作物防御UV-B辐射伤害的机理
2016-12-09李彦生王光华刘晓冰
李彦生,金 剑,王光华,刘晓冰
(中国科学院 东北地理与农业生态研究所 黑土区农业生态重点实验室,黑龙江 哈尔滨 150081)
作物防御UV-B辐射伤害的机理
李彦生,金 剑,王光华,刘晓冰
(中国科学院 东北地理与农业生态研究所 黑土区农业生态重点实验室,黑龙江 哈尔滨 150081)
自上世纪80年代中期发现南极“臭氧洞”及平流臭氧层耗竭现象以来,有关UV-B影响生物和生态系统的研究逐渐成为气候变化研究关注的热点之一。过去40年里,针对UV-B增加对作物的潜在影响,研究者们展开了大量深入细致的研究。研究发现, 自然和增强的UV-B降低作物产量的幅度一般不会超过20%,这种有限的产量损失反映了作物自身特有的防御UV-B损伤的机制。本文从植(作)物对UV-B辐射增强表现的4个层次,即植物形态、生理、生物化学和分子水平变化的光形态建成和胁迫响应,对作物防御UV-B辐射伤害的可能机制予以综述,并提出了今后需要注重研究的几个方向,旨为抗UV-B育种和农艺措施制定提供具有现实价值的信息和理论依据。参117。
UV-B辐射;植物形态;UV-B吸收化合物;抗氧化防御系统;基因表达
0 引 言
地球表面紫外线(UV-B)(280 nm~315 nm)的增加是全球气候变化研究关注的一个重要热点[1-2]。大量研究表明:UV-B的增加对水生和陆地生物均有不良作用,并影响生态系统间的互作[3-7]。
GISS模型表明:相对于1979年-1992年时期,北半球2010年-2020年春季UV-B将增加14%,南半球增加40%[8]。McKenzie等[9]预测,随着蒙特利尔议定书成功的实施,大气平流层臭氧层将逐渐得到恢复,但是,即使到本世纪中叶也很难恢复到上世纪80年代的水平。而且,自然和人类活动引起的N2O释放仍将严重威胁臭氧层的稳定性[10]。此外,全球农业的集约化,包括作物种植密度的提高、施肥量的增加将强烈影响作物冠层和群体接受的UV-B水平,由此影响作物的生长发育[11-12]。
自上世纪80年代中期发现南极“臭氧洞”及平流臭氧层耗竭现象以来,有关UV-B影响生物和生态系统的研究逐渐成为热点[1,6]。过去40年里,针对UV-B长期增加对作物的潜在影响,研究者们展开了大量深入细致的研究。多数结果表明,UV-B辐射的增强降低光合作用,抑制作物蒸腾,损伤叶绿体功能,障碍花和花粉发育,减少蛋白质合成,引起核酸和脂质损伤,抑制关键生理过程及降低作物生物量和产量[13-19]。
研究发现,自然和增强的UV-B降低作物产量的幅度一般不会超过20%[1]。这种有限的产量损失反映了作物自身特有的防御UV-B损伤的机制,包括(1)UV-B吸收物质积累[20-21],(2)酶及非酶抗氧化防御[22-23],(3)DNA修复酶的活化[24]。
有关UV-B辐射对植物水分代谢、呼吸作用和矿质营养吸收等方面的影响已有相关专题评述[13-14],本文仅从植物对UV-B辐射增强表现的光形态建成和胁迫响应两大方面4个层次,即植物形态、生理、生物化学和分子水平变化,对作物防御UV-B辐射伤害的可能机制予以综述。
1 叶片形态、解剖和UV-B吸收物质
已有研究表明,光形态建成反应引起植物结构和化学组成的变化,从而改变UV辐射穿透植物的过程,是植物适应周边辐射变化的一种响应[1]。其中,叶片表皮对UV辐射的衰减作用是多数植物避免UV-B危害的主要机制。UV-B增强以及自然UV辐射降低了玉米、高粱等作物品种的叶面积和叶片厚度(比叶重)[25-26]。Qi等[27]发现叶片厚度与叶片UV-B吸收物质的总量呈显著正相关关系,这些物质主要出现在叶片的上表皮和下表皮中,但上表皮是UV-B衰减的主要场所。
叶片表皮层类黄酮的积累降低UV-B辐射表皮透射比[28],一般而言,透射比高的植物不耐UV-B辐射。Feng等[29]指出大豆品种晋豆耐UV-B增强的部分原因是叶片类黄酮含量高、叶片小和角质层厚。大麦类黄酮突变体对UV-B辐射的敏感性降低,尽管其叶片的类黄酮含量比突变后每株仅仅高出7%[30]。角质层的蜡质和木质素同样起着吸收UV辐射保护叶片的作用[31]。由于蜡质层是响应UV-B辐射的重要表皮特征,耐性强的类型在UV-B辐射时蜡质含量增加,而敏感型品种蜡质含量减少[5]。
许多研究指出,叶片中的苯丙烷类化合物,主要包括类黄酮、花色素苷和相关的酚类,是叶片叶肉组织防御UV-B损伤的重要物质[20,32]。UV-B增强的条件下,植物对UV-B辐射最一致的响应是UV-B吸收物质含量的增加。UV-B吸收物质在叶片的表皮毛状体和表皮细胞中积累最多[22]。这些物质具有有效的自由基净化能力,可以直接增强植物对UV-B辐射的光保护功能[23]。
对UV-B具有相当耐性的拟南芥突变体,主要表现就是类黄酮及其它酚类化合物的积累[33]。类黄酮不仅是UV-B的过滤物质,也是抗氧化物质,它可以通过吸收上表皮组织的UV-B辐射防止敏感器官受到伤害,而缺少这些物质会导致严重的氧化胁迫[34]。当受到UV-B辐射时,小麦、大豆叶片的类黄酮总量增加[20,35]。酚类化合物含量与UV-B吸收能力呈显著相关性,较高UV-B条件下,植物品种间产生这些化合物的能力不同[36]。
然而,Kreft等[37]表明,UV-B辐射增强降低荞麦芸香苷含量,而芸香苷是一种具有抗氧化特性的类黄酮物质。Yao等[38]研究发现UV-B对荞麦叶片芸香苷含量的影响,取决于叶片的位置和UV-B辐射的强度。但是无论UV-B强度高低,植株上部叶片的芸香苷含量均高于下部叶片,因为上部叶片比下部叶片受到更多的辐射。
研究发现,黄瓜、小麦和大豆品种间类黄酮化合物组成和含量存在差异[4,35,39]。Zu等[40]利用UV-B灯辐射分析了20个大豆品种的表现,发现7个品种类黄酮总量增加,5个品种含量降低,8个品种含量没有变化。由于没有考虑每一种类黄酮化合物的含量水平,UV-B辐射很可能在不增加类黄酮总量的同时影响着某种特定类黄酮化合物的合成。例如Warren等[41]报道了UV-B辐射后某些类黄酮化合物是选择性合成的。UV-B增强后,植物叶片表皮细胞、蜡质层和叶毛中不同类黄酮化合物含量和比例发生明显变化,尤其是具有邻位羟基B环的类黄酮物质,如栎皮黄酮葡萄糖苷、藤黄菌素和绿原酸等[42]。
