CoOOH用作阴极催化剂对非水性锂-氧气电池性能的影响
2016-12-20蔡生容王晓飞朱丁母仕佳张开放黄利武陈云贵
蔡生容 王晓飞 朱丁 母仕佳 张开放 黄利武 陈云贵*,
(1四川大学材料科学与工程学院,成都610065) (2四川大学新能源与低碳技术研究院,成都610065)
CoOOH用作阴极催化剂对非水性锂-氧气电池性能的影响
蔡生容1王晓飞1朱丁*,2母仕佳1张开放1黄利武1陈云贵*,1
(1四川大学材料科学与工程学院,成都610065) (2四川大学新能源与低碳技术研究院,成都610065)
利用工艺简单,成本低廉的共沉淀法制得CoOOH,并用作非水性锂-氧气电池阴极催化剂。通过恒流充放电、线性伏安扫描(LSV)和电化学阻抗(EIS)测试研究了电极的电化学性能。结果表明:由于CoOOH能够明显提高氧气还原反应(ORR)的催化活性,与未使用CoOOH的电极相比较,使用CoOOH为催化剂的电极首次放电容量高达5 093 mAh·g-1,提高了1.7倍。电池的充电过电压降低了约460 mV,充电可逆性得到增强,充放电可逆性提高,使得循环性能得到显著改善。
锂-氧气电池;CoOOH;催化剂;高性能
With the demands of energy storage in electric vehicles markets,Li-O2batteries have attracted worldwide attention due to its high theoretical specific energy up to 11 586 Wh·kg-1(excluding O2)[1],which is equal to that of the gasoline[2],and is about eight times higher than that of the Li-ion batteries[3].In general,the non-aqueous Li-O2battery consists of anode metal Li,high Li-ion conductive organic electrolyte,and the oxygen cathode.On the basis of the electrochemical reaction 2Li+O2⇌Li2O2,the ideal discharge&recharge process of Li-O2batteries is in essence of the oxygen reduction reaction(ORR)and oxygen evolution reaction(OER)[4],which involves no pollution,and makes the batteries environment-friendly.However,in spite of the great prospect,the practical application of Li-O2technology is restricted by several serious issues of the oxygen cathode, including low capacity,large polarization and extremely poor cycle capabilities[4-6].
Developing catalysts has been proved to be a feasible approach to improve the performance of Li-O2batteries[7-9].Among the reported catalysts,noble metal materials display efficient catalytsis for the ORR or the OER[10-11],butthe widespread applicationsare limited due to the high cost.Because of the low cost,ease of preparation and reasonably good catalytic activity, transition metal oxides have drawn more interest,such as Co3O4,MnO2,Fe3O4,NiO and so on[12-13].Except, metal oxyhydroxides which are important precursors for metal oxides have been considered as promising catalysts.Zhang et al.used high aspect ratioγ-MnOOH nanowires as catalysts for non-aqueous Li-O2batteries,enabling the cathode to obtain outstanding performances which were attributed to the cathode porosity and high catalystefficiency[14].Zhang etal.also reported that the lithium-air battery based onγ-MnOOH/MWCNTs composites gave high discharge capacity and low charge potential[15].Lietal.investigated β-FeOOH nanospindles coated on multi-walled carbon nanotubes(β-FeOOH/MWCNTs)in Li-O2batteries which afforded a high capacity of 6 000 mAh·g-1,and good rate capability[16].However,up to now,CoOOH has been rarely researched in Li-O2batteries.Considering the good catalyses of the Co-based oxides and the oxyhydroxides,the catalytic activity of CoOOH is urgent to be studied.
Herein,CoOOH was successfully prepared by a simple one-step co-precipitation method,and the Li-O2batteries using CoOOH as catalyst displayed high electrochemical performance.
1 Experimental
1.1Catalyst synthesis
The preparation of CoOOH was according to previous report[17].In a typicalsynthesis,2 g of CoCl2·6H2O was dissolved in 21 mL of deionized water (H2O).Next,1 g of NaOH was also dissolved in 32 mL of H2O,and dropped into the CoCl2solution with stirring.Subsequently,HCl was used to adjust the pH value to 12.And 50 mL of H2O2(30%)was added into the mixture.Then,the mixture was heated at 60℃for 2 h with magnetic stirring.After cooling to room temperature,the final products were filtered, washed and dried.
