MOLECULAR CLONING AND EXPRESSION CHARACTERIZATION OF HEAT SHOCK PROTEIN 70 FROM GOBIOCYPRIS RARUS
2017-02-15HUANGShuTingLIUQiuPingDENGChuanHEYeXIONGLiNIUChunQingCHENYunandLIUYan
HUANG Shu-Ting, LIU Qiu-Ping, DENG Chuan, HE Ye, XIONG Li, NIU Chun-Qing, CHEN Yun and LIU Yan
(Key Laboratory of Freshwater Fish Reproduction and Development, Key Laboratory of Eco-environments in Three Gorges Reservoir Region, Key Laboratory of Aquatic Science of Chongqing, School of Life Sciences, Southwest University, Chongqing 400715, China)
MOLECULAR CLONING AND EXPRESSION CHARACTERIZATION OF HEAT SHOCK PROTEIN 70 FROM GOBIOCYPRIS RARUS
HUANG Shu-Ting, LIU Qiu-Ping, DENG Chuan, HE Ye, XIONG Li, NIU Chun-Qing, CHEN Yun and LIU Yan
(Key Laboratory of Freshwater Fish Reproduction and Development, Key Laboratory of Eco-environments in Three Gorges Reservoir Region, Key Laboratory of Aquatic Science of Chongqing, School of Life Sciences, Southwest University, Chongqing 400715, China)
Heat shock protein (HSP) 70, as a molecular chaperone and extensively studied protein in environmental toxicology, plays a key role in membrane translocation, protein degradation, and regulatory process. However, as toxicology and risk assessment of freshwater, characterizations of HSP70 in rare minnow, Gobiocypris rarus, remain unknown. In this paper, we described the full-length cDNA of HSP70, designated as GrHSP70, by cloning through rapid-amplification of cDNA ends (RACE) technique from the liver of Gobiocypris rarus for the first time. The GrHSP70 shares high nucleotide sequence homolog, corresponding to amino acid sequence homology (>95%) with other fish species by NCBI blast analysis. Through quantitative real-time PCR (RT-PCR), we obtained the distribution of GrHSP70 in thirteen tissues of the untreated Gobiocypris rarus juveniles and its expression profile in liver after pentachlorophenol (PCP) exposure. The results indicated that mRNA transcription level of GrHSP70 is tissue-dependent, with the highest expression in the gonad tissue. Moreover, GrHSP70 is significantly up-regulated in liver by a concentration/time-dependent manner after PCP exposure. In summary, our results suggest that GrHSP70 may serve as a very sensitive biomarker for a toxicology and risk assessment of freshwater pollution in China.
Gobiocypris rarus; HSP70; Rapid-amplification of cDNA ends; Pentachlorophenol; Biomarker
Generally known as stress proteins, Heat Shock Proteins (HSPs) induced by heat shock, are triggered by a wide variety of physiological and environmental insults, such as heat stress, hyperthermia, toxicants, reduced oxygen, osmotic and oxidative stress, and heavy metal[1]. According to the molecular mass size and the degree of homology, HSPs have several families: HSP110, HSP90, HSP70, HSP60, HSP40, and small HSPs family[2,3]. HSP70 and HSP90 are the relatively conservative and abundant expression protein as a molecular chaperone and extensively studied proteins in the families, and play the key roles in membrane translocation, degradation of misfolded proteins and other regulatory processes[4].
HSP70, one of the firstly induced under stress conditions, is higher conservation across the taxa, and widely applied in environmental toxicology[5]. There were various effects of diverse endocrine disrupting chemicals (EDCs) on HSP70s in different experimental species. Transcription of HSP70s in Chironomus riparius increased significantly under bisphenol A (BPA)[6]and in Dreissena polymorpha treated with tributyltin[7], however, a decreased HSP70s mRNA expression was found in Chironomus riparius during 2, 4-D (at concentration of 1 μg/L) treatment[8], in the clam Ruditapes philippinarum under benzo (a) pyrene[4], and in Tigriopus japonicus treated with the NP and 4-t-octylpheno (OP)[9]. Strangely, the 1μmol/L PCP treatment was not able to activate HSP70 expression level in Chironomus riparius, but kill the larvaesome hours later, while the 25 μg/L PCP (96h) exposure induced significant increases on HSP70 transcript from C. riparius[10,11], indicating that those species could not be an effectively index. Owing to the significantly dose/time-dependent profile of mRNA expression, HSP70 has been a sensitive biomarker to assess the effect of EDCs toxicity[6—8,12]. HSP70s gene sequences and expression patterns in response to various stresses had been reported in many fish species, including zebrafish, Korean rockfish, tilapia fish, carp fish, rainbow trout[13—17].