Winter和 Rostas[43]证实,两种栎皮类黄酮物质含量在UV-B增强条件下显著增加,进一步研究发现UV-B增强有利于栎皮黄酮葡萄糖苷的积累,而不利于花色素葡萄糖苷的积累。Gould等[44]报道,离体纯化的花色素苷提取液具有极强的抗氧化物质特性,并能净化活细胞中的活性氧,提出花色素苷是唯一的提高净化H2O2速率的类黄酮类物质,其防御UV辐射的机制可能包括对UV的吸收或者净化活性氧,也可能是两者并存。
然而,由于有关类黄酮类化合物在植物体内的功能方面的知识相当有限,我们对苯丙烷类化合物水平自然差异的功能性意义理解不足,导致大田条件下该种物质积累总量和其特种化合物积累动态之间的关系尚未明确,且植物间或品种间吸收UV化合物含量存在差异,是否呈现出显著的生理代谢意义迄今尚不清晰。因此,有必要开展吸收UV-B化合物的组成、总体含量及其在叶片分布的相关研究。
2 抗氧化防御系统
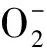
UV-B促进脂质氧化作用产物的形成,破坏自然脂质可溶性抗氧化物,诱导编码抗氧化物基因的表达[13]。研究证实,植物组织和细胞通过多种抗氧化物酶的上调来保护源于UV-B氧化胁迫的伤害[48]。组成抗氧化物防御系统的酶主要有超氧化物歧化酶(SOD; EC 1.15.1.1),过氧化氢酶(CAT; EC1.11.1.6),愈伤木酚过氧化物酶(POD;EC1.11.1.7),抗坏血酸过氧化物酶(APX; EC1.11.1.11),谷胱甘肽还原酶(GR; EC1.6.4.2)和脱氢抗坏血酸还原酶 (DHAR; EC1.8.5.1)[49]。
SOD快速将自由基氧转化为H2O2,并继而由CAT转化为水和氧气。SOD对UV-B 辐射响应的报道结果并不一致。例如,UV-B辐射下,菜豆、小麦、拟南芥和水稻的SOD活性提高[3,50],大麦和大豆没有变化[51],而向日葵子叶的SOD活性降低[52]。
CAT是过氧化物酶体的主要组成,对底物亲和力较低。H2O2分解替代途径是通过细胞中的APX来催化H2O2。APX 是专一的过氧化物酶,其以氧化抗坏血酸成单脱氢抗坏血酸为代价催化H2O2分解[53]。APX同工酶至少分布在4个亚细胞结构中,即基质、类囊体膜、微体和细胞溶质[54]。通过系列反应去除H2O2的过程称之为抗坏血酸-谷胱甘肽循环。
有研究报道,UV-B辐射时,拟南芥幼苗合成抗氧化物的酶包括POD、APX 和 SOD[55]。Liu 和 McClure[56]发现为适应由于UV-B增强而导致的氧化胁迫,POD活性会增高,而SOD的活性变化则因UV-B辐射强度不同而具有差异。
虽然植物受UV-B辐射后是如何产生活性氧的原因尚不清楚,但至少NADPH 氧化酶参与了此过程[55]。直接证据是UV-B辐射后3个菜豆品种的叶片、茎秆和根系均诱导产生NADP-苹果酸酶[57]。而NADP-苹果酸酶可能通过为木质素和类黄酮的生物合成提供NADPH, 在防御UV-B辐射反应中起到主动作用。或许仅测定酶的总活性不能完全反映UV诱导的结构专一变化或者酶的其他组成的改变。例如,UV-B能够有区别的调节酶的同工酶,包括POD、CAT、SOD和 APX。因此,有必要深入研究解决这些问题。
Logemann等[58]发现UV诱导产生的酶为莽草酸途径提供碳骨架,而这些酶在UV-B胁迫下以ATP的形式为合成有利于细胞发挥正常功能的物质提供能量[59]。同时进行UV-B+Cd 处理会导致菠菜丙二醛(MDA)积累增加,表明处理导致细胞质膜发生氧化损害和功能的丧失[23]。Wang等[60]指出,MDA 含量的增加是植物响应不同胁迫包括UV-B胁迫的敏感指标之一。
层次聚类分析结果表明,SOD活性变化是大豆品种对UV-B增强最为敏感的生理指标,其他指标依次是膜渗透性、类黄酮含量、MDA含量、叶绿素a和叶绿素b含量[40]。Zu等[61]进一步研究认为,UV-B诱导的氧化胁迫主要通过间接作用影响植物体,如抑制抗氧化防御系统功能的发挥或激活可以产生活性氧的酶(如NADPH 氧化酶)。
虽然,Yannarelli等[62]证明HO 活性的增加与蛋白质表达和转录水平的增强有关。多数抗氧化物研究中相关抗氧化酶反应的酶活性研究结果和相应mRNA水平上的研究结果并不一致,而且植物如何响应、适应环境变化的机制尚不清楚[16,50]。
非酶反应的防御系统主要是低分子量的抗氧化物,包括脯氨酸、抗坏血酸、谷胱甘肽、α-生育酚和类胡罗卜素。脯氨酸是公认的渗透胁迫保护物质,研究发现它也有解毒活性氧的功能[63],而且大豆叶片脯氨酸积累的增加与UV-B诱导的氧化胁迫及其Ni的解毒作用相联[64]。抗坏血酸盐或谷胱甘肽,作为过氧化物酶反应的电子受体,也起到类似类黄酮的作用[65]。抗坏血酸(AsA)是主要的抗氧化物,其直接与羟基自由基、超氧化物和单价氧反应,并作为最有影响的抗氧化物质还原α-生育酚的氧化形态。已经观察到有些植物对UV-B的响应是AsA总量增加[3,66],而UV-B对玉米幼苗的 AsA 含量没有影响[67]。质外体中的AsA的一个主要功能是氧化还原缓冲作用,可以保护质膜免于氧化损伤,并触发分子响应机制[68]。谷胱甘肽是含硫化合物,其在植物防御氧化伤害中起着一定作用,Wefers 和 Sies[69]指出AsA和谷胱甘肽可能参与几种保护机制,氧化/还原形态的AsA和谷胱甘肽通过转运子在叶绿体膜上运转,而转运子的活性会受到胁迫而改变。
研究表明,UV-B辐射诱导生物膜、多不饱和脂肪酸和磷脂脂质体发生脂质氧化现象非常明显[70]。已有大量数据证明UV辐射改变膜结构和功能的途径,包括膜透性的变化、K-ATPase和过氧化脂质的抑制以及膜抗性的降低等[35]。有关UV辐射促进膜变化的生理效应尚不确定,没有证据表明UV辐射对膜的伤害与细胞死亡有关,因为UV辐射引起膜的变化可能在诱导花色素苷合成中起作用。过氧化氢在生物膜上扩散会导致细胞伤害,而UV-B处理后脂质过氧化作用和H2O2氧化作用增强[71-72]。
