Preparation and Identification of Cyclo (His-Pro) Precursors from Corn Protein
2016-12-06ZHANGYanrongFANHongxiuLIUHongchengDONGXinCollegeofFoodScienceandEngineeringJilinAgriculturalUniversityChangchun130118China
ZHANG Yanrong, FAN Hongxiu, LIU Hongcheng, DONG Xin(College of Food Science and Engineering, Jilin Agricultural University, Changchun 130118, China)
Preparation and Identification of Cyclo (His-Pro) Precursors from Corn Protein
ZHANG Yanrong, FAN Hongxiu, LIU Hongcheng, DONG Xin
(College of Food Science and Engineering, Jilin Agricultural University, Changchun 130118, China)
The value-added use of corn protein for production of the cyclo (His-Pro) precursors histidyl-proline (His-Pro) and prolyl-histidine (Pro-His) was investigated. The extracted γ-zein was hydrolyzed with single proteases (alcalase, protamex, flavourzyme and papain) and two proteases in sequence, respectively. The hydrolysate prepared with alcalase and flavourzyme in sequence exhibited the highest content of cyclo (His-Pro) precursors. Response surface methodology and an orthogonal array design L16(45) were applied to optimize the hydrolysis conditions. The optimum conditions for alcalase hydrolysis were as follows: substrate concentration of 32 mg/mL, enzyme/substrate (E/S) ratio of 12 100 U/g and hydrolysis time of 6.5 h, while the optimum conditions for flavourzyme hydrolysis were as follows: E/S ratio of 17 000 U/g, pH of 7.5, hydrolysis temperature of 55 ℃ and hydrolysis time of 6 h. Identification and quantification of cyclo (His-Pro) precursors in the hydrolysate were achieved by ultraperformance liquid chromatography (UPLC) and electrospray ionizationmass spectrometry (ESI-MS). The results showed that the hydrolysate contained a high content of Pro-His dipeptide (6.88 mg/g).
corn protein; enzymatic hydrolysis; cyclo (His-Pro) precursor; γ-zein
ZHANG Yanrong, FAN Hongxiu, LIU Hongcheng, et al. Preparation and identification of cyclo (His-Pro) precursors from corn protein[J]. 食品科学, 2016, 37(22): 52-59. DOI:10.7506/spkx1002-6630-201622008. http://www.spkx.net.cn
ZHANG Yanrong, FAN Hongxiu, LIU Hongcheng, et al. Preparation and identification of cyclo (His-Pro) precursors from corn protein[J]. Food Science, 2016, 37(22): 52-59. DOI:10.7506/spkx1002-6630-201622008. http://www.spkx.net.cn
Cyclo (His-Pro) is an endogenous cyclic dipeptide which is ubiquitous in tissues and body fluids of both man and animals[1]. It has numerous biological activities such as mitigation of ethanol-induced anesthesia[2], prevention of the neuronal death induced by free radicals and traumatic injury[3], suppression of food-intake[4]and anti-diabetic activity[5]. More recently, it was discovered that cyclo (His-Pro) is present in large quantities in some protein hydrolysates such as soybean protein hydrolysate[6], casein[7]hydrolysate, fish hydrolysate and shrimp hydrolysate[8]. It is thought to be
resulted from the nonenzymatic cyclization of His-Pro and/ or Pro-His dipeptides, and these linear dipeptides can be released from proteins by thermal manipulation or enzymatic hydrolysis[9]. Cyclo (His-Pro) derived from food proteins have attracted more attention due to its safety, low-price and wide distribution properties. Its health promoting properties have been studied extensively in the recent years. Faden et al.[10]reported that the dietary intake of cyclo (His-Pro)-rich casein hydrolysates in healthy volunteers can increase the levels of cyclo (His-Pro) in plasma far above the baseline values. Song et al.[11]also reported that oral intake of soybean protein hydrolysate containing cyclo (His-Pro) can control blood glucose metabolism in diabetic rats.
Corn gluten meal (CGM) is a major by-product of corn wet milling, which contains (on a dry basis) 50%-60% (m/m) of protein. However, the low water solubility and severely imbalanced amino acid compositions of CGM protein limit its use as food additive[12]. In China, over 840 000 t of CGM are produced every year. At present, CGM is mainly used as feedstuff or discarded directly, which caused environmental disposal problems in China[13-14]. More studies are needed to identify the beneficial effects of CGM protein. The amino acid sequence analysis, conducted using on-line software (GenBank), shows that γ-zein, a major protein fraction of CGM protein, is rich in His-Pro and Pro-His sequences in its primary structure[15]. This finding suggests that CGM is a valuable raw material to obtain cyclo (His-Pro) precursors. The objective of this study was to prepare γ-zein hydrolysates with the maximum amount of His-Pro and/or Pro-His by enzymatic hydrolysis. γ-Zein was extracted from CGM and different enzymes were screened for enzymatic hydrolysis. Response surface methodology and a L16(45) orthogonal experimental design were adopted to determine the optimum hydrolysis conditions. His-Pro and/ or Pro-His in the hydrolysate were further identified by ultra performance liquid chromatography-electrospray ionization mass spectrometer (UPLC-ESI-MS). To our knowledge, the present work first studied preparing cyclo (His-Pro) precursors, His-Pro and Pro-His, from corn protein.
1 Materials and Methods
1.1 Materials and reagents
Corn gluten meal (protein content of 55.76%, dry basis) was provided by Huanglong Corn Co. Ltd. (Jilin, China). L-histidyl-proline dipeptide (His-Pro, 98%, 252.5 D) and L-proline-histidyl dipeptide (Pro-His, 98%, 252.5 D) were purchased from GL Biochemistry Co. Ltd. (Shanghai, China). Ultrapure water was produced by a Milli-Q Plus system (Millipore Corporation, USA). Trifluoroacetic acid (TFA, 99%) were purchased from Sigma-Aldrich Corporation (Shanghai, China). Acetonitrile (ACN, HPLC grade) was purchased from Thermo Fisher Scientific Co. (Shanghai, China). Molecular weight cut-off (MWCO) ultra-filtration membranes with a 3 kD cut-off were purchased from Millipore (Bedford, MA, USA). All the other chemicals and solvents used were of analytical grade.
