一种SO2固定及用于制备BaSO3或BaSO4的方法
2016-12-20张飞刘丽华沙峰乔宪书张建斌
张飞 刘丽 华沙峰 乔宪书 张建斌
(内蒙古工业大学化工学院,呼和浩特010051)
一种SO2固定及用于制备BaSO3或BaSO4的方法
张飞刘丽华沙峰乔宪书张建斌*
(内蒙古工业大学化工学院,呼和浩特010051)
在常温常压下,由乙二胺(EDA)和乙二醇及其衍生物(EGs)组成的混合体系可捕集SO2并转化为一种SO2储集材料(SO2SM)。EDA+EGs体系呈现了强的捕集性能(0.364~0.662 gSO2·gabsorbent-1)。FTIR,XPS和XRD结果确证了SO2SM为一种烷基亚硫酸盐。以EG-SO2SM为原料制备具有多种形貌的BaSO3或BaSO4,在此过程中,EG-SO2SM不仅提供了原材料,而且可以释放EDA和EG用作表面活性剂,调控晶体的结晶化过程。
SO2捕集;SO2储集材料;烷基亚硫酸盐;BaSO4
0 Introduction
The emissions of SO2from the burning of fossil fuels cause severe environmental issues and adverse human health effects[1];hence,it is of significance to reduce,capture and recycle SO2.The current methods of SO2capture mainly include limestone scrubbing, ammonia scrubbing and organic solvents[2-4].In particularly,the ammonia scrubbing could efficiently minimize secondary pollution problems and present a high SO2capture capability,but it also exhibits some significant inherent drawbacks,including volatility, corrosiveness and occasional decomposition[5-8].Recently,Heldebrant et al.[9]developed a series of SO2binding organic liquids(SO2BOLs)based on blends of amidine/alcohol or guanidine/alcohol,by which theloss of amines was effectively reduced.Although the method could effectively capture SO2,the high cost might limit their industrial applications in large-scale SO2capture processes.Thus,developing a novel SO2capture and utilization technology with low-cost and excellentloading ability of SO2is highly desired.

Fig.1 Formative process of the SO2SMs by the equimolar of EDA and EGs to capture SO2in the ice-water bath under ambient pressure
In this work,to address high volatility and degradation problems of ammonia scrubbing,we developed an method to capture,store and transform SO2into a novel SO2-storage materials(SO2SMs)by using the system of 1,2-ethanediamine(EDA)/ethylene glycol derivatives(EGs)(Fig.1).In particular,the generated SO2SMs could release SO2when exposed to high temperature according to the TGA result.Subsequently, the EG-based SO2SM(EG-SO2SM)was used to prepare BaSO3with a“leaf”morphology through the vapor diffusion method.Since this approach avoided the direct mixing of Ba2+and SO32-,it is easy to control the nucleation and growth of BaSO3crystals without the addition surfactant.Moreover,the aqueous EGSO2SM solution was also employed to prepare BaSO4with a block morphology by using H2O2as the oxidant. Most importantly,the EG-SO2SM not only yielded alkylsulfite but also released EDA and EG that acted as efficient surfactants to guide the subsequent crystallization.As a result,the developed process offered an alternative approach to the comprehensive utilization of SO2and yielded well-controlled BaSO3and BaSO4with orderly crystal morphologies.
1 Experimental
1.1Materials
The analytical grade EDA was purchased from Tianjin Reagent Company(China,Residue on ignition≤0.1%).The analytical grade EG,BDO,DEG,TEG and PEG 200 were purchased from Beijing Reagent Company(China,Content≥99.0%).Compressed SO2(99.9%,V/V)was purchased from the Standard Thing Center(China).All reagents were obtained in the higher purity grade possibly and directly used as received withoutfurther purification.
1.2SO2capture
In a typicalexperiment,SO2absorption experiment was carried out with approximately 15 g mixture of EDA and EG(nEDA/nEG=1)that was charged into a glass container with an inner diameter of 2 cm and a height of 15 cm under ambient pressure and room temperature.Subsequently,the pure SO2was bubbled into the solution at a rate of 60 mL·min-1.The absorption amount of SO2in the absorbent was measured using an electronic balance with±0.1 mg and was determined according to the increased mass every 5 min.The electricalconductivity and temperature of the systems were monitored every 1 min with a high sensitivity conductivity meter(DDSJ-308A).