因此,植物对光氧化胁迫的适应是多因素、多因子参与的防御过程。活性氧形成和净化的深入探讨有助于理解植物防御过程的各种关系。鉴于越来越多的研究表明活性氧参与UV-B辐射导致的伤害,明确活性氧去除机制对UV研究显得相当重要[45]。但是,有关自然UV-B辐射和去除UV-B辐射状态下,UV-B辐射对酶活性和抗氧化物质的影响研究并不多见[18,50]。
3 植物激素对UV-B的响应
众所周知,植物激素在控制细胞分裂、伸长,调节生长发育和形态建成以及产量形成中起着关键作用[73]。已经确认高等植物有5大类植物激素,此外茉莉酸(JA)、水杨酸(SA)、油菜素内酯(BR)和多胺(PA)也被列入植物激素类[74]。
植物激素的合成与作用受到环境因子的调控,实际上,激素是植物对逆境响应基因表达的初始因子[75]。研究发现,UV-B辐射强度的微量增加就会对IAA、细胞分裂素和 ABA等内源激素的合成、转运和分配产生显著影响,其结果是细胞伸长受到限制、气孔关闭及光合速率下降。因为UV-B辐射增强导致的光氧化自由基伤害会降低IAA和GA含量,却又增强了IAA氧化酶的活性,从而进一步加深了来自于自由基的伤害[76-77]。
株高的降低是评估UV-B辐射敏感度的常用指标,UV-B 辐射显著矮化大豆,主要是节间长度变短而不是节数的减少[78]。向日葵幼苗表现出类似现象,Ros等认为是光氧化抑制内源激素IAA的合成,进而影响细胞壁的伸展[79]。UV-B 辐射可能直接影响细胞分裂和内在的生长特性,Caldwell[80]发现促进植物轴向生长而抑制伸长的乙烯在UV-B辐射条件下含量增多。但是,具体原因尚不清楚。
Dayan等[81]认为GA 信号是烟草节间伸长、形成层活性和纤维分化的基础。光敏色素调节着植物发芽和幼苗建成中的GA合成,但是,在UV光谱区,Pr和Pfr的吸收光谱没有区别,因此该类光感器不参与大豆的节间伸长。研究发现,突变体水稻品种的上部节间的GA含量明显高,说明GA参与节间的伸长[82-83]。UV-B去除以及外援喷施GA3苋菜幼苗同样有类似现象,包括下胚轴长度增加[84]。因此,UV-B辐射引起的节间长度伸长很可能是受到内源激素变化的调节,但是引起遗传机制和生物化学合成变化的过程尚不明了。
Peng和 Zhou[85]研究了UV-B增强条件下,稀土元素镧对大豆幼苗内源激素含量的影响,发现La (III) + UV-B 处理的植株IAA 和 GA 含量高于单纯UV-B处理,而IAA活性和GA 含量低于对照。ABA的一个功能是调节气孔保卫细胞的活性。一般而言,胁迫状态下,植株组织ABA的积累可以降低气孔传导、引起气孔关闭,抑制光合作用。已有研究表明,UV-B辐射导致气孔关闭并增加气孔阻力[86],其原因是保卫细胞的K+溢出以及气孔调节的激素ABA的变化[87]。ABA 诱导气孔的关闭需要H2O2以及NO的参与,而UV-B辐射提高了叶绿体膜的透性,导致膨压丧失,解除ABA合成的限制作用从而出现ABA的积累[88]。
SA被认为是重要的信号分子,其在胁迫状态下调节许多生理过程,在改善非生物胁迫对作物的伤害中起着重要作用[89]。Belkhadi等[90]发现浸泡SA 的幼苗避免了叶绿素的破坏,叶面喷施SA同样增加色素的含量[91]。研究表明,UV辐射状态下,植物大量积累SA,并直接为各种抗氧化反应提供信号[89]。已有报道表明,多种胁迫条件下SA 能够诱导抗氧化物活性[74,92],Choudhury 和 Panda[93]观察到SA处理导致植株 CAT,POD和 SOD 活性降低。
Li等[94]发现 SA能够缓解UV-B 对大豆幼苗生长、色素含量的不利影响。SA处理可以显著降低由于UV-B 辐射引起的脂质过氧化作用,致使SOD活性提高,POD活性降低,而CAT活性不受影响。由此提出,SA不仅是潜在的抗氧化物而且是保持细胞质膜完整性的稳定剂,以此提高植物对UV-B胁迫的抗性。Ervin[95]也报道外源SA施用可以缓解UV-B辐射对草地早熟禾的危害程度。Mahdavian等[96]指出SA能够通过增加叶绿素含量降低UV辐射对植株光合器官的胁迫程度。Stratmann等[97]报道UV辐射影响JA含量,导致基因表达和昆虫采食交叠。然而,UV-B辐射诱导的植物激素包括SA和JA提高植物抗性的机理尚不清楚。
4 DNA损伤与基因表达
DNA的吸收光谱使其成为UV-B辐射伤害的主要靶标,即使很低的辐射剂量也能杀死缺少DNA修复途径的突变体[98]。研究认为,UV-B辐射的直接伤害源于DNA分子对UV-B辐射的吸收,导致DNA产生二聚体和单聚体的DNA光产物引起细胞伤害,并直接伤害蛋白质[99],而间接伤害则是通过自由基和活性氧的产生[100]。Hargreaves等[101]指出DNA并非直接吸收UV-A 辐射,但仍然通过存在的DNA光产物产生次级光反应或者是间接的光致敏反应,引起DNA损伤。DNA损伤测定表明,UV-B辐射引发较多荧光穿透叶片致使细胞大面积受到干扰[20]。
植物修复机制包括DNA伤害的切补修复或嘧啶二聚体作为光裂合酶的修复(由UV-A和光合有效辐射活化)[102]。DNA吸收UV-B辐射引起光致转换,生成环丁(烷)嘧啶二聚体(CPDs)和嘧啶二聚体。由于DNA 和 RNA 聚合酶不能辨识这些光生产物,经过CPD光裂合酶的消除对于DNA复制和转录就十分必要[98]。
现已明确,UV-B增强引起参与苯丙烷类化合物途径的苯丙烷类化合物基因和酶的上调[103]。由于查耳酮(花色素)合酶(CHS; EC 2.3.1.74)是催化类黄酮生物合成的第一步反应,遗传改进CHS的表达就可能增加防御UV-B辐射伤害的类黄酮类化合物的产生。大豆的CHS是由多基因家族(至少8个)编码(GmCHS1-GmCHS8)。Shimizu等[104]报道,除GmCHS2外,其它成员的表达都受到白光的诱导并在UV-B作用下增强。研究表明,在现实的UV-B水平下,Rubisco 水平的减少是导致光合速率降低的主要原因[14],而且光合基因表达很可能是下调的[105]。