Alcalase AF 2.4 L, protamex and flavourzyme 500MG were purchased from Novo Enzyme Co. (Bagsvaerd, Denmark). Papain was purchased from Pangbo Biological Engineering Co. Ltd. (Nanning, China). The optimum pH and temperature of commercial proteases were provided by the product supplier. Protease activity was determined according to the method of Balti et al.[16]using casein as a substrate. One unit of protease activity (U) was defined as the amount of enzyme required to liberate 1 μg of tyrosine per min under the experimental conditions used. Protease activities of alcalase, protamex, flavourzyme and papain were determined to be 286 000, 160 000, 247 000 U/g and 200 000 U/g, respectively.
1.2 Instruments and equipments
Supercritical fluid extraction apparatus (HA121-50-02, Hua’an Supercritical Extraction Co., Nantong, China); Water bath (DZKW-4, Zhongxing Weiye Instrumental Co. Ltd., Beijing, China); pH meter (PHS-3BW, Shanghai Lida Instrumental plant, China); Freeze drier (FD-1B-50, Boyikang Instrumental Co. Ltd., Beijing, China); UPLC system (ACQUITY H-Class UPLC, Waters, USA); UPLCESI-MS system (Thermo LCQ XP, Thermo Scientific Inc., USA); High speed refrigerated centrifuge (CT15RT, Tianmei Scientific Instrumental Co. Ltd., Shanghai, China).
1.3 Methods
1.3.1 Extraction of γ-zein from corn gluten meal
γ-Zein was extracted by the method of Malumba et al.[17]with some modifications. CGM was defatted with supercritical CO2extraction at 25 MPa, 45 ℃ for 2 h. The defatted meal was suspended in 10 volumes of 70% ethanol (V/V) and stirred for 90 min at 20 ℃. The mixture was then centrifuged. This operation was repeated three times and all the supernatants were combined as α-and β-zeins. The residue was then treated with 10 volumes of 70% ethanol plus 0.5% sodium acetate (m/V) and 0.6% 2-mercaptoethanol (V/V) at 20 ℃ for 90 min, followed by centrifugation. The supernatant was collected, centrifuged and the pellet was further processed twice using the same method. All the supernatants
were pooled and evaporated to 1/20th of its original volume at 55 ℃. After evaporation, the sample was dialyzed against distilled water at 4 ℃ and then freeze-dried to obtain γ-zein. α- and β-zeins can be used for coatings, plastics, or carrier materials[18], while the dried γ-zein sample was collected for enzymatic hydrolysis. The protein content of γ-zein, determined by Kjeldahl method, was 85%.
1.3.2 Preparation of γ-zein hydrolysates
1.3.2.1 Single protease hydrolysis
Four proteases, including alcalase, flavourzyme, protamex and papain, were used to hydrolyze γ-zein, respectively. γ-Zein was suspended with distilled water to a final concentration of 50 mg/mL, and the mixture was heated at 90 ℃ for 10 min before hydrolysis. The hydrolysis was initiated by addition of protease at the required E/S ratio and incubated, aided with constant agitation, under the optimum temperature and pH for each protease. After digestion with setting time, the enzymatic hydrolysis was stopped by heating at 95 ℃ for 10 min. At different time intervals during the hydrolysis process, an aliquot of hydrolysate was withdrawn and heated at 95 ℃ for 10 min to inactivate the enzyme. The collected aliquots and final hydrolysates were centrifuged (4 000 r/min, 15 min, 4 ℃), and the supernatants were collected and lyophilized for determination of degree of hydrolysis (DH) and content of cyclo (His-Pro) precursors.
1.3.2.2 Hydrolysis of γ-zein by two proteases
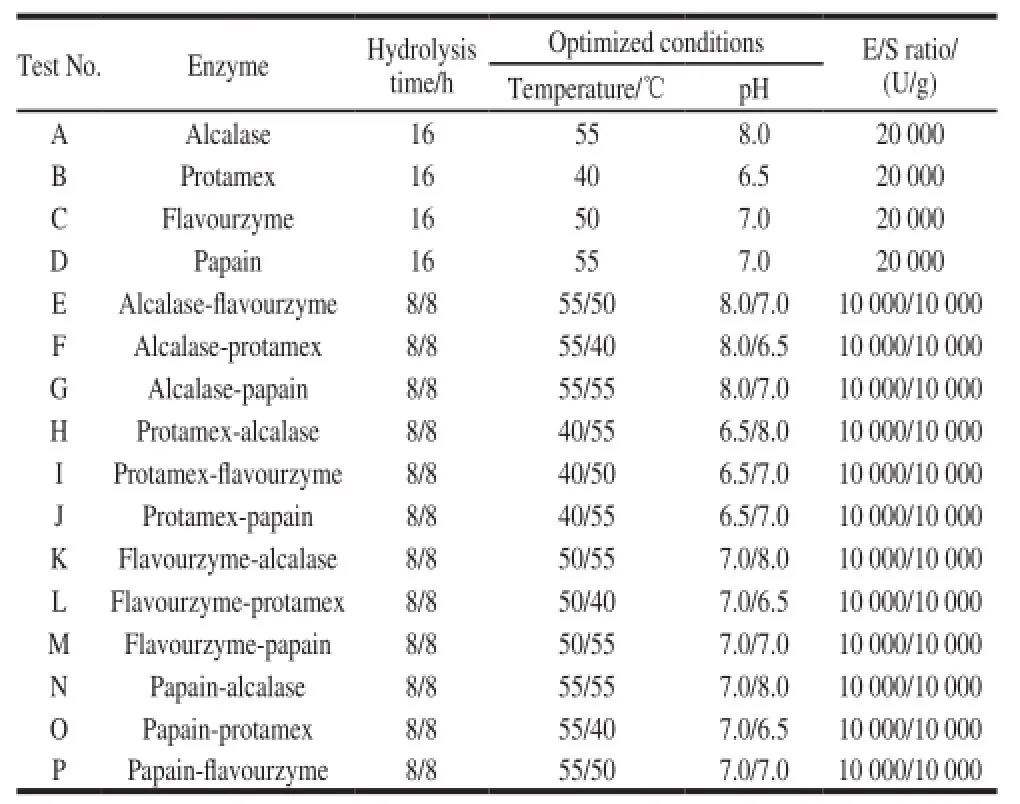
Table1 Parameters for enzymatic hydrolysis of γ-zein with single proteases and two proteases in sequence
Among the four proteases, combination of two proteases was used to hydrolyze γ-zein. In first step, pretreated γ-zein was hydrolyzed under the optimum temperature and pH of a protease (alcalase, flavourzyme, protamex, and papain), and second step the hydrolysis conditions were adjusted. The second protease was added for further hydrolysis. The hydrolysis conditions for single and two proteases are summarized in Table1.