1.3Fixation of SO2into SO2SMs
SO2was bubbled into the system EDA+EGs in the ice-water bath under ambient pressure for about 90 min.After the bubbling,the white solid was collected and washed with ethanol several times to dissolve the residual EDA and EGs,dried under vacuum at 60℃for 10 h and then stored at room temperature.The SO2SMs were characterized by X-ray photoelectron spectroscopy(XPS),X-ray diffraction (XRD),fourier transform infrared spectroscopy(FTIR) and thermogravimetry analysis(TGA).XPS data were obtained with a KRATOS Axis ultra X-ray photoelectron spectrometer with a monochromatized Al KαX-ray(hν=1 486.6 eV)operated at150 W.XRD patterns were collected on a powder X-ray diffractometer (Siemens D/max-RB)with Cu Kα(λ=0.154 06 nm) radiation and scanning rate of 0.05°·s-1operated at working voltage of 40 kV and working current of 40 mA.FTIR spectra of SO2SMs were taken as 1% dispersion in KBr powder using a Nexus 670 FTIR spectrometer with a resolution of 1 cm-1in the range of 4 000~400 cm-1and a base line correction was made for the spectra that were recorded in air at room temperature.TGA(Q50 V20.6 Build 31)was employed to measure the weight percentage of SO2SMs.In the thermal stability experiment,the sample was heated up to 400℃with a rate of 10℃·min-1in an atmosphere nitrogen.Morphologies of the obtained BaSO3or BaSO4were examined via scanning electron microscopy(SEM,Quanta FEG 650)with an accelerating voltage of 20 kV.
1.4Preparation of BaSO3and BaSO4with the EG-SO2SM
In a typical experiment,BaSO3was prepared by hydrothermal synthesis method.Specifically,a certain concentration of SO2SM was dissolved in 20 mL distilled water.At the same time,10 mL of Ba(OH)2saturated limpid solution was added to a smalljar that located in the reaction vessel(Fig.S1).The reaction temperature was controlled at 100℃for 2.5 h in an oven;BaSO4was prepared by the dripping method.A 50 mL solution of BaCl2(2.5 mmol·L-1)and H2O2(0.15 mol·L-1)were added into 50 mL EG-SO2SM(0.1 g)aqueous solution in drop wise with an addition speed of 15 drop·min-1.After the reaction,the resulting precipitates were separated from the mother liquids by filtration,washed with distilled water for severaltimes and then dried at120°C for 5 h.
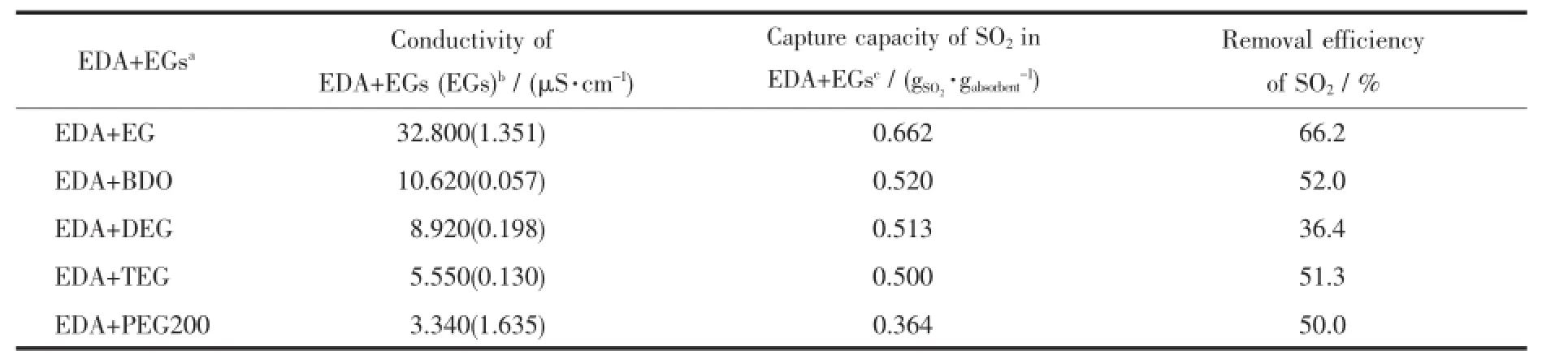
Table 1 Comparison of conductivity,capture capacity of SO2,the removal efficiency of SO2of the EDA+EGs systems
2 Results and discussion
2.1SO2capture