Pentachlorophenol (PCP), as an organochlorine compound in a kind of endocrine disrupting chemicals (EDCs), is classified as a priority environmental contamination by the United States Environmental Protection Agency (US-EPA)[18]. PCP has been widely used as pesticide, herbicide, algaecide, defoliant, biocide, wood preservative, fungicide and molluskacide in China as well as in the entire world[19]. It can exist in water, soil and air for a long time, and is hard to be degraded or decomposed, PCP even can be absorbed into human body across skin, lungs, gastrointestinal lining[20,21], damaging the immune system, increasing body temperature, disrupting normal sexual and cognitive, even implicating in the incidence of liver tumors[22,23].
As early as 1980s, the concern about the toxicity of PCP and the potential adverse effects on human being, animals, plants, aquatics led to a regulatory action to limit its use[20,24]. Previous studies found that a low dose concentration PCP was highly toxic to aquatic fishes, e.g. apoptosis in Carassius carassius[20], DNA damage in Channa punctatus[25], the decreasing spawning capacity and fertility in Japanese Medaka[26]. In addition, PCP could also disrupt the thyroid endocrine system or normal endocrine function in some fishes[9,27]. Recent papers reported that GrHSP90 and Vitellogenin (VTG) mRNA expression levels altered under PCP exposure[27,28]. The underlying molecular mechanism of PCP-induced toxicity is unknown, therefore, more researches on GrHSP70 are needed.
Rare minnow (Gobiocypris rarus), is an endangered freshwater fish specie of the family Cyprinidae, predominantly distributing in upstream waters in the Yangtze River, Sichuan Province, China[29]. The minnows have several advantages: 30—80 mm small in length, easily cultured in lab, a relatively short life cycle, high fertilization and hatching rates in spawning hundreds of eggs, and their life-history stages are readily identified[27—29]. Most importantly, due to their sensitivity to many kinds of environmental endocrine disrupting chemicals[30,31], rare minnows become a potential model organism in aquatic toxicological assessment testing in China[32].
1 Materials and methods
1.1 Chemicals, fish and culture conditions
PCP (purity>99%) was purchased from Chem. Service in analytical grade. The 17α-ethynylestradiol (EE2) (purity>98%) was purchased from Sigma. Dimethysufoxide (DMSO) ordered from Sanland-Chem. Both PCP and EE2 powders were dissolved in DMSO to final concentration of 1 mg/mL and stored at 4℃. Then dilute the stock solution to the desired concentration for experiment.
Gobiocypris rarus were purchased from Institute of Hydrobiology, Chinese Academy of Sciences in Wuhan, and had been raised in our laboratory for more than 6 years. The fish used in the experiment were domesticated in flow-through system with dechlorinated tap water (pH 7.6±1.5, OD>5 mg/L, water hardness 7.8±0.7° dH) and subjected to a 12h∶12h of light: dark at (25±2)℃. The brood stock fed newly hatched brine shrimp (Artemia salina) twice a day. The waste and feed residue were removed daily and the test equipment and chamber were cleaned once a week.
1.2 Molecular cloning of GrHSP70
According to the manufacturer's instructions, total RNA was isolated from the liver tissue of untreated Gobiocypris rarus using RNA simple Total RNA Kit (TIANGEN, China). The first-strand cDNA was synthesized by the Prime ScriptTMRT reagent kit with a gDNA Eraser (TaKaRa, China). Based on the conserved sequences of HSP70 genes from closely related species, two degenerate primers (HSP70-R, HSP70-F) (Tab. 1) were designed by Primer Premier 5.0. PCR system was performed in 25 μL reaction volume containing 0.25 μL Ex TaqTM(TaKaRa), 2.5 μL Ex TaqTMbuffer (Mg2+free), 1.5 μL Mg2+, 2 μL dNTP, 1 μL of each degenerate primer and 15.75 μL RNasefree water and the above synthesized cDNA was acted as a template. The PCR products were migrated on a 1% agarose gel and observed on a gel imaging system. The obtained core fragment was purified by a gel extraction kit (OMEGA), subcloned into pMD19-T Vectors (TaKaRa), and sequenced (Invitrogen, China).
The full-length cDNA of GrHSP70 was determined by using the SMARTerTMRACE cDNA amplification kit (Clontech, USA). Two pairs of gene-specific primers (HSP70-3′F1, HSP70-3′F2, HSP70-5′R1, HSP70-5′R2) (Tab. 1) were designed based on the above obtained core fragment. The nest-polymerase chain reaction (PCR) amplification was choseto extend the 5′/3′ GrHSP70 cDNA sequence. The 5′and 3′ cDNA template was separately synthesized according to the manufacturer's protocol and ultimately stored at -20℃.