1.3.3 Determination of DH
The DH of γ-zein was determined using Tanzadehpanah et al.[19]method. DH is defined as the percent ratio of the number of peptide bonds broken to the total number of bonds per unit weight. Free amino groups obtained from the hydrolysis reaction were measured spectrophotometrically by the trinitrobenzenesulfonic acid method. The total numbers of amino groups were determined in 6 mol/L HCl at 100 ℃ for 24 h. DH was calculated according to the equation (1):

Where Ltis the amount of α-amino acid released at time t/mmol; L0is the amount of α-amino acid in the original acidsolubilized protein substrate/mmol; Lmaxis the total numbers of amino groups/mmol.
1.3.4 Determination of contents of His-Pro and Pro-His dipeptides by UPLC
For UPLC analysis, γ-zein hydrolysate solution (10.0 mg/mL) was filtered through a 3 kD MWCO ultrafiltration membrane to remove protein. The resulting peptides was collected, lyophilized and re-dissolved in 2% ACN and 0.05% TFA in deionized water (V/V) to the concentration of 0.2 mg/mL and then filtered through a 0.2 μm syringe filter.
An ACQUITY H-Class UPLC system was used for the determination of the contents of His-Pro and Pro-His dipeptides. Samples were loaded onto a reversed-phase ACQUITY UPLC HSS T3 column (100 mm × 2.1 mm, 1.7 µm). The mobile phases were water containing 0.05% TFA (A) and acetonitrile (B). The elution condition was 98% A-2% B. The injection volume was 5 μL, and the flow rate was 0.2 mL/min. The column temperature was set at 35 ℃. His-Pro and Pro-His were measured at 220 nm and quantified by the external standard.
The linearity of detection for each analyte was examined using 5 different standard solutions (50, 100, 200, 300, and 400 μg/mL). A calibration curve was constructed by performing linear regression of the peak area vs. analyte concentration. The equations of His-Pro and Pro-His
dipeptides are y1= 479 329x1-173 (R2= 0.999 8) and y2= 19 050x2-207 (R2= 0.999 8), respectively, in which y1and y2are the peak areas of His-Pro and Pro-His dipeptides respectively, and x1and x2are the concentrations of His-Pro and Pro-His dipeptides, respectively (mg/mL). The chromatograms obtained were processed to get the contents of His-Pro and Pro-His dipeptides.
The content of cyclo (His-Pro) precursors for each hydrolysate was calculated as equation (2):
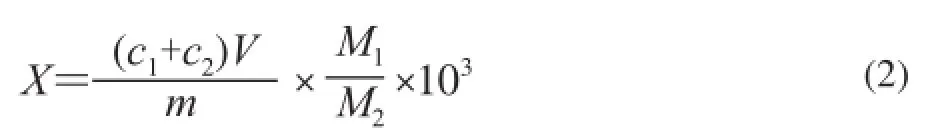
Where X is the content of cyclo (His-Pro) precursors in the hydrolysate/(mg/g); c1and c2are the concentrations of His-Pro and Pro-His in the sample obtained by standard curve respectively/(mg/mL); V is the volume of the sample/mL; m is the weight of the sample/g; M1is the weight of peptides sample of Mw< 3 kD; and M2is the weight of γ-zein hydrolysate.
1.3.5 Optimization of first-stage hydrolysis conditions by response surface methodology (RSM)
The software Design-Expert Trial Version 8.0.7.1 from Stat-Ease Inc. (Minneapolis, USA) was employed for experimental design, data analysis, and model building. A Box-Behnken design with five replicates at the central point and four axial points (with 12 runs) was used to determine the response pattern and then to establish a model. E/S ratio (X1), hydrolysis time (X2), and substrate concentration (X3) were chosen for independent variables. Other hydrolysis parameters, such as hydrolysis temperature and pH, were not considered because their optimum levels are provided by the manufacturer. The range and central point values of the independent variables were based on the results of preliminary single-factor experiments (data not shown). The DH was the dependent variable (Y) used to evaluate the effects on γ-zein hydrolysates.
1.3.6 Optimization of second-stage hydrolysis conditions by orthogonal test design
An orthogonal experiment L16(45) was applied to optimize the hydrolysis conditions. Four levels of each of the four selected factors (E/S ratio, hydrolysis time, hydrolysis temperature and pH) were examined in 16 experimental runs. The values chosen for each level were based on the appropriate range determined in the preliminary singlefactor experiments (data not shown) and the blank column was considered to be the experimental error. The content of cyclo (His-Pro) precursors was the dependent variable. Analysis of variance (ANOVA) was also used to determine how much of the variation was contributed by each factor and the significant effect. Statistical analyses were conducted by SPSS 16.0 software.