A series of EDA+EGs were used to capture SO2and the results are shown in Table 1.In the capture processes,the compressed 99.9%SO2was continuously bubbled into 15 g of EDA+EGs solution for 90 min.The results indicated that the volatility of EDA was effectively reduced.This phenomenon can be explained by the formation of hydrogen bonding between-OH group and the-NH2group thatresults in the ionization of EDA[10].In a typical experiment,the EDA+EG system was monitored by the changes in mass,temperature and electrical conductivity as shown in Fig.2.Fig.2a indicates that the capture capacity of SO2rapidly increased before 80 min and then slowly increased with the prolonged capture time. Simultaneously,the solution became sticky.Specifically,the capture reached about90%saturation atthe initial 80 min and there was no additional uptake from 80 to 110 min,which could be attributed to the reduction of mass transfer rate and the generation of alkyl sulfite.Fig.2b illustrates that SO2capture process was exothermal,in which the temperature rapidly increased to 116.3℃,then slowly decreased with prolonged time.As shown in Fig.2c,the conductivity of absorbent increased from 33 to 661μS·cm-1after SO2was bubbled,which suggested that the capture of SO2in absorbents was very quick.When the reaction was conducted for 30 min,the electrical conductivity increased to 146 800μS·cm-1,indicatingthe ionization degree ofthe system was very significant. Meanwhile,the temperature reached the maximum. With the proceeding of reaction,the solution became sticky and finally converted into white solid.At the end of the reaction,the conductivity value decreased to 4 020μS·cm-1,which clearly demonstrated that there was an apparent charge change between the low and high ionic strength solutions.With increasing system viscosity,the conductivity values of the solution decreased and the solid-state SO2SM was formed at90 min.
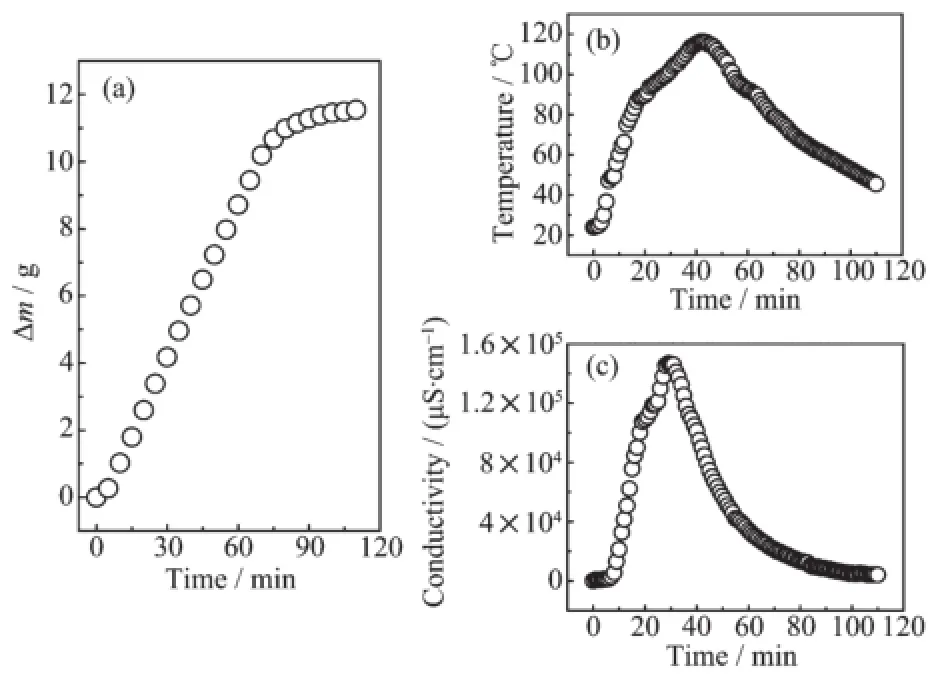
Fig.2 Changes of weight(a),temperature(b)and conductivity(c)for the EDA+EG system at 60 mL·min-1SO2flow rate under 101.325 kPa and 25℃
To control the reaction temperature,all the EDA+EGs systems were kept in an ice-water bath and the SO2capture process was monitored by measuring their mass change,as shown in Fig.3.It was evident that the capture capacity of SO2in these systems was in the order of EDA+EG>EDA+BDO>EDA+DEG>EDA+TEG>EDA+PEG 200.As can be seen from Fig. 3,the systems of EDA+EGs possessed various SO2capture capabilities ranging from 0.364 to 0.662 gSO2· gabsorbent-1.Compared this result with the data in the literature,the maximum capture capability(0.662 gSO2·gabsorbent-1)of the EDA+EG system was lower than results from Yang et al.[11](1.09 gSO2·g[EE3AE]-1),but higher than the results reported by Heldebrant et al.[9](0.54 gSO2·gDBUA-1),Wu et al.[1](0.31 gSO2·g[TMG]L-1),Lee etal.[12](0.28 g·g-1),Yang etal.[13](0.10 gSO2[Bztmeda][MeSO3]SO2·gFA-1)and Huang etal(0.02 g·g-1)[14].SO2[TMG][BF4]