Casati和 Walbot[59]发现UV-B辐射导致几种与光合有关的玉米基因表达减弱,而抗氧化物相关的基因表达增强。研究同样发现UV-B增强了参与脂肪酸代谢和氧脂类生物合成的基因表达[106]。利用微芯片技术已经分别鉴定出玉米和拟南芥中100多个响应基因[107]。Yannarelli等[62]表明HO mRNA 上调方式与UV-B响应的其他基因类似。但是,UV-B辐射活化实际信号转导的途径并不十分清楚[97]。
早期研究表明,植物MYB转录因子调节植物花色素苷和栎鞣红生物合成,刚毛的分化,决定表皮细胞的形状和GA-基因的活化[108-109]。Shimizu[104]分离鉴定了GmMYB29 基因亚家族的基因表达,发现其对UV-B辐射呈现出显著的上调特征。GmMZB29 基因家族至少由4个紧密相关的基因组成,可以分成2组,A组基因表达在开始UV-B辐射两个小时后达到高峰,且GmCUS mRNA积累保持增加。
有研究发现,在UV-B处理之前喷施抗氧化物质能够阻碍与病原菌有关基因的转录增加并降低光合基因转录[110]。说明对UV-B辐射响应而言,活性氧参与导致转录水平变化的途径。为评估活性氧诱导HO-1转录水平中的作用,Yannarelli等[16]探讨了AsA 处理对UV-B影响的作用方式,与活性氧调节HO-1基因表达对UV-B响应一致,AsA处理干扰了转录的增加。
显然,测定高等植物的DNA伤害,分析DNA伤害修复与生产力的关系,对评估UV-B增强的影响相当重要。但植物对UV-B响应的分子基础尚不完全明了,一般认为是受体分子和响应信号转导对细胞器的信号感知的结果,并部分调节着基因表达。Xu等[21]并未观察到蛋白质影响信号转导,因为多数参与信号转导的蛋白质丰度极低。另外,mRNA 水平的研究并不能解释最终蛋白质的数量和质量变化。mRNA和蛋白质水平之间的关系并不紧密,尤其是对叶绿体基因而言,其通常是受到后转录水平的控制[111]。此外,许多蛋白质经翻译后修饰,诸如,信号肽去除、磷酸化和糖酵解过程,其对蛋白质的活性及其亚细胞的局部化至关重要。因此,mRNA水平变化本身并不足以评估对UV-B的响应,有必要在蛋白质水平研究UV-B的影响。而这方面的研究相对有限,多数研究集中在单个蛋白质,诸如PR-1蛋白,GR、抗坏血酸过氧化酶和SOD、NR 和血红素氧化酶[45,55,62]。所以,有必要从蛋白组角度深入研究UV-B响应的分子基础。
综上,研究者们从光形态建成和胁迫响应方面探讨了作物对UV-B辐射的适应或防御机制,但是多数结果趋于少数因素的研究,综合进行系统评价的研究鲜有报道,而这对未来选育具有抗性作物品种具有积极的意义。
5 今后研究方向
很多植物和作物品种能够适应UV-B辐射的增强。Sullivan等[32]研究了400种植物(品种),指出有2/3对UV-B敏感,1/3表现为较高的耐(抗)性。小麦、玉米、大豆、水稻和高粱对UV-B辐射的响应品种间存在明显差异[26,39,61,112-116]。品种间对UV-B敏感性的差异可能源于对UV-B辐射不同的适应或防御机制,为利用遗传工程培育品种或改进农艺措施应对UV-B辐射增强降低作物产量的潜在危险奠定了重要基础。
总体来讲,植物具有系列响应UV-B的机制来防御、缓解或修复UV-B伤害,包括DNA修复、表皮层过滤、酶和非酶反应的抗氧化防御以及植物激素的适应作用等。然而,这些可能的作用机制在何等程度上起着作用,是否过滤筛选机制及适应机制(诸如抗氧化物质和内源激素)之间是否存在交替作用或互作,尚有待深入研究。当然,剖析这种机制的路径极具挑战性。
由此,我们建议今后在测定分析相对伤害指数、形态变化、叶片上表皮蜡质含量和产量等表观评价指标基础上,应该系统深入开展耐UV-B的机理的研究,包括:
(1)叶片主要UV-B吸收物质及抗氧化物对UV-B辐射的响应,包括黄酮、酚类化合物抗坏血酸盐、α-生育酚、脯氨酸、花色素苷和谷胱甘肽等变化。
(2)UV-B辐射对叶片氧化状态及主要抗氧化酶活性的影响,包括2-硫代巴比土酸反应物(TBARS)、过氧化氢含量、膜渗漏变化、超氧化物歧化酶(SOD)、过氧化氢酶(CAT)、过氧化物酶(POD)、抗坏血酸过氧化物酶(APX)、谷胱甘肽还原酶(GR)和脱氢抗坏血酸还原酶(DHAR)变化。
(3)植物内源激素种类及其活性对UV-B辐射的反应,包括脱落酸(ABA)、细胞分裂素(Cyt)活性、赤霉素(GA)、生长素(IAA)、乙烯、水杨酸(SA)活性变化。
(4)叶片响应UV-B辐射的分子基础,包括UV-B去除对叶片DNA 和 RNA 的影响、UV-B增强对叶片DNA 和 RNA 的伤害和叶片蛋白质组对UV-B辐射响应的分析等。
此外,由于现今的多数研究是在温室、植物生长箱或大田条件下,利用包层材料探讨较低水平光合有效辐射的UV-B增强对某种植(作)物或品种的影响,由于UV-B和PAR的自然光谱的变化,所处的条件区别于正常的大田环境,从而影响伤害与修复机制的平衡[6]。而从UV-B去除的角度探讨作物的响应只是近十几年的事情[117]。实际上,UV-B 去除可以更为现实地评价作物及其品种对现有UV辐射水平的敏感性,为作物适应气候变化提供更有实用价值的信息。然而,大田条件下有关农作物UV-B去除的研究很少。
因此,开展大田条件下UV-B去除的研究,是一个很好的研究途径,将为植物防御UV-B辐射机理和选择抗性品种或应对措施提供更加现实可靠的数据。由此,分析UV-B对作物抗性生理、DNA损伤以及蛋白代谢过程的影响,全面解析UV-B条件下,作物的基因-代谢-生理协调变化,揭示防御UV-B伤害的品种间差异的生理生化基础,明确作物抗(耐)UV-B基因型的关键特性和UV-B辐射诱导防御系统表达的机制,将为生物技术、传统育种和农艺栽培提供极有现实价值的信息,从而为抗UV-B育种和农艺措施制定提供深入的理论依据。
[1] Ballaré C L,Caldwell M M,Flint S D,et al.Effects of solar ultraviolet radiation on terrestrial ecosystems.Patterns,mechanisms,and interactions with climate change[J].Photochemical and Photobiological Sciences,2011,10(2):226-241.