1.3.7 Identification of His-Pro and/or Pro-His by UPLCESI-MS
Freeze dried peptides that have been passed through MWCO 3 kD ultrafiltration membrane was dissolved in 5% ACN and 0.05% TFA in deionized water for UPLC-ESIMS analysis. The ESI spectrometer was operated in positive ion mode. Conditions were spray voltage of 5.0 kV; sheath gas (nitrogen) flow rate of 50 mL/min; capillary voltage of 25.0 V; heated capillary temperature of 250 ℃; tube lens offset voltage of 85.0 V. Detection of His-Pro and/or Pro-His was performed through selected ion monitoring (SIM) by selecting the precursor ion at m/z 253.5 (isolation width ±1).
UPLC conditions were described in Section 1.3.4.
2 Results and Analysis
2.1 Selection of the most appropriate proteases for hydrolysis of γ-zein
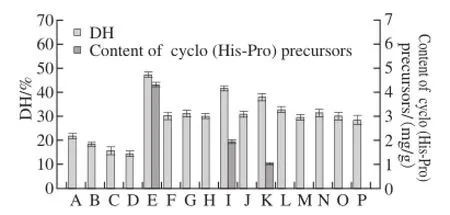
Fig.1 DH and of cyclo (His-Pro) precursors content of γ-zein hydrolysates prepared with single or two proteases (n = 3)
The effects of γ-zein hydrolysis by single protease and two proteases are shown in Fig.1. Results showed that the DH of hydrolysates prepared by single protease was lower than those prepared by two proteases, and no His-Pro or Pro-His was observed during the single protease hydrolysis. Compared to the hydrolysis with single protease, more intensive hydrolysis and increase in the content of cyclo (His-Pro) precursors were observed when two proteases were used such as alcalase-flavourzyme, protamex-flavourzyme and flavourzyme-alcalase. The two-stage hydrolysis with alcalaseflavourzyme produced the highest DH value and content of cyclo (His-Pro) precursors. It was also observed that significant differences in DH and content of cyclo (His-Pro) precursors between alcalase-flavourzyme and flavourzymealcalase. The differences in DH and content of cyclo (His-Pro)
precursors between alcalase-flavourzyme and flavourzymealcalase imply that flavourzyme and alcalase have cleaved γ-zein at different sites. Flavourzyme had higher hydrolysis effect on the alcalase hydrolysate, while alcalase showed lower hydrolysis effect on the flavourzyme hydrolysate.
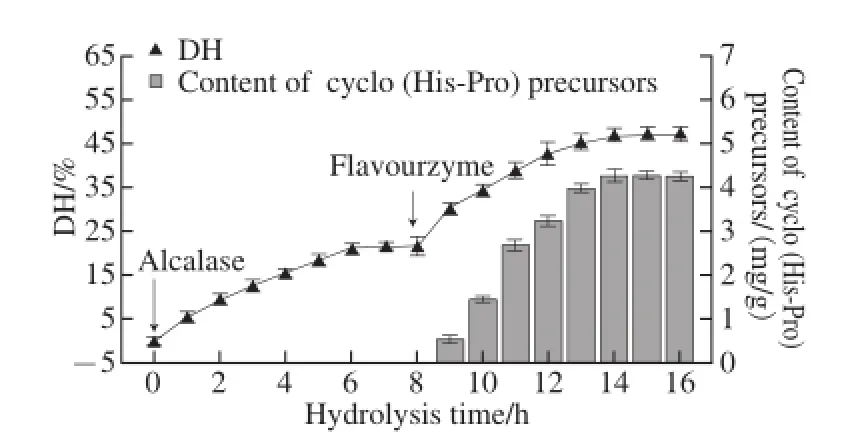
Fig.2 Variations in DH and cyclo (His-Pro) precursors content of γ-zein hydrolysate with hydrolysis time using alcalase and flavourzyme in sequence (n = 3)
The effect of DH on the content of cyclo (His-Pro) precursors was also investigated. DH and content of cyclo (His-Pro) precursors during the alcalase-flavourzyme hydrolysis were determined. As shown in Fig.2, when alcalase, an endopeptidase, was added first, the DH of γ-zein increased to reach its maximum after 6 h of hydrolysis. After this time the DH remained constant. However, no His-Pro or Pro-His dipeptide was observed with DH below 22%. At 8 h flavourzyme, an exopeptidase, was added to continue the hydrolysis. This increased the DH from 22% to 47.14%. The cyclo (His-Pro) precursors began to be released when DH was higher than 30% and the content of cyclo (His-Pro) precursors increased with the increase of DH. The highest content of cyclo (His-Pro) precursors was obtained with a DH of 47.14%, but extensive hydrolysis of γ-zein did not result in increase in DH and content of cyclo (His-Pro) precursors. These results indicated that DH may exert important effects on the content of cyclo (His-Pro) precursors. The hydrolysate with high DH exhibited higher content of cyclo (His-Pro) precursors compared to the hydrolysate with low DH. It is also found that one enzyme cannot achieve such a high DH in a reasonable period of time. Further digestion of alcalase hydrolysis with flavourzyme greatly contributed to the release of His-Pro and/or Pro-His dipeptides.
Since aclalase-flavourzyme was the most appropriate combination to prepare cyclo (His-Pro) precursors, it was selected to perform the optimization studies of γ-zein hydrolysis.
2.2 Optimization of alcalase hydrolysis conditions by RSM
The conditions for the alcalase hydrolysis were optimized by RSM based on a Box-Behnken design. The combined effects of E/S ratio (X1), hydrolysis time (X2), and substrate concentration (X3) on the DH (Y) of γ-zein are presented in Table2.