1.3 Real-time PCR reaction
The RT-PCR was used to analyze tissue distribution and gene expression of GrHSP70. For this amplification, the total RNA was obtained from the untreated Gobiocypris rarus blood, brain, fin, gill, gonad, heart, intestine, kidney, liver, muscle, skin, spleen and swim bladder tissues, respectively. The special primers (HSP70-rF, HSP70-rR, β-actin-rF, βactin-rR) (Tab. 1) were used to separately amplify a fragment of GrHSP70 and Grβ-actin. β-actin, the most reliable endogenous reference gene, which was mentioned in some researches[30,31]. Grβ-actin was acted as an internal control to calibrate the expression of GrHSP70 by the 2-∆∆Ctmethod[33]. The RT-PCR cycle profile was: an initial denaturation at 95℃ for 60s, it was followed by 40 cycles of denaturation at 95℃ for 15s, annealing at 58℃ for 10s, extension at 72℃ for 20s. The total volume of reaction system was described as following: 2 μL of cDNA template, 5 μL SYBR®Premix Ex TaqTMⅡ (2×, TaKaRa, China), 0.8 μL of each primer (Tab. 1) and added deionized water up to 20 μL. The experiment was performed in triplicate.
1.4 PCP-induced effect on GrHSP70 gene expression
The genetically uniform juvenile male and female Gobiocypris rarus [weight=(0.53±0.15) g, length=(36.6±3.7) mm; n=60] were the offspring from the same pair of brood stock. They were initially acclimated by the normal method of feeding in 10 L tanks with 6 L dechlorinated tap water. After a period of growth, 60 fish were randomly divided into twelve groups (five fish per group). Concentration choice was according to the pilot experiments under similar exposure scenarios in Gobiocypris rarus in our previous research in our lab. Four experimental groups (concentration of PCP at 8, 16, 80, 160 μg/L), a positive control group (EE2, 50 ng/L) and a solvent control group (0.001% DMSO, v/v) and all were exposed for 7 days. The other six groups were exposed to 80 µg/L PCP for different time (0, 0.5, 1, 3, 5, 7 days), respectively. For this exposure experiment, half of the water (3 L) in every tank was replaced daily with de-chlorinated tap water that dosed with appropriate amount of chemicals. Water quality parameters were recorded daily, and no mortality was observed during exposure concentration.
The experimental fish were euthanized and dissected with sterile instruments. Total RNA of each group was extracted from liver tissue, the first strand cDNA was synthesized as above. The RT-PCR was performed with the primers (HSP70-rF, HSP70-rR, βactin-rF, β-actin-rR) (Tab. 1) using SYBR®Green Master Mix Reagent Kit amplification on Iq5 Multicolor real-time PCR system (Bio-Rad, USA). A melting curve was run to analyze the quality of the PCR reaction by the end of each cycle. In order to ensure the accuracy of these results, every sample was analyzed individually and processed in triplicate.
1.5 Statistical analysis
In this research, charts were generated by the Origin 8.0 software, and all experimental data were expressed as mean±standard error of mean (SEM). The Software SPSS13.0. was used for statistical analysis of variance (ANOVA) and a probability of P<0.05 was considered to be significant, P<0.01 was considered to be statistically extremely significant.
2 Results
2.1 Sequence analysis of GrHSP70
The core fragment of GrHSP70 (Fig. 1a) was1783 bp, and the length corresponding to the 3′ partial fragment (Fig. 1b) and the 5′ partial fragment (Fig. 1c) was 1271 bp and 685 bp, respectively. A 2399 bp nucleotide sequence representing the complete cDNA sequence of GrHSP70 gene was highly similar to other species by BLAST search at National Center for Biotechnical Institute (NCBI). The cDNA sequence of GrHSP70 was submitted in GenBank with an accession no. KR232359.

Tab. 1 Sequences of primers in this research
The nucleotide sequence of a full-length cDNA was analyzed by ExPASy- ProtParam tool. The results were as following: the length of 3′UTR was 263 bp containing a polyA tail, the length of 5′UTR was 204 bp which located in upstream of a start codon (ATG) and the open reading frame was 1932 bp which encoded a polypeptide of 643 amino acids.
2.2 Phylogenetic analysis of GrHSP70
Highest similarity between GrHSP70 and HSP70 in Coreius guichenoti is 98%. GrHSP70 holds 96%, 95%, 86% and 85% similarity to that of Tanichthys albonubes, Danio rerio, Mus musculus and Homo sapiens, respectively. A neighbor-joining phylogenetic tree was constructed according to GrHSP70 and other known HSP70 amino acid sequences (Fig. 2). GrHSP70 possessed the closest evolutionary relationship with HSP70 in Coreius guichenoti. Node numbers were used to determine the confidence indices of this evolutionary tree by the bootstrap value 10000 method.
2.3 Tissue distribution of GrHSP70
The mRNA transcription levels of GrHSP70 were detected in thirteen tissues of the untreated Gobiocypris rarus juvenile by RT-PCR. GrHSP70 showed highly expressed in gonad and skin, the moderate expression in brain and kidney, but relatively low expression in blood, fin, intestine, gill, heart, muscle, spleen, liver, and swim bladder. Fig. 3 indi-cated that GrHSP70 had the highest expression in the gonad (P<0.05).