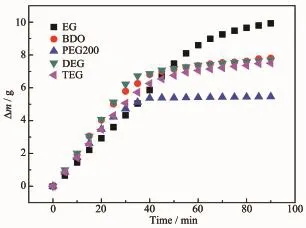
Fig.3 Changes of weight for the EDA+EGs systems at 60 mL·min-1SO2flow rate under 101.325 kPa and 0℃
2.2Characterization of the SO2SMs
Considering the EDA+EG system possessed a more remarkable SO2capture capacity than other systems,we next characterized the structure of EGSO2SM using XPS and FTIR techniques(Fig.4 and 5). Fig.4 shows the deconvolution of C1s,N1s,S2p and O1s XPS spectra of the EG-SO2SM.The C1s spectra could be deconvoluted into three different peaks:C-C/ C-H(284.8 eV),C-O(286.5 eV)and C-NH2(287.9 eV)[13,15].Moreover,the N1s spectrum indicated the presence of C-N/N-H at 401.4 eV[15-16].Taken together, the C1s and N1s spectra demonstrated that the EGSO2SM contained the moieties of C-H,C-O,C-N and N-H.To gain the chemical state of S atom in the interaction process of SO2with the EDA+EG system, the O1s and S2p spectra were deconvoluted according to the references[17-18].The peak of O1s was observed at 531.3 eV,which was assigned to oxygen of C=O and C-O in EG and/or O=S and O-S(sulfonic groups,sulfate and sulfite)in SO2[17,19].The sulfur doublet for SO32-was found at166.3 and 168.1 eV,which corresponded to the S2p3/2and S2p1/2transitions,respectively[18,20].
Subsequent,as shown in Fig.5,the FTIR spectrum of EG-SO2SM shows that the peaks at3 430, 2 170 and 1 574 cm-1corresponded to N-H of-NH3+in EG-SO2SM[21-23],suggesting EG-SO2SM was a primary amine salt.The peaks at 1 353,1 144,1 088,989, 943 and 636 cm-1were assigned to the structures of S=O and S-O[24-26],which were in good agreement with the characteristic peaks of S=O and S-O in standardsubstance Na2SO3and BaSO3(Fig.S2).It should be noted that the peak of 1 353 cm-1was not in agreementwith standard substance(1 444 cm-1).This phenomenon may be related to the EDA and EG from SO2SM,which had an effect on the absorption peak. The results indicated the EG-SO2SM contained the similar structure as-SO3-,well consistent with the results of XPS.Heldebrant[9]puts forward the reaction mechanism of amidine/alcohol or guanidine/alcohol. The characteristic of this reaction is that the raw materials contain amino group and hydroxyl group.In our work,EDA and EG possess a similar structure with Heldebrant reported raw materials.At the same time,based on the above experimental results and the study results of Heldebrant,the EG-SO2SM was confirmed as an alkyl sulfite.Moreover,the results of XRD analysis(17.08°,20.18°,20.66°,24.5°,27°and 29.04°)revealed that the SO2SMs possessed a high degree of crystallinity(Fig.S3).More characterization results ofother SO2SMs are shown in Fig.S3~5.