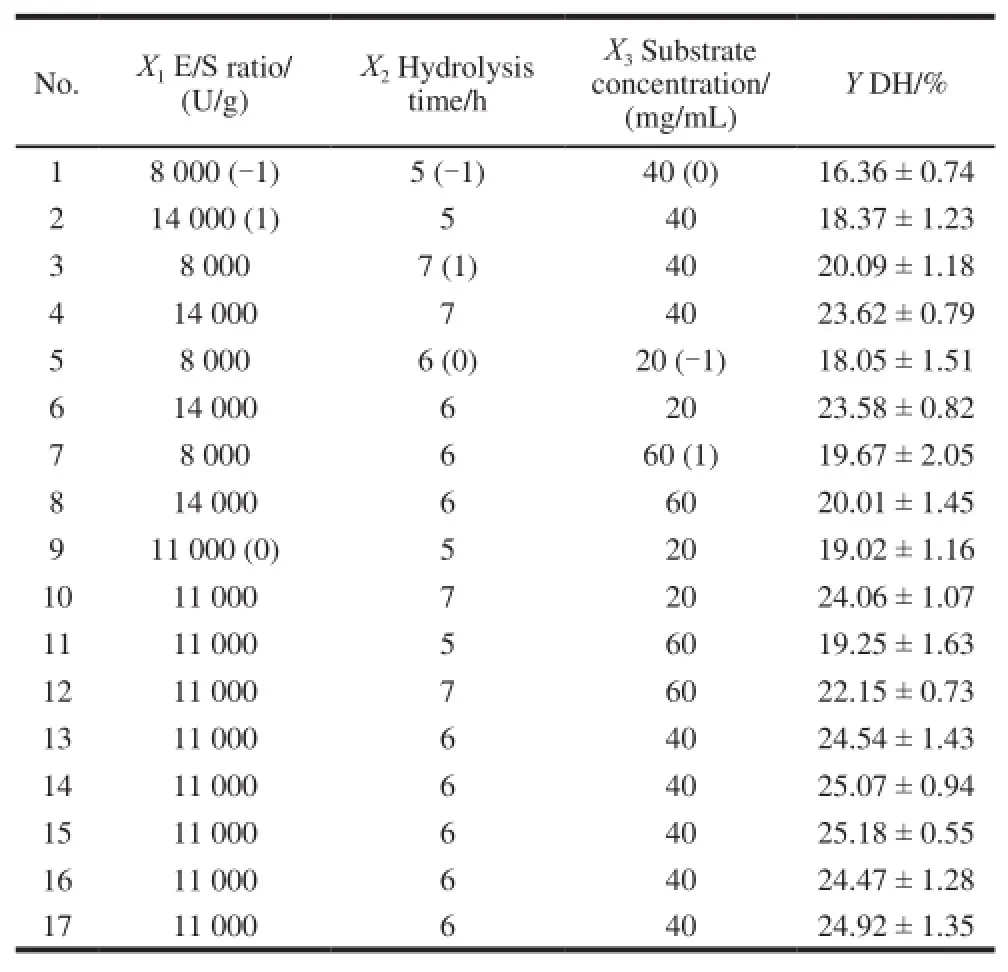
Table2 Box-Behnken experimental design of the independent variables along with the observed values for the response
Based on the data in Table2, the regression equation (coded levels) for the DH (Y) of alcalase hydrolysate as a function of the three independent variables was derived as follows:
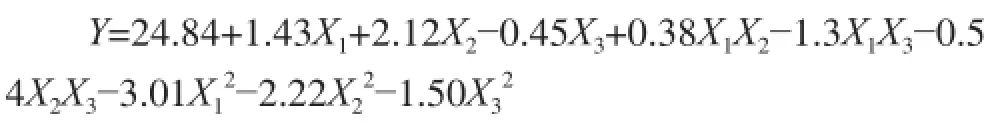
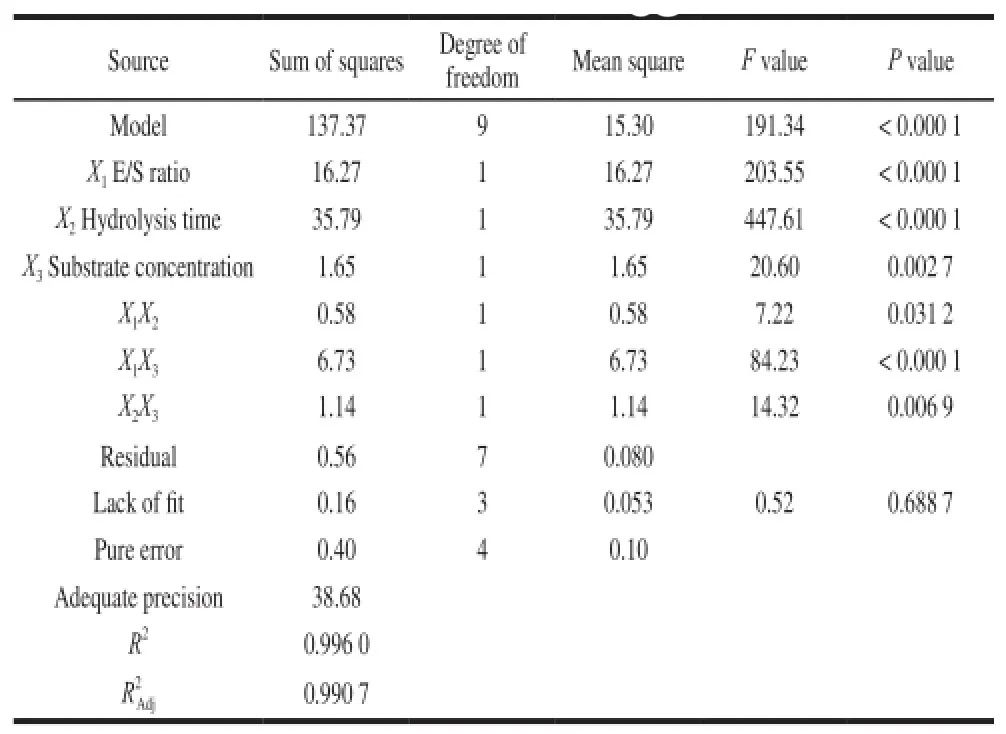
Table3 Analysis of variance (ANOVA) for response surface quadratic model for the DH of hydrolysates
The analysis of variance (Table3) showed that the P value of the model was lower than 0.000 1, which indicated that the model was significant. The P value of lack of fit was
0.688 7, which was higher than 0.05. This further indicated that the model was significant. The determination coefficient (R2) was close to 1 (0.996 0) and the adjusted determination coefficientwas 0.990 7, indicating that the model explained 99.07% of the variation in the data. The adequate precision measures the signal-to-noise ratio, and usually a value greater than 4 is desirable[20]. The adequate precision values for the model was 38.68, indicating adequate signalto-noise ratios. Therefore, the model proved to be powerful for navigating the design space. The significance of each parameter coefficient was determined by P values, the smaller the P values the more significance of the coefficient. In this case, all factors have significant effects on DH (P<0.05). The interaction effects of E/S ratio-hydrolysis time, E/S ratio-substrate concentration and hydrolysis time-substrate concentration on DH (P < 0.05) were also significant.