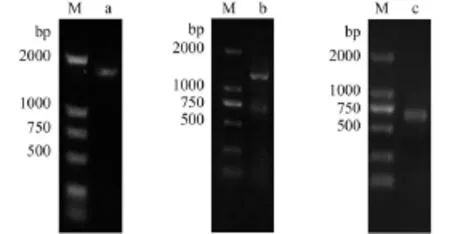
Fig. 1 Electrophoresis of HSP70 from Gobiocypris rarus amplification by PCRa. core fragment was cloned by RT-PCR; b. fragment of 3′ RACE; c. fragment of 5′ RACE (M: DNA Marker DL2000)
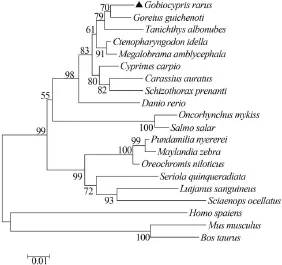
Fig. 2 Phylogenetic analysis of HSP70 sequencesThe neighbor-joining tree was generated using HSP70 amino acids of 19 related species. The percentage bootstrap values obtained from 10000 re-samplings were shown in nodes. The GenBank accession numbers for HSP70s are: ▲Gobiocypris rarus (KR 232359), Coreius guichenoti (AHA44483), Tanichthys albonubes (ADM15726), Ctenopharyngodon idella (ADX32514), Megalobrama amblycephala (ACG63706), Danio rerio (AAH 56709), Carassius auratus (BAC67184), Cyprinus carpio (AEO44578), Oncorhynchus mykiss (ABO62281), Salmo salar (ACI34374), Oreochromis niloticus (NP_001298261), Sciaenops ocellatus (ADL18372), Pundamilia nyere-rei (XP_005747773), Schizothorax prenanti (AHB19296), Lutjanus sanguineus (ADO 32584), Seriola quinqueradiata (BAG82850), Maylandia zebra (XP_ 004556510), Mus musculus (BAE40342), Bos Taurus (NP_976067), Homo sapiens (AAK17898)
2.4 mRNA expression of GrHSP70 in response to PCP
The mRNA expression levels of GrHSP70 were significantly up-regulated in four experimental groups with concentration of PCP at 8, 16, 80, and 160 μg/L as PCP concentration increasing compared with the control group (0.001% DMSO, v/v) (Fig. 4a). In transcription level, the peak of GrHSP70 was observed at the relatively high concentration of 160 μg/L. The expression profile of GrHSP70 mRNA was an obvious dose-dependent (P<0.05) in above six groups. The other six groups of Gobiocypris rarus were treated under concentration of PCP at 80 μg/L for 0.5, 1, 3, 5, and 7 days (Fig. 4b). The GrHSP70 level increased gradually with time, and peaked on the seventh day, indicating that mRNA expression of the GrHSP70 was in a time-dependent manner (P<0.05).
3 Discussion
HSP70s are a group of most conservative and abundant proteins among the HSPs family, which are the earliest widely studied molecular chaperone[34,35]. Recently, some essential functions of HSP70s havebeen reported[36—38], playing important roles in membrane translocation, assisting translation and maintaining cell homeostasis, protein metabolism under normal and stress conditions[1,8,36],and potential applications in disease-resistant and genetic breeding[34].
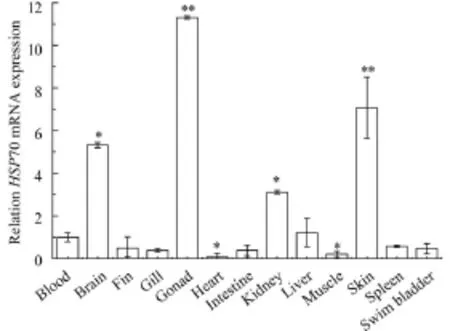
Fig. 3 Relative HSP70 mRNA expression of Gobiocypris rarus in untreated blood, brain, fin, gill, gonad, heart, intestine, kidney, liver, muscle, skin, spleen and swim bladder tissuesThe GrHSP70 mRNA expression of blood utilized as the control group, and Grb-actin explored as an internal control. Each bar was the mean±SEM obtained from three independent experiments each with three replicates. *P<0.05 and **P<0.01 indicate significant differences between exposure group and control group, respectively
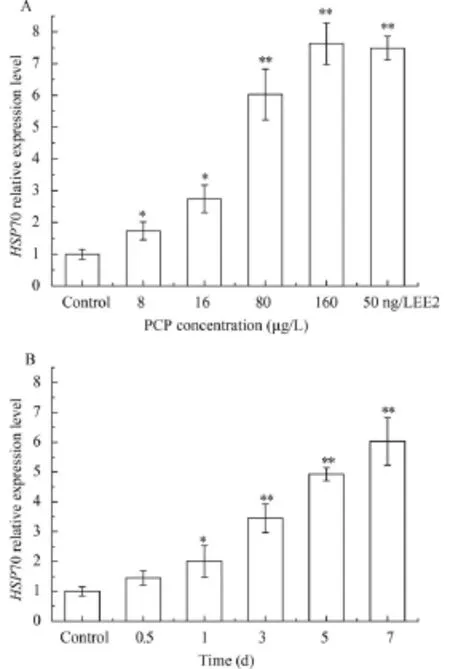
Fig. 4 Effect of PCP on HSP70(a) mRNA expressions in liver at different concentrations in Gobiocypris rarus; Effect of PCP on HSP70(b) mRNA expressions in liver at different time in Gobiocypris rarus and the fish were exposed at 80 μg/L PCP for different 7 daysAll the experimental data was calibrated by the expression level of β-actin. Each bar represented the mean±SEM for three independent experiments. Every experiment was conducted in triplicate. *P<0.05 and **P<0.01 indicate significant differences between exposure group and the control group