[2] Zhang L,Allen Jr L H,Vaughana M M,et al.Solar ultraviolet radiation exclusion increases soybean internode lengths and plant height[J].Agricultural and Forest Meteorology,2014,184:170-178.
[3] Dai Q,Yan B,Huang S,et al.Response of oxidative stress defense systems in rice (Oryzasativa) leaves with supplemental UV‐B radiation[J].Physiologia Plantarum,1997,101(2):301-308.
[4] 梁 滨,周 青.UV-B辐射对植物类黄酮影响的研究进展[J].中国生态农业学报,2007,15(3):191-194.
[5] Kakani V G,Reddy K R,Zhao D,et al.Senescence and hyperspectral reflectance of cotton leaves exposed to ultraviolet‐B radiation and carbon dioxide[J].Physiologia Plantarum,2004,121(2):250-257.
[6] Caldwell M M,Bornman J F,Ballaré C L,et al.Terrestrial ecosystems,increased solar ultraviolet radiation,and interactions with other climate change factors[J].Photochemical and Photobiological Sciences,2007,6(3):252-266.
[7] Rastogi R P,Singh S P,Incharoensakdi A,et al.Ultraviolet radiation-induced generation of reactive oxygen species,DNA damage and induction of UV-absorbing compounds in the cyanobacteriumRivulariasp.HKAR-4[J].South African Journal of Botany,2014,90:163-169.
[8] Taalas P,Kaurola J,Kylling A,et al.The impact of greenhouse gases and halogenated species on future solar UV radiation doses[J].Geophysical Research Letters,2000,27(8):1127-1130.
[9] McKenzie R L,Aucamp P J,Bais A F,et al.Ozone depletion and climate change:impacts on UV radiation[J].Photochemical & Photobiological Sciences,2011,10(2):182-198.
[10] IPCC 2007.Climate change[D].2007:The physical science basis.Agenda,2007,6.
[11] Tevini M.UV-B effects on plants[M].In:Agrawal S B,Agrawal M(eds).Environmental pollution and plant responses.Lewis Publishers,Boca Raton,2000.
[12] Zepp R G,Erickson Iii D J,Paul N D,et al.Effects of solar UV radiation and climate change on biogeochemical cycling:interactions and feedbacks[J].Photochemical & Photobiological Sciences,2011,10:261-279.
[13] 彭 祺,周 青.植物次生代谢响应UV-B辐射胁迫的生态学意义[J].中国生态农业学报,2009,17(3):610-615.
[14] 张君玮,周 青.UV-B辐射对植物水分代谢的影响[J].中国生态农业学报,2009,17(4):829-833.
[15] He J M,She X P,Meng Z N,et al.Reduction of rubisco amount by UV—B radiation is related to increased H2O2content in leaves of mung bean seedlin[J].Journal of Plant Psiology and Molecular Biology,2004,30(3):291-296.
[16] Yannarelli G G,Gallego S M,Tomaro M L.Effect of UV-B radiation on the activity and isoforms of enzymes with peroxidase activity in sunflower cotyledons[J].Environmental and Experimental Botany,2006,56(2):174-181.
[17] Feng H,Li S,Xue L,et al.The interactive effects of enhanced UV-B radiation and soil drought on spring wheat[J].South African Journal of Botany,2007,73(3):429-434.
[18] Xu C P,Natarajan S,Sullivan J H.Impact of solar ultraviolet-B radiation on the antioxidant defense system in soybean lines differing in flavonoid contents[J].Environmental and Experimental Botany,2008,63(1-3):39-48.
[19] Fan C X,Hu H Q,Wang L H,et al.Enzymological mechanism for the regulation of lanthanum chloride on flavonoid synthesis of soybean seedlings under enhanced ultraviolet-B radiation[J].Environmental Science and Pollution Research International,2014,21(14):8792-8800.
[20] Mazza C A,Boccalandro H E,Giordano C V,et al.Functional significance and induction by solar radiation of ultraviolet-absorbing sunscreens in field-grown soybean crops[J].Plant Physiology,2000,122(1):117-125.
[21] Xu C,Sullivan J H,Garrett W M,et al.Impact of solar ultraviolet-B on the proteome in soybean lines differing in flavonoid contents[J].Phytochemistry,2008,69(1):38-48.
[22] Zancan S,Suglia I,La Rocca N,et al.Effects of UV-B radiation on antioxidant parameters of iron-deficient barley plants[J].Environmental and Experimental Botany,2008,63(1-3):71-79.
[23] Li X,Zhang L,Li Y,et al.Changes in photosynthesis,antioxidant enzymes and lipid peroxidation in soybean seedlings exposed to UV-B radiation and/or Cd[J].Plant and Soil,2012,352(1):377-387.
[24] Kim B G,Kim J H,Kim J,et al.Accumulation of flavonols in response to ultraviolet-B irradiation in soybean is related to induction of flavanone 3-beta-hydroxylase and flavonol synthase[J].Molecules and Cells,2008,25(2):247-252.
[25] Correia C M,Areal E L V,Torres-Pereira M S,et al.Intraspecific variation in sensitivity to ultraviolet-B radiation in maize grown under field conditions:II.Physiological and biochemical aspects[J].Field Crops Research,1999,62(2-3):97-105.
[26] Kataria S,Guruprasad K.Intraspecific variations in growth,yield and photosynthesis of sorghum varieties to ambient UV (280-400nm) radiation[J].Plant Science,2012,196:85-92.
[27] Qi Y,Bai S,Heisler G M.Changes in ultraviolet-B and visible optical properties and absorbing pigment concentrations in pecan leaves during a growing season[J].Agricultural and Forest Meteorology,2003,120(1-4):229-240.
[28] Tevini M,Braun J,Fieser G.The protective function of the epidermal layer of rye seedlings against ultraviolet-B radiation[J].Photochemistry and Photobiology,1991,53(3):329-333.
[29] Feng H,An L,Chen T,et al.The effect of enhanced ultraviolet-B radiation on growth,photosynthesis and stable carbon isotope composition (δ13C) of two soybean cultivars (Glycinemax) under field conditions[J].Environmental and Experimental Botany,2003,49(1):1-8.
[30] Reuber S,Bornman J,Weissenböck G.A flavonoid mutant of barley(HordeumvulgareL.) exhibits increased sensitivity to UV‐B radiation in the primary leaf[J].Plant,Cell and Environment,1996,19(5):593-601.