From the computer prediction, the optimum conditions to obtain the highest DH for alcalase hydrolysate were hydrolysis time of 6.5 h, E/S ratio of 12 100 U/g and substrate concentration of 32 mg/mL. After alcalase hydrolysis under these optimum conditions, the DH of the hydrolysate was (25.68 ± 0.77)%, which were not significantly different from the predicted value (25.31%).
2.3 Optimization of flavourzyme hydrolysis conditions by orthogonal test design

Table4 Factors and levels of the orthogonal array design L16(45) and experimental results for flavourzyme hydrolysis
Flavourzyme was used to further hydrolyze the alcalase hydrolysate previously optimized by RSM. In order to liberate high amounts of His-Pro and/or Pro-His from γ-zein, an orthogonal experiment L16(45) was applied to optimize the hydrolysis conditions. The effects of E/S ratio, hydrolysis time, hydrolysis temperature and pH on the formation of cyclo (His-Pro) precursors are shown in Table4.
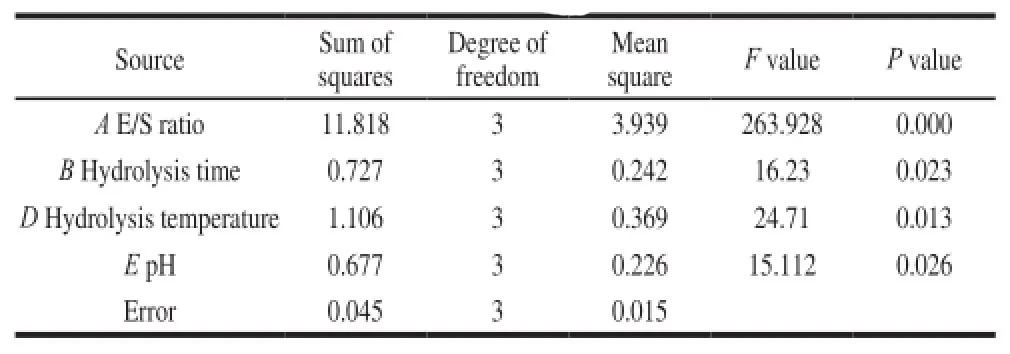
Table5 Analysis of variance (ANOVA) and significance test of orthogonal array experimental results
According to the R values, the orders of the four effecting factors were E/S ratio > hydrolysis temperature >hydrolysis time > pH. The results of ANOVA (Table5) indicated that all the four factors showed significant (P < 0.05) effect on the content of cyclo (His-Pro) precursors. According to the k values (Table4), the optimum flavourzyme hydrolysis condition was A3B3D3E4, i.e. E/S ratio of 17 000 U/g, hydrolysis time of 6 h, hydrolysis temperature of 55 ℃ and pH of 7.5. Through verification tests, the maximum content of cyclo (His-Pro) precursors reached to (6.88 ± 0.104) mg/g under the optimum hydrolysis condition. It was reported that the content of cyclo (His-Pro) in several common foods were 1.46 μg/g in dried shrimp and 1.18 μg/g in fish sauce[8]. Koo et al.[21]also reported that the soybean protein hydrolysate contained 8.1 mg/g (dry weight of hydrolysate) of cyclo (His-Pro). If all the precursors were cyclized to cyclo (His-Pro) in γ-zein hydrolysate, the content of cyclo (His-Pro) would be up to 6.88 mg/g, which is slightly lower than that of the reported soybean protein hydrolysate. However, soybean production in China is only 12–13 millions tons each year, which is not able to meet the demand of domestic consumption. In China approximately 70 million tons of soybeans are imported annually, which accounts for 80% of its total consumption[22-23]. Researchers predicted that by 2020, domestic soybean demand-supply gap would reach up to 13.83 million t[24]. CGM, however, is a huge protein resource and has not been effectively utilized. Annual discards of CGM in China is estimated to be approximately 80 000 t[14]. Development of cyclo (His-Pro) precursors from CGM may open up new economic opportunities for increasing valueadded utilization of corn protein.
The UPLC-UV chromatograms of mixed standards and hydrolysate samples were shown in Fig.3 and Fig.4. In the optimized alcalase-flavourzyme hydrolysates only the signal of Pro-His dipeptide (fraction 1) was detected at the expected retention times. The purity and identity of Pro-His dipeptide in this hydrolysate were also confirmed by UPLC-ESI-MS.