In this study, we cloned the full-length cDNA sequence (GenBank accession No. KR232359) from liver of Gobiocypris rarus by 5′/3′ RACE for the first time. The complete nucleotide sequence shared 85%—98% identities with HSP70 sequences of Coreius guichenoti, Tanichthys albonubes, Cyprinus carpio, Danio rerio, Mus musculus, Homo sapiens and Bos Taurus, providing GrHSP70 protein is encoded by GrHSP70 gene that might function as a molecular chaperone just like the HSP70s from other organisms[15, 16, 39].
Our phylogenetic tree analysis clearly displayed that GrHSP70 was clustered with the HSP70 of Coreius guichenoti, while the GrHSP90 was cluster with the HSP90 of Tanichthys albonubes[28]. Both Gr-HSP70 and GrHSP90, however, grouped in the ortholog of cyprinid species, indicating that the Gr-HSP70 is homologue of the known fish HSP70s[40].
The tissue-dependent of GrHSP70 mRNA transcripts revealed that as an important molecular chaperone molecule, which played a certain role in keeping membrane translocation and protein metabolism, assisting translation and maintainning cell homeostasis under untreated situation[39,41]. The similar expression profile was also happened to the GrHSP90 gene[28], demonstrating that the genes of HSPs owned the alike function such as a chaperone, which have a vital role in the formation or maintenance of the na-tive conformation of cytosolic proteins[42].
In addition, HSP70 was reported universally expressed in Chironomus riparius and Sebastes schlegeli[7,15], and the expression may associate with corresponding functions of GrHSP70. For example, a relatively high expression level of HSP70 gene in kidney indicated that HSP70 might be correlated with immune response[43,44]. Some previous studies had proposed that high expression in some special organs might be associated with the process of mitosis, permatogenesis, endocytosis[45—47]. In this study, GrHSP70 was higher expression found in gonad, brain than in intestine, spleen and swim bladder, but the reason is not clear. In the future, we will conduct a further investigation to figure out a certain relationship between the function and organization distribution.
As early as 2010, researchers had begun to pay extensive attention to HSPs function as a biomarker
[1]Hightower L E. Heat shock, stress proteins, chaperones, and proteotoxicity [J]. Cell, 1991, 66(2): 191—197
[2]Feeder M E, Hofmann G E. Heat shock protein, molecular chaperones, and the stress response: evolutionary and ecological physiology [J]. Annual Review of Physiology, 1999, 61(1): 243—282
[3]Lindquist S, Craig E A. The Heat shock proteins [J]. Annual Review of Genetics, 1988, 22(1): 631—677
[4]Liu T, Pan L, Cai Y, et al. Molecular cloning and sequence analysis of Heat shock proteins 70 (HSP70) and 90 (HSP90) and their expression analysis when exposed to benzo (a) under some stress situation, including HSP70 and HSP90[28,48]. For example, the gradually increased expression level of HSP70 from C. riparius under BPA between 12h to 24h and the HSP70 from C. japoinica following NP between 24h to 96 h[6,49]. In addition to the above-mentioned species, many reports of HSP70 in other species as a biomarker: zebra mussel, Chironomus tentans, carp fish, Gracilaria lemaneiformis, barnacle larvae, and earthworm Lumbricus terrestris[7,16,39,44,50]. Certainly, HSP70s in response to PCP from different species might be distinctive[10—12,51,52], for example, HSP70 was more sensitive to the PCP exposure compared with 2, 4, 6-TCP exposure in the Cracian carp brown tissue[51], but PCP did not consistently enhance HSP70 expression of Baikalian sponges[52].