1.2Li-O2cellassembly and electrochemical measurements
According to our previous report[18],a swageloktype Li-O2battery was assembled in an argon-filled glove box by placing a lithium foil,two celgard 2500 separators,70μL organic electrolyte(0.9 mol·L-1LiTFSI in tetraethylene glycol dimethyl ether (TEGDME)solvents),and a dried porous cathode into a custom-designed cell.The cathode was prepared by coating a mixture of 50%Super P carbon(SP),30% CoOOH and 20%polyvinylidene fluoride(PVDF)on a nickel foam current collector(1.5 cm-2),and marked as CSP electrode.The cathode was dried at 120℃under vacuum for 12 h.The carbon loading was about 1.5 mg,and the capacity for the Li-O2battery was calculated based on the weight of SP.After standing for 2 hours,the galvanostatic tests were carried out on a LAND 2001A at 25℃under 101.325 kPa pressure of O2.The test voltage was from 2.0 to 4.5 V versus Li+/Li,and the current density was 0.07 mA·cm-2.A cathode composed of SP and PVDF was also made for comparison,and named SP electrode.
1.3Characterizations
After electrochemical test,the battery was disassembled for further analysis,in the glove box.X-ray diffraction(XRD)was carried outusing a Dandong DX-2600 diffractometer,with Cu Kαradiation(λ= 0.154 18 nm)ata scan speed of0.04°·s-1.The operating voltage was 35 kV and the current was 25 mA.The micrograph was collected by scanning electron microscopy(SEM,JSM-7500F)and the operating voltage was 5 kV.Electrochemical impedance spectroscopy(EIS) was measured by a PARSTAT2273 and the frequency was from 100 kHz to 10 mHz.Linear sweep voltammetry(LSV)was also carried out on a PARSTAT2273 with the scan speed of 0.2 mV·s-1.
2 Results and discussion
Fig.1a is the XRD pattern of prepared CoOOH. The main diffraction peaks of the products are well indexed to the(003),(101),(012),(104),(015),(107), (110)and(113)planes of CoOOH(PDF No.07-0169). The morphology was detected by SEM,as shown in Fig.1b.
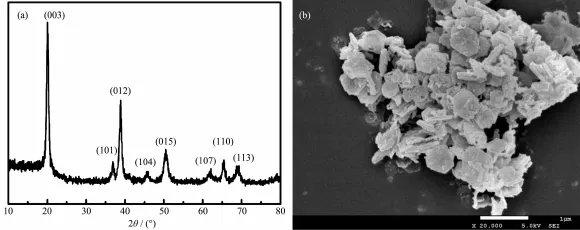
Fig.1 Characterization of prepared CoOOH:(a)XRD pattern;(b)SEM image
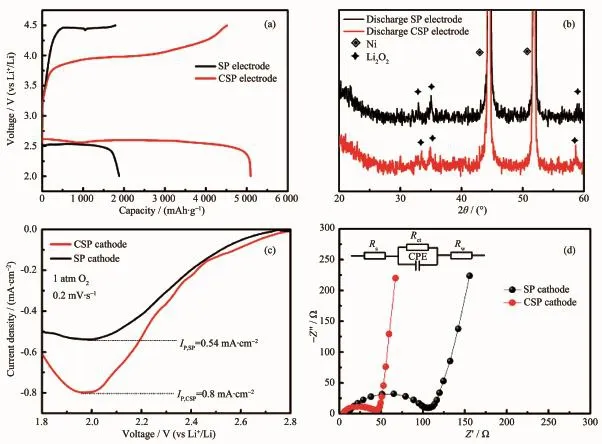
Fig.2 Electrochemical performance of Li-O2batteries with the SP and the CSP electrodes:(a)the first discharge-charge curves;(b)XRD patterns;(c)cathodic LSV curves;(d)EIS spectra
Fig.2a shows the typical discharge-recharge curves of both the SP and the CSP electrodes.At the discharge terminal,the CSP electrode delivers a specific capacity of5 093 mAh·g-1,which is 1.7 times higher than thatofthe SP electrode(1 883 mAh·g-1). The discharge voltage of the CSP electrode(2.6 V)is also 70 mV higher than that of the SP electrode(2.53 V).Obviously,the introduction of CoOOH greatly enhances the energy output of the oxygen cathode. Fig.2b exhibits the XRD patterns of the discharge products for both electrodes.The main peaks around 33°,35°and 58°can be assigned to the Li2O2phase (PDF No.73-1640),indicating that the formation of Li2O2on both the SP and the CSP electrodes duringthe discharge process.Fig.2c presents the cathodic LSV curves of the SP and CSP electrodes.Apparently, the peak current(Ip)ofthe CSP cathode(0.8 mA·cm-2) is much higher than thatof the SP cathode(0.54 mA· cm-2),suggesting a high ORR catalysis of the asprepared CoOOH.Fig.2d displays the EIS spectra of two kinds of electrodes.Two plots exhibita semicircle and a line.According to the literature[16],the high frequency intercept of the semicircle on the real axis is Rs,which includes resistance from electrodes and their contacts to the current collectors and electrolyte resistance.The semicircle at medium frequency is charge-transfer resistance(Rct),corresponding to the kinetic reaction at the air electrode surface.The line atlow frequency is Rw,which is a finite length Warburg element that may be attributed to the diffusion in the cathode.It can be found that the charge-transfer resistance(Rct)in the pristine CSP electrode(36Ω)is much smaller than that in the pristine SP electrode (91Ω),indicating an enhanced kinetics of the ORR, which is in agreement with the result of the LSV(Fig. 2c).The greatly enhanced ORR kinetics significantlyimproves the energy output(Fig.2a).Moreover,the charge voltage plateau of the CSP electrode is 3.99 V, which is 460 mV smaller than that of the SP electrode,suggesting a significantly enhanced recharge-ability ofthe CSP electrode.