[31] Caldwell M M,Robberecht R,Flint S D.Internal filters:prospects for UV-acclimation in higher plants[J].Physiologia Plantarum,1983,58(3):445-450.
[32] Sullivan J H,Gitz D C,Liu-Gitz L,et al.Coupling short-term changes in ambient UV‐B levels with induction of UV-screening compounds[J].Photochemistry and Photobiology,2007,83(4):863-870.
[33] Bieza K,Lois R.An Arabidopsis mutant tolerant to lethal ultraviolet-B levels shows constitutively elevated accumulation of flavonoids and other phenolics[J].Plant Physiology,2001,126(3):1105-1115.
[34] Peng Z F,Strack D,Baumert A,et al.Antioxidant flavonoids from leaves ofPolygonumhydropiperL[J].Phytochemistry,2003,62(2):219-228.
[35] Zu Y Q,Li Y,Chen H Y,et al.Intraspecific responses in crop growth and yield of 20 wheat cultivars to enhanced ultraviolet-B radiation under field conditions[J].Field Crops Research,2000,67(1):25-33.
[36] Levizou E,Manetas Y.Spectrophotometric assessment of leaf UV-B absorbing compounds and chemically determined total phenolic levels are strongly correlated[J].Canadian Journal of Botany,2002,80(6):690-694.
[37] Kreft S,Strukelj B,Gaberscik A,et al.Rutin in buckwheat herbs grown at different UV‐B radiation levels:comparison of two UV spectrophotometric and an HPLC method[J].Journal of Experimental Botany,2002,53(375):1801-1804.
[38] Yao Y,Xuan Z,Li Y,et al.Effects of ultraviolet-B radiation on crop growth,development,yield and leaf pigment concentration of tartary buckwheat (Fagopyrumtataricum) under field conditions[J].European Journal of Agronomy,2006,25(3):215-222.
[39] 冯虎元,安黎哲,徐世健,等.紫外线-B辐射增强对大豆生长、发育、色素和产量的影响[J].作物学报,2001,3(3):319-323.
[40] Zu Y Q,Li Y,Chen H Y,et al.Intraspecific differences in physiological response of 20 soybean cultivars to enhanced ultraviolet-B radiation under field conditions[J].Environmental and Experimental Botany,2003,50(1):87-97.
[41] Warren J M,Bassman J H,Fellman J K,et al.Ultraviolet-B radiation alters phenolic salicylate and flavonoid composition of Populus trichocarpa leaves[J].Tree Physiology,2003,23(8):527-535.
[42] Harborne J B,Williams C A.Advances in flavonoid research since 1992[J].Phytochemistry,2000,55(6):481-504.
[43] Winter T R,Rostás M.Ambient ultraviolet radiation induces protective responses in soybean but does not attenuate indirect defense[J].Environmental Pollution,2008,155(2):290-297.
[44] Gould K S,McKelvie J,Markham K R.Do anthocyanins function as antioxidants in leaves? Imaging of H2O2in red and green leaves after mechanical injury[J].Plant,Cell and Environment,2002,25(10):1261-1269.
[45] Kalbina I,StridA.Supplementary ultraviolet-B irradiation reveals differences in stress responses between Arabidopsis thaliana ecotypes[J].Plant,Cell and Environment,2006,29(5):754-763.
[46] Green R,Fluhr R.UV-B-induced PR-1 accumulation is mediated by active oxygen species[J].The Plant Cell,1995,7(2):203-212.
[47] Mackerness S A H,Surplus S L,Blake P,et al.Ultraviolet-B-induced stress and changes in gene expression in Arabidopsis thaliana:role of signalling pathways controlled by jasmonic acid,ethylene and reactive oxygen species[J].Plant,Cell and Environment,1999,22(11):1413-1423.
[48] Chen K,Feng H,Zhang M,et al.Nitric oxide alleviates oxidative damage in the green algaChlorella pyrenoidosa caused by UV-B radiation[J].Folia Microbiologica,2003,48(3):389-393.
[49] 闫生荣,周 青.紫外辐射与复合胁迫对植物抗氧化酶系统的影响[J].中国生态农业学报,2007,15(3):195-197.
[50] Agrawal S B,Rathore D.Changes in oxidative stress defense system in wheat (TriticumaestivumL.) and mung bean (VignaradiataL.) cultivars grown with and without mineral nutrients and irradiated by supplemental ultraviolet-B[J].Environmental and Experimental Botany,2007,59(1):21-33.
[51] Mazza C A,Battista D,Zima A M,et al.The effects of solar ultraviolet‐B radiation on the growth and yield of barley are accompanied by increased DNA damage and antioxidant responses[J].Plant,Cell and Environment,1999,22(1):61-70.
[52] Costa H,Gallego S M,Tomaro M L.Effect of UV-B radiation on antioxidant defense system in sunflower cotyledons[J].Plant Science,2002,162(6):939-945.
[53] Jiménez A,Hernández J A.,del Río L A,et al.Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves[J].Plant Physiology,1997,114(1):275-284.
[54] 梁婵娟,陶文沂,周 青.UV-B辐射增强的环境植物学效应研究[J].中国生态农业学报,2004,12(4):46-48.
[55] Rao M V,Paliyath G,Ormrod D P.Ultraviolet-B-and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana[J].Plant Physiology,1996,110(1):125-136.
[56] Liu L,McClure J W.Effects of UV-B on activities of enzymes of secondary phenolic metabolism in barley primary leaves[J].Physiologia Plantarum,1995,93(4):734-739.
[57] Pinto M E,Edwards G E,Riquelme A A,et al.Enhancement of nodulation in bean (Phaseolusvulgaris) by UV-B irradiation[J].Functional Plant Biology,2002,29(10):1189-1196.
[58] Logemann E,Wu S C,Schröder J,et al.Gene activation by UV light,fungal elicitor or fungal infection in Petroselinum crispum is correlated with repression of cell cycle-related genes[J].The Plant Journal,1995,8(6):865-876.
[59] Casati P,Walbot V.Gene expression profiling in response to ultraviolet radiation in maize genotypes with varying flavonoid content[J].Plant Physiology,2003,132(4):1739-1754.
[60] Wang G H,Chen K,Chen L Z,et al.The involvement of the antioxidant system in protection of desert cyanobacterium Nostoc sp.against UV-B radiation and the effects of exogenous antioxidants[J].Ecotoxicology and Environmental Safety,2008,69(1):150-157.
[61] Zu Y G,Pang H H,Yu J H,et al.Responses in the morphology,physiology and biochemistry of Taxus chinensis var.mairei grown under supplementary UV-B radiation[J].Journal of Photochemistry and Photobiology B:Biology,2010,98(2):152-158.