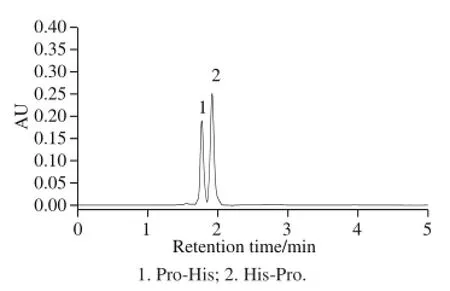
Fig.3 UPLC-UV chromatograms of dipeptide standards
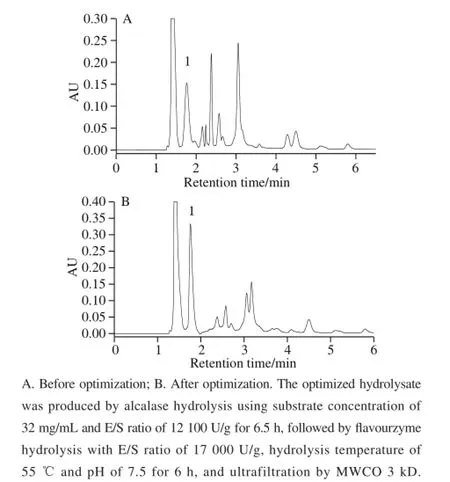
Fig.4 Comparison of UPLC-UV chromatograms of alcalaseflavourzyme hydrolysates before and after optimization of hydrolysis conditions
2.4 Identification of cyclo (Pro-His) precursors by UPLCESI-MS
In Fig.5 the selected ion chromatogram of hydrolysate prepared under optimum conditions was given. The sample showed only one peak at retention time of 1.76 min, and the retention time was found to be similar to that found in the UPLC-UV chromatogram (Fig.4B), which proved that the peak at retention time of 1.76 min in the UPLC-ESIMS chromatogram were fraction 1. The corresponding mass spectrum of fraction 1 was shown in Fig.5. The detected molecular masse of 252.7 D ([M+H]+253.7 D) agreed well with the masse of Pro-His dipeptide standards. Thereby, the UPLC-UV results were confirmed and the presence of the Pro-His dipeptide in γ-zein hydrolysate was proven.
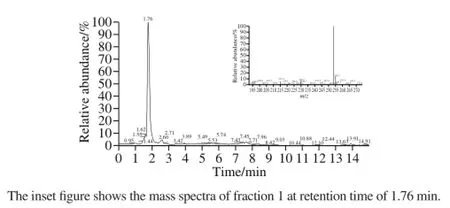
Fig.5 UPLC-ESI-MS chromatogram of alcalase-flavourzyme hydrolysates prepared under the optimum conditions
3 Conclusion
The present study was the first to report preparation of cyclo (His-Pro) precursors from corn protein using enzymatic hydrolysis method. Results showed that the amount of cyclo (His-Pro) precursors of γ-zein protein hydrolysates was closely related to the enzyme type and the sequence of hydrolysis. Sequential hydrolysis using alcalaseflavourzyme was shown to be the most effective one. The optimum hydrolysis conditions were as follows: alcalase hydrolysis was performed with E/S ratio of 12 100 U/g and substrate concentration of 32 mg/mL for 6.5 h, followed by flavourzyme hydrolysis with E/S ratio of 17 000 U/g and pH of 7.5 at 55 ℃ for 6 h. The cyclo (His-Pro) precursors with 6.88 mg/g content could be readily obtained under the optimum conditions. The presence of His-Pro and/or Pro-His in hydrolysate was further confirmed by UPLC-ESIMS by comparison of the retention times and the molecular mass with reference compounds. This method provided a promising bioprocessing route for production of cyclo (His-Pro) by using CGM protein.
[1] MINELLI A, BELLEZZA I, GROTTELLI S, et al. Focus on cyclo (His-Pro): history and perspectives as antioxidant peptide[J]. Amino Acids, 2008, 35(2): 283-289. DOI:10.1007/s00726-007-0629-6.
[2] IMAMURA M, PRASAD C. Cyclo (His-Pro) potentiates GABA/ ethanol-mediated chloride uptake by neurosynaptosomes[J]. Peptides, 2003, 24(3): 445-448. DOI:10.1016/S0196-9781(03)00060-3.
[3] ABIRAM A, KOLANDAIVEL P. Structural analysis and the effect of cyclo (His-Pro) dipeptide on neurotoxins-a dynamics and density functional theory study[J]. Journal of Molecular Model, 2010, 16(2): 193-202. DOI:10.1007/s00894-009-0531-0.
[4] SONG M K, ROSENTHAL M J, SONG A M, et al. Body weight reduction in rats by oral treatment with zinc plus cyclo-(His-Pro)[J]. British Journal of Pharmacology, 2009, 158(2): 442-450. DOI:10.1111/ j.1476-5381.2009.00201.x.
[5] CHOI S A, YUN J W, PARK H S, et al. Hypoglycemic dipeptide cyclo (His-Pro) significantly altered plasma proteome in streptozocininduced diabetic rats and genetically-diabetic (ob/ob) mice[J]. Molecular Biology Reports, 2013, 40(2): 1753-1765. DOI:10.1007/ s11033-012-2229-0.
[6] MOON K S, MARK J R, ALBERT M S, et al. Raw vegeTablefood containing high cyclo (His-Pro) improved insulin sensitivity and body weight control[J]. Metalolism Clinical and Experimental, 2005, 54(11): 1480-1489. DOI:10.1016/j.metabol.2005.05.014.
[7] PARK Y, LEE H J, CHOI J W, et al. Anti-diabetic effect of Cyclo-His-Pro (CHP)-enriched yeast hydrolysate in streptozotocin-induced diabeticmice[J]. African Journal of Biotechnology, 2013, 12(35): 5473-5479. DOI:10.5897/AJB12.1556.
[8] HILTON C W, PRASAD C, VO P, et al. Food contains the bioactive peptide, cyclo(His-Pro)[J]. Journal of Clinical Endocrinology and Metabolism, 1992, 75(2): 374-381. DOI:10.1210/jc.75.2.375.
[9] MINELLI A, GROTTELLI S, MIERL A, et al. Cyclo (His-Pro) exerts anti-inflammatory effects by modulating NF-κB and Nrf 2 signalling[J]. The International Journal of Biochemistry and Cell Biology, 2012, 44(3): 525-535. DOI:10.1016/j.biocel.2011.12.006.
[10] FADEN A I, FOX G B, DI X, et al. Neuroprotective and nootropic actions of a novel cyclized dipeptide after controlled cortical impact injury in mice[J]. Journal of Cerebral Blood Flow and Metabolism, 2003, 23(3): 355-363. DOI:10.1097/00004647-200303000-00010.