Our investigation of HSP70 expression in Gobiocypris rarus juveniles exposed to PCP, presented a more typical dose/time-dependent pattern compared with HSP90[28]. Differential expression of the relative HSP70 and HSP90 mRNA found from male Gobiocypris rarus exposed to p, p-DDE (after concentration: 2 μg/L) treatment[53]. An occurrence of malformation or deformity happened when HSP90 expression altered sharply[8,54]. HSP70 served as a biomarker in aquatic biology commonly[5,6], while the HSP90 may act as a capacitor of phenotypic variation or morphological evolution rather than a bioma-ker[55,56]. Compared to GrHSP90, our results more reasonably confirm that GrHSP70 may act as a much better biomarker in evaluating PCP pollution.
In conclusion, the complete cDNA sequence of HSP70 was isolated from the liver of Gobiocypris rarus for the first time and its mRNA transcription level under PCP exposure were analyzed. As a better biomarker HSP70, the cloning and expression analysis will provide more values of developing Gobiocypris rarus as an ideal experimental model animal in Chinese aquatic toxicology research.
pyrene in the clam Ruditapes philippinarum [J]. Gene, 2015, 555(2): 108—118
[5]Mukhopadhyay I, Nazir A, Saxena D K, et al. Heat shock response: HSP70 in environmental monitoring [J]. Journal of Biochemical and Molecular Toxicology, 2003, 17(5): 249—254
[6]Planelló J L, Martínez-Guitarte, Morcillo G. The endocrine disruptor bisphenol A increases the expression of HSP70 and ecdysone receptor genes in the aquatic larvae of Chironomus riparius [J]. Chemosphere, 2008, 71(10): 1870—1876 [7]Clayton M E, Steinmann R, Fent K. Different expression patterns of Heat shock proteins HSP60 and HSP70 in zebra mussels (Dreissena polymorpha) exposed to copper and tributyltin [J]. Aquatic Toxicology, 2000, 47(3): 213—226
[8]Park K, Park J, Kim J, et al. Biological and molecular responses of Chironomus riparius (Diptera, Chironomidae) to herbicide 2, 4-D (2, 4-dichloro phenoxyaceticacid) [J]. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 2010, 151(4): 439—446
[9]Rhee J S, Raisuddin S, Lee K W, et al. Heat shock protein (HSP) gene responses of the intertidal copepod Tigriopus japonicus to environmental toxicants [J]. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 2009, 149(1): 104—112
[10]Morales M, Planelló R, Martínez-Paz P, et al. Characterization of HSP70 gene in Chironomus riparius: expression in response to endocrine disrupting pollutants as a marker of ecotoxicological stress [J]. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 2011, 153(1): 150—158
[11]Morales M, Martínez-Paz P, Martín R, et al. Transcriptional changes induced by in vivo exposure to pentachlorophenol (PCP) in Chironomus riparius (Diptera) aquatic larvae [J]. Aquatic Toxicology, 2014, 157: 1—9
[12]Köhler H R, Knödler C, Zanger M. Divergent kinetics of HSP70 induction in Oniscus asellus (Isopoda) in response to four environmentally relevant organic chemicals (B[a]P, PCB52, γ-HCH, PCP): Suitability and limits of a biomarker [J]. Archives of Environmental Contamination and Toxicology, 1999, 36(2): 179—185
[13]Scheil V, Zürn A, Köhler H R, et al. Embryo development, stress protein (Hsp70) responses, and histopathology in zebrafish (Danio rerio) following exposure to nickel chloride, chlorpyrifos, and binary mixtures of them [J]. Environmental Toxicology, 2010, 25(1): 83—93
[14]Mu W, Wen H, Li J, et al. Cloning and expression analysis of a HSP70 gene from Korean rockfish (Sebastes schlegeli) [J]. Fish & Shellfish Immunology, 2013, 35(4): 1111—1121
[15]Molina A, Biemar F, Müller F, et al. Cloning and expression analysis of an inducible HSP70 gene from tilapia fish [J]. FEBS Letters, 2000, 474(1): 5—10
[16]Shen H, Sun Y Y. Dynamic effects of low-level 2-nitro-4'-hydroxydiphenylamine on the induction of HSP70 in thecarp fish livers and brains [J]. Journal of Lake Science, 2005, 17: 188—192
[17]Feng Q, Boone A N, Vijayan M M. Copper impact on Heat shock protein 70 expression and apoptosis in rainbow trout hepatocytes [J]. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 2003, 135(3): 345—355