[62] Yannarelli G G,Noriega G O,Batlle A,et al.Heme oxygenase up-regulation in ultraviolet-B irradiated soybean plants involves reactive oxygen species[J].Planta,2006,224(5):1154-1162.
[63] Matysik J,Bhalu B,Mohanty P.Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants[J].Current Science,2002,82(5):525-532.
[64] Prasad S M,Dwivedi R,Zeeshan M.Growth,photosynthetic electron transport,and antioxidant responses of young soybean seedlings to simultaneous exposure of nickel and UV-B stress[J].Photosynthetica,2005,43(2):177-185.
[65] Takahama U,Oniki T.Flavonoids and some other phenolics as substrates of peroxidase:physiological significance of the redox reactions[J].Journal of Plant Research,2000,113(3):301-309.
[66] Galatro A,Simontacchi M,Puntarulo S.Free radical generation and antioxidant content in chloroplasts from soybean leaves exposed to ultraviolet-B[J].Physiologia Plantarum,2001,113(4):564-570.
[67] Carletti P,Masi A,Wonisch A,et al.Changes in antioxidant and pigment pool dimensions in UV-B irradiated maize seedlings[J].Environmental and Experimental Botany,2003,50(2):149-157.
[68] Pignocchi C,Foyer C H.Apoplastic ascorbate metabolism and its role in the regulation of cell signalling[J].Current Opinion in Plant Biology,2003,6(4):379-389.
[69] Wefers H,Sies H.The protection by ascorbate and glutathione against microsomal lipid peroxidation is dependent on vitamin E[J].European Journal of Biochemistry,1988,174(2):353-357.
[70] Kochevar I E.UV-induced protein alterations and lipid oxidation in erythrocyte membranes[J].Photochemistry and Photobiology,1990,52(4):795-800.
[71] Mishra V,Mishra P,Srivastava G,et al.Effect of dimethoate and UV-B irradiation on the response of antioxidant defense systems in cowpea (VignaunguiculataL.) seedlings[J].Pesticide Biochemistry and Physiology,2011,100(2):118-123.
[72] Yang Y,Yao Y,Xu G,et al.Growth and physiological responses to drought and elevated ultraviolet‐B in two contrasting populations of Hippophae rhamnoides[J].Physiologia Plantarum,2005,124(4):431-440.
[73] Liu B,Liu X B,Wang C,et al.Endogenous hormones in seed,leaf,and pod wall and their relationship to seed filling in soybeans[J].Crop and Pasture Science,2010,61(2):103-110.
[74] Saruhan N,Saglam A,Kadioglu A.Salicylic acid pretreatment induces drought tolerance and delays leaf rolling by inducing antioxidant systems in maize genotypes[J].Acta Physiologiae Plantarum,2012,34(1):97-106.
[75] Zaffari G R,Peres L E P,Kerbauy G B.Endogenous levels of cytokinins,indoleacetic acid,abscisic acid,and pigments in variegated somaclones of micropropagated banana leaves[J].Journal of Plant Growth Regulation,1998,17(2):59-61.
[76] Huang S,Dai Q,Peng S,et al.Influence of supplemental ultraviolet-B on indoleacetic acid and calmodulin in the leaves of rice (OryzasativaL.)[J].Plant Growth Regulation,1997,21(1):59-64.
[77] 王春霞,刘亚力,李凤梅,等.稀土硝酸盐对生成超氧阴离子自由基的抑制作用[J].中国稀土学报,2000,18(3):286-288.
[78] Teramura A H,Murali N.Intraspecific differences in growth and yield of soybean exposed to ultraviolet-B radiation under greenhouse and field conditions[J].Environmental and Experimental Botany,1986,26(1):89-95.
[79] Ros J,Tevini M.Interaction of UV-radiation and IAA during growth of seedlings and hypocotyl segments of sunflower[J].Journal of Plant Physiology,1995,146(3):295-302.
[80] Caldwell M M,Teramura A H,Tevini M.Effects of increased solar ultraviolet radiation on terrrestrial plants[J].Ambio,1995,24(3):166-73.
[81] Dayan J,Voronin N,Gong F,et al.Leaf-induced gibberellin signaling is essential for internode elongation,cambial activity,and fiber differentiation in tobacco stems[J].The Plant Cell,2012,24(1):66-79.
[82] Luo A,Qian Q,Yin H,et al.EUI1,encoding a putative cytochrome P450 monooxygenase,regulates internode elongation by modulating gibberellin responses in rice[J].Plant and Cell Physiology,2006,47(2):181-191.
[83] Zhu Y,Nomura T,Xu Y,et al.Elongated uppermost internode encodes a cytochrome P450 monooxygenase that epoxidizes gibberellins in a novel deactivation reaction in rice[J].The Plant Cell,2006,18(2):442-456.
[84] Sharma A,Guruprasad K.Similarities in the biochemical changes between solar UV exclusion and GA application in Amaranthus caudatus[J].Physiology and Molecular Biology of Plants,2009,15(4):367-370.
[85] Peng Q,Zhou Q.The endogenous hormones in soybean seedlings under the joint actions of rare earth element La (III) and ultraviolet-B stress[J].Biological Trace Element Research,2009,132(1-3):270-277.
[86] Björn L O.Effects of ozone depletion and increased UV‐B on terrestrial ecosystems[J].International Journal of Environmental Studies,1996,51(3):217-243.
[87] Yang J H,Chen T,Wang X L.Effect of enhanced UV-B radiation on endogenous ABA and free proline contents in wheat leaves[J].Acta Ecologica Sinica,1999,20(1):39-42.
[88] Wang X Q,Ullah H,Jones A M,et al.G protein regulation of ion channels and abscisic acid signaling in Arabidopsis guard cells[J].Science,2001,292(5524):2070-2072.
[90] Belkhadi A,Hediji H,Abbes Z,et al.Effects of exogenous salicylic acid pre-treatment on cadmium toxicity and leaf lipid content inLinumusitatissimumL[J].Ecotoxicology and Environmental Safety,2010,73(5):1004-1011.
[91] Hayat S,Fariduddin Q,Ali B,et al.Effect of salicylic acid on growth and enzyme activities of wheat seedlings[J].Acta Agronomica Hungarica,2005,53(4):433-437.
[92] Mutlu S,Atici Ö,Nalbantoglu B.Effects of salicylic acid and salinity on apoplastic antioxidant enzymes in two wheat cultivars differing in salt tolerance[J].Biologia Plantarum,2009,53(2):334-338.
[93] Choudhury S,Panda S K.Role of salicylic acid in regulating cadmium induced oxidative stress inOryzasativaL.roots[J].Bulgarian Journal of Plant Physiology,2004,30(3-4):95-110.
[94] Li X M,Ma L J,Bu N,et al.Effects of salicylic acid pre-treatment on cadmium and/or UV-B stress in soybean seedlings[J].Biologia Plantarum,2014,58(1):195-199.