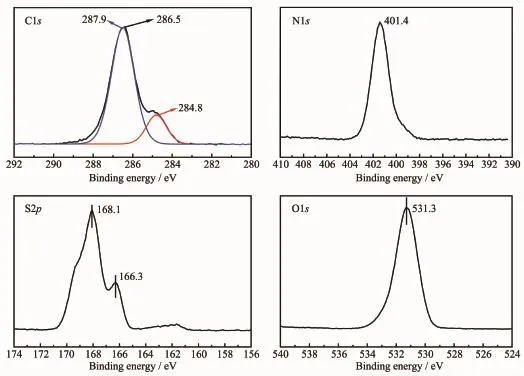
Fig.4 XPS spectra of the EG-SO2SM
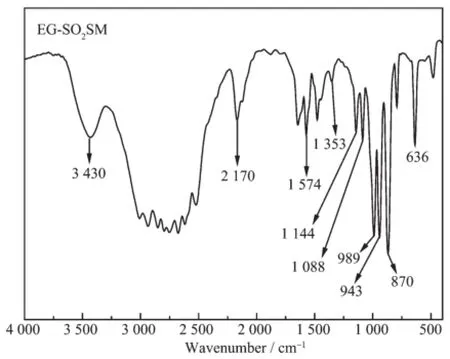
Fig.5 FTIR spectrum of EG-SO2SM
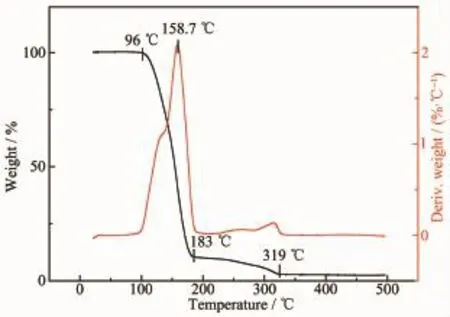
Fig.6 TGA of EG-SO2SM
Besides,as shown in Fig.6,the TGA results shows that the weight loss of EG-SO2SM is divided into two processes.The first weight loss of EG-SO2SM is observed from 96 to 183℃due to the decomposition of SO2and EDA.The second weight loss of EGSO2SM is observed from 183 to 319℃due to thedecomposition of EG.The resultds of TGA could provide evidence which the reaction mechanism of SO2an the systems of EDA+EG is similar to Heldebrant′s study results;meanwhile,the results of TGA also indicate the SO2SM could release SO2by heating.
2.3Preparation of BaSO3and BaSO4with the EG-SO2SM
On the basis of TGA result,SO2could be readily and gradually released from the EG-SO2SM at elevated temperature.Therefore,the released SO2slowly reacted with Ba2+ions to form leaf-like BaSO3at 100℃for 2.5 h(Fig.7a).Furthermore,the aqueous EG-SO2SM solution was employed to prepare blocklike BaSO4by adding H2O2as an oxidant(Fig.7b).Fig. 8 and 9 show thatthe XRD patterns and FTIR spectra results of BaSO3and BaSO4samples and their IR characteristic peaks are in good agreement with references[24-27].Notably,in these processes,the released EDA and EG from the EG-SO2SM were acted as novel surfactants during the formation of crystals.The detailed processes for the formation of BaSO3and BaSO4and the mechanistic studies are under investigation and the result will be published in due course.

Fig.7 Representative applications of EG-SO2SM
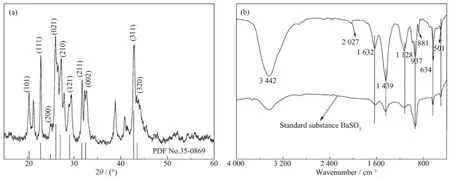
Fig.8 XRD pattern(a)and FTIR spectrum(b)of BaSO3sample

Fig.9 XRD pattern(a)and FTIR spectrum(b)of BaSO4sample
3 Conclusions
In summary,a novel SO2capture,storage and utilization method was successfully developed by utilizing EDA-EGs systems.In this process,SO2can react efficiently with the EDA-EGs systems under mild conditions to form the solid SO2SMs,which could be used as the raw materials to prepare BaSO3or BaSO4with different morphologies.Moreover,the released EDA and EG from the EG-SO2SM were acted as facilitative surfactants to guide the crystallization process ofcrystals.
Acknowledgments:This work was supported by the National Natural Science Foundation of China(Grants No. 21666027,21166017),the Natural Science Foundation of Inner Mongolia Autonomous Region(Grant No.2016JQ02),the Inner Mongolia Science and Technology Key Projects,the Program for Grassland Excellent Talents of Inner Mongolia Autonomous Region,Program for New Century Excellent Talents in University(Grant No.NCET-12-1017)and training plan of academic backbone in youth of Inner Mongolia University of Technology.