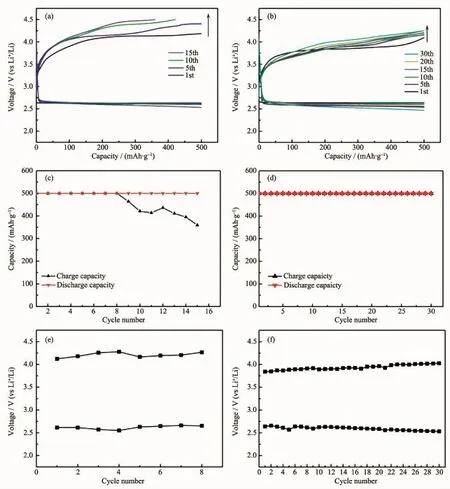
Fig.3 Discharge-charge curves,cycling performances and discharge-charge potential plateaus of the Li-O2battery with the SP(a,c,e)and the CSP(b,d,f)electrodes
In order to investigate the cycling performance of Li-O2batteries,the SP and CSP electrodes were tested following the widely used capacity-limited way[19],with a capacity restriction of 500 mAh·g-1.Fig.3a exhibits the discharge-charge curves of the Li-O2battery with the SP electrode.The related discharge-charge capacity and potential plateau against the cycle number are displayed in Fig.3c and 3e,respectively.The battery with the SP electrode can just completely be operated for 8 cycles.It notes that the recharge capacity starts to decrease atthe 9th cycle when the recharge voltage reaches 4.5 V.From the 9th to 15th cycle,only the discharge capacity meets the fixed value indicating the recharge is incomplete.The 15th coulombic efficiency of the SP electrode is simply 72%.On the contrary,the CSP electrode shows the superior cycling performance.It can be found that the battery with the CSP electrode has no fading of discharge and charge capacities during continued 30 cycles and the coulombic efficiency always keeps 100%,as shown in Fig.3b and 3d.These results show thatthe introduction of CoOOH enhances the cycling performance of the Li-O2batteries.The 1st discharge voltage of the CSP electrode(2.64 V)is about 30 mV higher than that of the SP electrode(2.61 V),and the 1stcharge voltage of the CSP electrode is approximately 3.84 V that is 280 mV smaller than that of the SP electrode(4.12 V).Although the charge voltage increases during the subsequent cycling process,the performance of the CSP electrode is still better than that of the SP electrode.When the SP electrode does not achieve the fixed charge capacity reaching the cut-off voltage at the 15th cycle,the charge voltage of the CSP electrode with the 100%coulombic efficiency is about 3.92 V.After 30 cycles,the charge voltage of the CSP electrode is just 4.03 V.Meanwhile,the discharge voltage is as high as 2.53 V(as shown in Fig.3f).

Fig.4 SEM images of the SP(a,b,c)and CSP electrodes(d,e,f)at pristine,discharged and recharged,respectively
SEM was used to investigate the changes in morphology at different discharge-recharge states,as shown in Fig.4.Fig.4b reveals that the typical smooth toroidal Li2O2in the large size coats on the discharged SP cathode,as revealed by the earlier studies[20].At the end of the recharge process,some toroidal Li2O2can still be clearly observed(Fig.4c).That means the SP electrode is irreversible.Dramatically differing from the SP electrode,there is relatively rough chain morphology attached to the surface of the discharged CSP electrode,as exhibited in Fig.4e.In contrast to the SP electrode,the generated Li2O2almostcompletelydecomposes and the surface is relatively clear(Fig.4f). The electrode nearly returns to the pristine.