[95] Ervin E H,Zhang X,Fike J H.Ultraviolet-B radiation damage on Kentucky Bluegrass II:Hormone supplement effects[J].HortScience,2004,39(6):1471-1474.
[96] Mahdavian K,Kalantari K,Ghorbanli M,et al.The effects of salicylic acid on pigment contents in ultraviolet radiation stressed pepper plants[J].Biologia Plantarum,2008,52(1):170-172.
[97] Stratmann J.Ultraviolet-B radiation co-opts defense signaling pathways[J].Trends in Plant Science,2003,8(11):526-533.
[98] Britt A B,May G D.Re-engineering plant gene targeting[J].Trends in Plant Science,2003,8(2):90-95.
[99] Bray C M,West C E.DNA repair mechanisms in plants:crucial sensors and effectors for the maintenance of genome integrity[J].New Phytologist,2005,168(3):511-528.
[100] Latifi A,Ruiz M,Zhang C C.Oxidative stress in cyanobacteria[J].FEMS Microbiology Reviews,2009,33(2):258-278.
[101] Hargreaves A,Taiwo F,Duggan O,et al.Near-ultraviolet photolysis of β-phenylpyruvic acid generates free radicals and results in DNA damage[J].Journal of Photochemistry and Photobiology B:Biology,2007,89(2-3):110-116.
[102] Taylor R M,Tobin A K,Bray C M.DNA damage and repair in plants[M].In:Lumsden PJ (Ed.).Plants and UV-B responses to environmental change.Cambridge:Cambridge University Press,1997.
[103] Ryan K G,Swinny E E,Markham K R,et al.Flavonoid gene expression and UV photoprotection in transgenic and mutant Petunia leaves[J].Phytochemistry,2002,59(1):23-32.
[104] Shimizu T,Fujibe R,Senda M,et al.Molecular cloning and characterization of a subfamily of UV-B responsive MYB genes from soybean[J].Breeding science,2000,50(2):81-90.
[105] Jordan B R.Review:Molecular response of plant cells to UV-B stress[J].Functional Plant Biology,2002,29(8):909-916.
[106] Izaguirre M M,Scopel A L,Baldwin I T,et al.Convergent responses to stress.Solar ultraviolet-B radiation and Manduca sexta herbivory elicit overlapping transcriptional responses in field-grown plants of Nicotiana longiflora[J].Plant Physiology,2003,132(4):1755-1767.
[107] Casati P,Walbot V.Rapid transcriptome responses of maize (Zeamays) to UV-B in irradiated and shielded tissues[J].Genome Biology,2004,5(3):R16-R16.
[108] Oppenheimer D G,Herman P L,Sivakumaran S,et al.A myb gene required for leaf trichome differentiation in Arabidopsis is expressed in stipules[J].Cell,1991,67(3):483-493.
[109] Noda K I,Glover B J,Linstead P,et al.Flower colour intensity depends on specialized cell shape controlled by a Myb-related transcription factor[J].Nature,1994,369:661-664.
[110] Mackerness S A H,Surplus S L,Blake P,et al.Ultraviolet-B-induced stress and changes in gene expression in Arabidopsis thaliana:role of signaling pathways controlled by jasmonic acid,ethylene and reactive oxygen species[J].Plant Cell and Environment,1999,22(11):1413-1423.
[111] Mackerness S A H,Thomas B,Jordan B R.The effect of supplementary ultraviolet-B radiation on mRNA transcripts,translation and stability of chloroplast proteins and pigment formation inPisumsativumL[J].Journal of Experimental Botany,1997,48(3):729-738.
[112] Dai Q,Shaobing P,Chavez A Q,et al.Intraspecific responses of 188 rice cultivars to enhanced UVB radiation[J].Environmental and Experimental Botany,1994,34(4):433-442.
[113] Kumagai T,Hidema J,Kang H-S,et al.Effects of supplemental UV-B radiation on the growth and yield of two cultivars of Japanese lowland rice (OryzasativaL.) under the field in a cool rice-growing region of Japan[J].Agriculture,Ecosystems and Environment,2001,83(1):201-208.
[114] Li Y,He L,Zu Y.Intraspecific variation in sensitivity to ultraviolet-B radiation in endogenous hormones and photosynthetic characteristics of 10 wheat cultivars grown under field conditions[J].South African Journal of Botany,2010,76(3):493-498.
[115] Singh M,Singh S,Agrawal S.Intraspecific responses of six cultivars of wheat(TriticumaestivumL.) to supplemental ultraviolet-B radiation under field conditions[J].Acta Physiologiae Plantarum,2012,34(1):65-74.
[116] Baroniya S S,Kataria S,Pandey G,et al.Intraspecific variations in antioxidant defense responses and sensitivity of soybean varieties to ambient UV radiation[J].Acta Physiologiae Plantarum,2013,35(5):1521-1530.
[117] Zhang W W,Wang G H,Liu X B,et al.Effects of elevated O3exposure on seed yield,N concentration and photosynthesis of nine soybean cultivars (Glycinemax(L.)Merr.) in Northeast China[J].Plant Science,2014,226:172-181.
Preventive Mechanisms to UV-B Radiation Damages in Crops
LI Yansheng,JIN Jian,WANG Guanghua,LIU Xiaobing
(KeyLaboratoryofMollisolsAgroecology,NortheastInstituteofGeographyandAgroecology,CAS,Harbin150081,China)
Since the discovery of the Antarctic ‘ozone hole’ and general depletion of the stratospheric ozone layer in the mid-1980 s,significant interest in documenting the effects of UV-B radiation (280 nm~315 nm) on organisms and ecosystems has been one of the most important concern of global change.A substantial number of studies have been intensively conducted over the last several decades to assess the potential impacts of long-term increases in ultraviolet-B radiation on crop plants,and have shown a diverse range of responses.Direct effects of natural or enhanced levels of UV-B radiation on plant yield have been detected to be modest,with growth reductions generally not exceeding 20% under field conditions.This limited impact reflects the activity of protective mechanisms in crop plants.This article summarizes changes at the physiological,morphological,biochemical and molecular levels,and proposes several future avenues with an aim to better understand the mechanisms whereby solar UV-B radiation boosts the expression of natural plant defenses.It could provide important elements for biotechnological,traditional crop breeding and viable cropping strategies programs.
UV-B radiation; plant morphology; UV-absorbing compounds; antioxidant defense system; gene expression
10.11689/j.issn.2095-2961.2016.04.003
2095-2961(2016)04-0223-11
2016-01-25;
2016-04-14.
科技部支撑项目(2014BAD11B01-A01);黑龙江省科技厅重大项目(GA14B101-A01).
李彦生(1983-),男,吉林长春人,助理研究员,研究方向为作物生理生态.
刘晓冰(1963-),男,黑龙江肇源人,研究员,博士生导师,研究方向为耕作与栽培.
X503.231
A