[11] SONG M K, HWANG I K, ROSENRHAL M J, et al. Antihyperglycemic activity of zinc plus cyclo (His-Pro) in genetically diabetic Goto-Kakizaki and aged rats[J]. Experimental Biology and Medicine, 2004, 228(11): 1338-1345.
[12] LIU Xiaolan, ZHENG Xiqun, SONG Zhanlan, et al. Preparation of enzymatic pretreated corn gluten meal hydrolysate and in vivo evaluation of its antioxidant activity[J]. Journal of Functional Foods, 2015, 18: 1147-1157. DOI:10.1016/j.jff.2014.10.013.
[13] HUANG Weihao, HE Hui, DONG Huawei, et al. Study on continuous preparation of corn pepide by enzyme membrane coupling technology and corn pepides’ structures[J]. Journal of the Chinese Cereals and Oils Association, 2011, 26(4): 24-30. DOI:10.7666/d.y2004235.
[14] CHEN Xin, CHEN Qingsen, PANG Guangchang. Present situation and tendency of preparing bioactive peptides by enzymolysis in corn gluten meal[J]. Food Science, 2004, 25(7): 202-206. DOI:10.3321/ j.issn:1002-6630.2004.07.049.
[15] WOO Y M, HU D W N, LARKINS B A, et al. Genomics analysis of genes expressed in maize endosperm identifies novel seed proteins and clarifies patterns of zein gene expression[J]. The Plant Cell, 2001, 13(10): 2297-2317. DOI:10.2307/3871509.
[16] BALTI R, ARROUME N N, BOUGATEF A, et al. Three novel angiotensin I-converting enzyme (ACE) inhibitory peptides from cuttlefish (Sepia officinalis) using digestive proteases[J]. Food Research International, 2010, 43(4): 1137-1143. DOI:10.1016/ j.foodres.2010.02.013.
[17] MALUMBA P, VANDERGHEM C, DEROANNE C, et al. Influence of drying temperature on the solubility, the purity of isolates and the electrophoretic patterns of corn proteins[J]. Food Chemistry, 2008, 111(3): 564-572. DOI:10.1016/j.foodchem.2008.04.030.
[18] ANDERSON T J, LAMSAL B P. Development of New method for extraction of α-zein from corn gluten meal using different solvents[J]. Cereal Chemistry, 2011, 88(4): 356-362. DOI:10.1094/ CCHEM-08-10-0117.
[19] THEANSUNGNOEN T, YARAKSA N, DADUANG S, et al. Purification and characterization of antioxidant peptides from leukocyte extract of Crocodylus siamensis[J]. 2014, 33(1): 24-31. DOI:10.1007/s10930-013-9536-8.
[20] PERIČIN D, RADULOCIĆ-POPOVIĆ L, VAŠTAG Ž, et al. Enzymatic hydrolysis of protein isolate from hull-less pumpkin oil cake: application of response surface methodology[J]. Food Chemistry, 2009, 115(2): 753-757. DOI:10.1016/j.foodchem.2008.12.040.
[21] BON K K, SUH H J, RA K S, et al. Protective effect of cyclo (His-Pro) on streptozotocin-induced cytotoxicity and apoptosis in vitro[J]. Journal of Microbiology and Biotechnology, 2011, 21(2): 2218 -2227. DOI:10.4014/jmb.1012.12003.
[22] YANG Weiqiao, LI Xihong, LIU Xia, et al. Improvement of soybean quality by ground source heat pump (GSHP) cooling system[J]. Journal of Stored products Research, 2015, 64: 113-119. DOI:10.1016/ j.jspr.2015.09.002.
[23] LIU Xiaobing, JIN Jian, WANG Guanghua, et al. Soybean yield physiology and development of high-yielding practices in Northeast China[J]. Field Crops Research, 2008, 105(3): 157-171. DOI:10.1016/ j.fcr.2007.09.003.
[24] YANG Shuguo. Economics of soybean industry in China from industry chain perspective[D]. Beijing: China Agricultural University, 2014.
玉米蛋白环(组氨酸-脯氨酸)二肽前体的制备及其鉴定
张艳荣,樊红秀,刘鸿铖,董 欣
(吉林农业大学食品科学与工程学院,吉林 长春 130118)
以玉米蛋白为原料,制备活性环(组氨酸-脯氨酸)二肽(cyclo(His-Pro))前体——His-Pro和Pro-His二肽。分别采用碱性蛋白酶、复合蛋白酶、风味蛋白酶和木瓜蛋白酶对提取的玉米γ-醇溶蛋白进行单酶和双酶分步水解,筛选出最佳酶解工艺。采用碱性蛋白酶和风味蛋白酶分步水解的水解产物中cyclo(His-Pro)前体的含量比其他水解产物要高。采用响应面和L16(45)正交试验对碱性蛋白酶和风味蛋白酶分步水解γ-醇溶蛋白的工艺进行了优化,最佳水解条件为:底物质量浓度32 mg/mL,先加入12 100 U/g的碱性蛋白酶水解6.5 h,再加入17 000 U/g的风味蛋白酶于pH 7.5、55 ℃条件下水解6 h。采用超高效液相色谱法和电喷雾质谱法对cyclo(His-Pro)前体进行定量和定性分析,结果表明水解产物中含有较高含量的脯氨酸-组氨酸二肽(6.88 mg/g)。
玉米蛋白;酶解;环(组氨酸-脯氨酸)二肽前体;γ-醇溶蛋白
Q516
A
1002-6630(2016)22-0052-08
2016-06-30
“十二五”国家科技支撑计划项目(2013BAD16B08);吉林省重大科技攻关项目(2012ZDGG007)
张艳荣(1965—),女,教授,博士,研究方向为粮油植物蛋白工程及功能食品。E-mail:xcpyfzx@163.com
10.7506/spkx1002-6630-201622008