3 Conclusions
In summary,CoOOH has been successfully prepared by a simple one-step co-precipitation method. Compared with the SP electrode,the CSP electrode displayed a specific capacity as high as 5 093 mAh· g-1,significantly reduced recharge potential and enhanced cycling stability.The CSP electrode had an improved ORR catalysis,and enhanced rechargeability.Our findings indicate that CoOOH is potentially good catalyst for high performance Li-O2batteries.
References:
[1]Bruce P G,Freunberger S A,Hardwick L J,etal.Nat.Mater., 2012,11:19-29
[2]YANG Feng-Yu(杨凤玉),ZHANG Lei-Lei(张蕾蕾),XU Ji-Jing(徐吉静),et al.Chinese J.Inorg.Chem.(无机化学学报),2013,29(8):1563-1573
[3]Ogasawara T,Débart A,Holzapfel M,et al.J.Am.Chem. Soc.,2006,128(4):1390-1393
[4]Gao R,Zhu J Z,Xiao X L,et al.J.Phys.Chem.C,2015, 119(9):4516-4523
[5]Su W W,Wang W,Li Y L,et al.Mater.Lett.,2016,180:203 -206
[6]Park M,Sun H,Lee H,et al.Adv.Energy Mater.,2012,2(7): 780-800
[7]Prabu M,Ketpang K,Shanmugam S.Nanoscale,2014,6(6): 3173-3181
[8]Kwak W J,Lau K C,Shin C D,et al.ACS Nano,2015,9(4): 4129-4137
[9]Hu X F,Han X P,Hu X,et al.Nanoscale,2014,6(7):3522-3525
[10]JIANG Jie(蒋颉),LIU Xiao-Fei(刘晓飞),ZHAO Shi-Yong (赵世勇),et al.Acta Chim.Sinica(化学学报),2014,4:417-426
[11]Jiang J,He P,Tong S F,etal.NPG Asia Mater.,2016,8:e239
[12]Kuang P,Li L Y,Chen C G,et al.Mater.Lett.,2016,176: 97-100
[13]Tong S F,Zheng M B,Lu Y,et al.J.Mater.Chem.A,2015,3 (31):16177-16182
[14]Zhang L L,Zhang X B,Wang Z L,et al.Chem.Commun., 2012,48(61):7598-7600
[15]Zhang M,Xu Q,Sang L,et al.Chin.Sci.Bull.,2014,59(24): 2973-2979
[16]Li J X,Wen W W,Zou M Z,et al.J.Alloys Compd.,2015, 639:428-434
[17]Zhu L,Wu W Y,Zhu Y S,et al.J.Phys.Chem.C,2015, 119(13):7069-7075
[18]Zhu D,Zhang L,Song M,et al.J.Solid State Electron., 2013,17(11):2865-2870
[19]Wang X F,Cai S R,Zhu D,et al.RSC Adv.,2015,5(107): 88485-88491
[20]Black R,Lee J H,Adams B,et al.Angew.Chem.Int.Ed., 2013,125(1):410-414
CoOOH as Cathode Catalyst for High Performance Non-Aqueous Li-O2Batteries
CAISheng-Rong1WANG Xiao-Fei1ZHU Ding*,2MU Shi-Jia1ZHANG Kai-Fang1HUANG Li-Wu1CHEN Yun-Gui*,1
(1College of Materials Science and Engineering,Sichuan University,Chengdu 610065,China) (2Institute of New Energy and Low-Carbon Technology,Sichuan University,Chengdu 610065,China)
CoOOH was synthesized by a simple one-step co-precipitation method,and used as cathode catalyst in non-aqueous Li-O2batteries.The introduction of CoOOH could catalyze the oxygen reduction reaction(ORR) upon discharge,making the CoOOH-contained cathode display a high discharge capacity of 5 093 mAh·g-1, which was about 1.7 times higher than that of the CoOOH-free cathode.Furthermore,the CoOOH-contained cathode exhibited a significantly reduced recharge overpotential(460 mV),enhancing recharge-ability.Thus,the reversibility was improved,and the cycling performance was ameliorated.
Li-O2battery;CoOOH;catalyst;high performance
TM912;O614.81+2
A
1001-4861(2016)12-2082-06
10.11862/CJIC.2016.272
2016-07-26。收修改稿日期:2016-10-07。
中物院——川大协同创新联合基金(No.XTCX2014001)和四川大学青年教师科研启动基金(No.2015SCU11055)资助项目。
*通信联系人。E-mail:zhuding@scu.edu.cn,ygchen60@aliyun.com,Tel:+86 28 62138375,+86 28 85407335