[18]Gupta S S, Stadler M, Noser C A, et al. Rapid total destruction of chlorophenols by activated hydrogen peroxide [J]. Science, 2002, 296(5566): 326—328
[19]Zimbron J A, Reardon K F. Continuous combined Fenton's oxidation and biodegradation for the treatment of pentachlorophenol-contaminated water [J]. Water Research, 2011, 45(17): 5705—5714
[20]Chang H. Advances in endocrine disrupting effects of pentachlorophenol [J]. Journal of Environment and Health, 2002, 19(3): 279—281
[21]Gao J, Liu L, Liu X, et al. Levels and spatial distribution of chlorophenols-2, 4-dichlorophenol, 2, 4, 6-trichlorophenol and pentachlorophenol in surface water of China [J]. Chemosphere, 2008, 71(6): 1181—1187
[22]Proudfoot A T. Pentachlorophenol poisoning [J]. Toxicological Reviews, 2003, 22(1): 3—11
[23]Montaño M, Gutleb A C, Murk A T J, et al. Persistent toxic burdens of halogenated phenolic compounds in humans and wildlife [J]. Environmental Science & Technology, 2013, 47(12): 6071—6081
[24]Reigart J R. Recognition and Management of Pesticide Poisonings [M]. DIANE Publishing. 2009
[25]Farah M, Ateeq A B, Ali M N, et al. Studies on lethal concentrations and toxicity stress of some xenobiotics on aquatic organisms [J]. Chemosphere, 2004, 55(2): 257—265
[26]Sun L, Zha J, Wang Z. Effects of binary mixtures of estrogen and antiestrogens on Japanese medaka (Oryzias latipes) [J]. Aquatic Toxicology, 2009, 93(1): 83—89
[27]Zhang X, Xiong L, Liu Y, et al. Histopathological and estrogen effect of pentachlorophenol on the rare minnow (Gobiocypris rarus) [J]. Fish Physiology and Biochemistry, 2014, 40(3): 805—816
[28]Liu Q, Huang S, Deng C, et al. Molecular characterization of Heat shock protein 90 gene and its expression in Gobiocypris rarus juveniles exposed to pentachlorophenol [J]. Fish Physiology and Biochemistry, 2015, 41(5): 1279—1291
[29]Cao W X, Wang J W. Rare minnow: A new laboratory animal in China [J]. Laboratory Animal Science and Management, 2003, 20(S1): 96—99
[30]Cao M, Duan J, Cheng N, et al. Sexually dimorphic and ontogenetic expression of dmrt1, CYP19a1a and CYP19a1b in Gobiocypris rarus [J]. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology, 2012, 162(4): 303—309
[31]Qin F, Wang X, Liu S, et al. Gene expression profiling of key genes in hypothalamus-pituitary-gonad axis of rare minnow (Gobiocypris rarus) in response to EE2 [J]. Gene, 2014, 552(1): 8—17
[32]Zhou Y X, Cheng S P, Hu W, et al. A new toxicity test organism-Gobiocypris rarus [J]. Zoological Research, 1995, 16(1): 59—63
[33]Livak K J, Schmittgen T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCTmethod [J]. Methods, 2001, 25(4): 402—408
[34]Basu N, Todgham A E, Ackerman P A, et al. Heat shock protein genes and their functional significance in fish [J]. Gene, 2002, 295(2): 173—183
[35]Gupta S C, Sharma A, Mishra M, et al. Heat shock proteins in toxicology: how close and how far [J]? Life Sciences, 2010, 86(11): 377—384
[36]Kiang J G, Tsokos G C. Heat shock protein 70 kD: molecular biology, biochemistry and physiology [J]. Pharmacology and Therapeutics, 1998, 80(2): 183—201
[37]Mayer M P, Bukau B. HSP70 chaperones: cellular functions and molecular mechanism [J]. Cellular and Molecular Life Sciences, 2005, 62(6): 670—684
[38]Daugaard M, Rohde M, Jäättelä M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions [J]. FEBS Letters, 2007, 581(19): 3702—3710
[39]Gu Y, Zhang X, Lu N. Cloning and transcription analysis of HSP70-1 and HSP70-2 of Gracilaria lemaneiformis under heat shock [J]. Aquaculture, 2012, 358: 284—291
[40]Smith T R, Tremblay G C, Bradley T M, et al. Characterization of the Heat shock protein response of Atlantic salmon (Salmo salar) [J]. Fish Physiology and Biochemistry, 1999, 20(3): 279—292
[41]Wu X, Tan J, Cai M, et al. Molecular cloning, characterization, and expression analysis of a Heat shock protein 70 (HSP70) gene from Paphia undulate [J]. Gene, 2014, 543(2): 275—285
