hepatology.2017;66:1125-1143.
92.Lou G,Song X,Yang F,et al.Exosomes derived from miR-122-modi fied adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma.J Hematol Oncol.2015;8:122.
93.Tan CY,Lai RC,Wong W,Dan YY,Lim SK,Ho HK.Mesenchymal stem cellderived exosomes promote hepatic regeneration in drug-induced liver injury models.Stem Cell Res Ther.2014;5:76.
94.Nong K,Wang W,Niu X,et al.Hepatoprotective effect of exosomes from human-induced pluripotent stem cell-derived mesenchymal stromal cells against hepatic ischemia-reperfusion injury in rats.Cytotherapy.2016;18:1548-1559.
95.Ma B,Ren J,Jiang HF,Jia J.Antitumor activities against hepatocellular carcinoma induced by bone marrow mesenchymal stem cells pulsed with tumorderived exosomes.Beijing Da Xue Xue Bao.2008;40:494-499.
96.Chen L,Xiang B,Wang X,Xiang C.Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure.Stem Cell Res Ther.2017;8:9.
97.Johnsen KB,Gudbergsson JM,Skov MN,Pilgaard L,Moos T,Duroux M.A comprehensive overview of exosomes as drug delivery vehicles-endogenousnanocarriersfortargeted cancertherapy.BiochimBiophysActa.2014;1846:75-87.
98.Srivastava A,Babu A,Filant J,et al.Exploitation of exosomes as nanocarriers for gene-,chemo-,and immune-therapy of cancer.J Biomed Nanotechnol.2016;12:1159-1173.
99.Marcus ME,Leonard JN.FedExosomes:Engineering therapeutic biological nanoparticles that truly deliver.Pharmaceuticals(Basel).2013;6:659-680.
100.Alvarez-Erviti L,Seow Y,Yin H,Betts C,Lakhal S,Wood MJ.Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes.Nat Biotechnol.2011;29:341-345.
101.Bala S,Marcos M,Kodys K,et al.Up-regulation of microRNA-155 in macrophages contributes to increased tumor necrosis factor{alpha}(TNF{alpha})production via increased mRNA half-life in alcoholic liver disease.J Biol Chemi.2011;286:1436-1444.
102.Bala S,Csak T,Momen-Heravi F,et al.Biodistribution and function of extracellular miRNA-155 in mice.Sci Rep.2015;5:10721.
103.Momen-Heravi F,Bala S,Bukong T,Szabo G.Exosome-mediated delivery of functionally active miRNA-155 inhibitor to macrophages.Nanomedicine.2014;10:1517-1527.
104.Marleau AM,Chen CS,Joyce JA,Tullis RH.Exosome removal as a therapeutic adjuvant in cancer.J Transl Med.2012;10:134.
杂志排行
Liver Research的其它文章
- Interleukin-22 in the pathogenesis and potential treatment of liver diseases☆
- Recent development and gene therapy for glycogen storage disease type Ia☆
- FOXO transcription factors in non-alcoholic fatty liver disease☆
- Long non-coding RNA in liver metabolism and disease:Current status☆
- Interaction between stress responses and circadian metabolism in metabolic disease☆
- Guide for Authors