Supporting information is available athttp://www.wjhxxb.cn
References:
[1]Wu W,Han B,Gao H,et al.Angew.Chem.Int.Ed.,2004, 43:2415-2417
[2]Renedo M J,Fernandez J.Ind.Eng.Chem.Res.,2002,41: 2412-2417
[3]Ortiz F J G,Vidal F,Ollero P,et al.Ind.Eng.Chem.Res., 2006,45:1466-1477
[4]Zheng Y J,Kiil S,Johnsson J E.Chem.Eng.Sci.,2003,58: 4695-4703
[5]Tailor R,Ahmadalinezhad A,Sayari A.Chem.Eng.J.,2014, 240:462-468
[6]Tailor R,Abboud M,Sayari A.Environ.Sci.Technol.,2014, 48:2025-2034
[7]Uyanga I J,Idem R O.Ind.Eng.Chem.Res.,2007,46:2558-2566
[8]Bollini P,Didas S A,Jones C W.J.Mater.Chem.,2011,21: 15100-15120
[9]Heldebrant D J,Koech P K,Yonker C R.Energy Environ. Sci.,2010,3:111-113
[10]Zhao T X,Guo B,Han L M,et al.ChemPhysChem,2015, 16:2106-2109
[11]Yang D Z,Hou M Q,Ning H,et al.Phys.Chem.Chem. Phys.,2013,15:18123-18127
[12]Lee H J,Jung Y M,Lee K I,et al.RSC Adv.,2013,3:25944 -25949
[13]Yang J,Hu D X.RSC Adv.,2012,2:11410-11418
[14]Huang J,Riisager A,Wasserscheid P,et al.Chem.Commun., 2006,38:4027-4029
[15]Desimoni E,Casella G I,Cataldi T R I,et al.Surf.Interface Anal.,1992,18:623-630
[16]Wang T J,Shemood P M A.Chem.Mater.,1995,7:1020-1030
[17]Kante P K,Bandosz T J.Carbon,2010,48:654-667
[18]Baltrusaitis J,Usher C R,Grassian V H.Phys.Chem.Chem. Phys.,2007,9:3011-3024
[19]Shen J G C,Kalantar T H,Herman R G,et al.Chem.Mater., 2001,13:4479-4485
[20]Smirnov M Y,Kalinkin A V,Pashis A V,et al.J.Phys. Chem.B,2005,109:11712-11719
[21]Heldebrant D J,Koech P K,Ang M T C,et al.Green Chem., 2010,12:713-721
[22]Wang H B,Jessop P G,Liu G J.ACS Macro Lett.,2012,1: 944-948
[23]Shang J P,Liu S M,Ma X Y,et al.Green Chem.,2012,14: 2899-2906
[24]Wang C M,Cui G K,Luo X Y,et al.J.Am.Chem.Soc., 2011,133:11916-11919
[25]Cui G K,Lin W J,Ding F,et al.Green Chem.,2014,16: 1211-1216
[26]Heldebrant D J,Koech P K,Yonker C R.Energy Environ. Sci.,2010,3:111-113
[27]Wang Q,Zhang D,Yang X L,et al.Green Chem.,2013,15: 2222-2229
A SO2Fixation Method and Its Use in Preparation of BaSO3or BaSO4
ZHANG Fei LIU Li-Hua SHA Feng QIAO Xian-Shu ZHANG Jian-Bin*
(College of Chemical Engineering,Inner Mongolia University of Technology,Huhhot 010051,China)
An innovative SO2fixation into solid SO2-storage materials(SO2SMs)under mild condition was developed by employing the low-cost amine-glycol systems consisting of 1,2-ethanediamine(EDA)and ethylene glycolseries(EGs).In particularly,the systems of EDA+EGs possessed a remarkable SO2capture capability from 0.364 to 0.662 gSO2·gabsorbent-1,which formed solid SO2SMs after washing with alcohol and drying under vacuum. The resulting SO2SMs were confirmed as alkyl sulfite by extensive characterization using FTIR,XPS and XRD techniques.Subsequently,the EG-based SO2SM(EG-SO2SM)was utilized to prepare BaSO3or BaSO4with different morphologies.The gradual release of EDA and EG from EG-SO2SM not only yielded raw material but also generated EDA and EG thatacted as efficientsurfactants for the subsequentrecrystallization process.
SO2capture;SO2-storage materials;alkyl sulfite;BaSO4
O611.4
A
1001-4861(2016)12-2205-07
10.11862/CJIC.2016.256
2016-07-29。收修改稿日期:2016-10-10。
国家自然科学基金(No.21666027,21166017)、教育部新世纪优秀人才项目(No.NCET-12-1017)、草原英才项目、内蒙古自然科学基金(No.2016JQ06)和内蒙古科委攻关项目资助。
*通信联系人。E-mail:tadzhang@pku.edu.cn,Tel:+86-471-6575722,Fax:+86-471-6575722;会员登记号:S06N9134M1005。