[42]Boston R S, Viitanen P V, Vierling E, et al. Molecular Chaperones and Protein Folding in Plants [M]. Post-Transcriptional Control of Gene Expression in Plants. Springer Netherlands. 1996, 191—222
[43]Pockley A G. Heat shock proteins as regulators of the immune response [J]. The Lancet, 2003, 362(9392): 469—476
[44]Cheng P, Liu X, Zhang G, et al. Cloning and expression analysis of a HSP70 gene from Pacific abalone (Haliotis discus hannai) [J]. Fish & Shellfish Immunology, 2007, 22(1): 77—87
[45]Rosario M O, Perkins S L, O'Brien D A, et al. Identification of the gene for the developmentally expressed 70 kDa Heat shock protein of mouse spermatogenic cells [J]. Developmental Biology, 1992, 150(1): 1—11
[46]Sconzo G, Palla F, Agueli C. Constitutive HSP70 is essential to mitosis during early cleavage of Paracentrotus lividus embryos: the blockage of constitutive HSP70 impairs mitosis [J]. Biochemical and Biophysical Research Communica-tions, 1999, 260(1): 143—149
[47]Tedeschi J N, Kennington W J, Berry O, et al. Increased expression of HSP70 and HSP90 mRNA as biomarkers of thermal stress in loggerhead turtle embryos (Caretta Caretta) [J]. Journal of Thermal Biology, 2015, 47: 42—50
[48]Karouna-Renier N K, Rao K R. An inducible HSP70 gene from the midge Chironomus dilutus: characterization and transcription profile under environmental stress [J]. Insect Molecular Biology, 2009, 8(1): 87—96
[49]Park K, Kwak I S. Expression of stress response HSP70 gene in Asian paddle crabs, Charybdis japonica, exposure to endocrine disrupting chemicals, bisphenol A (BPA) and 4-nonyl phenol (NP) [J]. Ocean Science Journal, 2013, 48(2): 207—214
[50]Nadeau D, Omeau S, Plante I. Evaluation for HSP70 as a biomarker of pollutants on the earthworm Lumbricus terrestris [J]. Cell Stress & Chaperones, 2001, 6(2): 153—163
[51]Su Y, Ren L, Luo Y, et al. Effects of two kinds of typical chlorophenols on the induction of heat shock protein 70 (HSP70) in the Cracian carp brown tissue [J]. China Environmental Science, 2007, 27(2): 260—263
[52]Efremova S M, Margulis B A, Guzhova I V, et al. Heat shock protein 70 (HSP70) expression and DNA damage in Baikalian sponges exposed to model pollutants and wastewater from Baikalsk Pulp and Paper Plant [J]. Aquatic Toxicology, 2002, 57(4): 267—280
[53]Yang L, Zha J, Zhang X, et al. Alterations in mRNA expression of steroid receptors and Heat shock proteins in the liver of rare minnow (Gobiocypris rarus) exposed to atrazine and p, p'-DDE [J]. Aquatic Toxicology, 2010, 98(4): 381—387
[54]Ruden D M, Xiao L, Garfinkel M D, et al. HSP90 and environmental impacts on epigenetic states: a model for the trans-generational effects of diethylstibesterol on uterine development and cancer [J]. Human Molecular Genetics, 2005, 14(1): 149—155
[55]Rutherford S L, Lindquist S. HSP90 as a capacitor for morphological evolution [J]. Nature, 1998, 396(6709): 336—342
[56]Queitsch C, Sangster T A, Lindquist S, et al. HSP90 as a capacitor of phenotypic variation [J]. Nature, 2002, 417(6889): 618—624
稀有鲫热激蛋白70的分子克隆及表达特征分析
黄书婷 刘秋萍 邓 川 和 野 熊 力 牛纯青 陈 韵 刘 堰
(西南大学生命科学学院, 淡水鱼类资源与生殖发育教育部重点实验室, 三峡库区生态环境教育部重点实验室, 水产科学重庆市市级重点实验室, 重庆 400715)
为研究稀有鮈鲫(Gobiocypris rarus) HSP70用于淡水毒理学和风险评估方面的可行性, 通过cDNA末端快速扩增的方法首次从稀有鮈鲫肝脏中成功克隆出HSP70基因的cDNA全长, 命名为GrHSP70。NCBI比对分析结果显示, GrHSP70核苷酸序列与其他鱼类的HSP70基因的核苷酸序列相似性极高, 相应的氨基酸序列同源性也高(大于95%)。通过实时定量PCR获得未经污染物暴露的稀有鮈鲫幼鱼体内13种组织中GrHSP70的分布以及PCP暴露后肝脏中该基因的表达图谱, 结果表明GrHSP70在稀有鮈鲫体内的表达呈现出组织依赖性,在性腺组织中的表达最高。此外, 在不同时间﹑不同浓度的PCP暴露下稀有鮈鲫肝脏中GrHSP70的表达模式呈现出显著的时间-剂量依赖效应。总之, GrHSP70可作为一种非常敏感的生物标志物, 适用于中国淡水环境污染的毒理学研究及风险评估。
稀有鮈鲫; 热激蛋白70; cDNA末端快速扩增; 五氯酚; 生物标志物
Q344+.1
A
1000-3207(2017)01-0018-08
10.7541/2017.3
Received date: 2016-03-28; Accepted date: 2016-10-12
Foundation item: Supported by the National Program on Key Basic Research Project (973 Program; 2012CB723205); the Financial Support Received from the National Natural Science Foundation of China (21147002)
Brief introduction of author: Huang Shu-Ting, E-mail: huangshutingelves@163.com
Liu Yan, E-mail: liuyan@swu.edu.cn; Tel.: +86 23 68252023; Fax: +86 23 68252365
