大气汞浓度升高对水稻叶片生理效应的影响研究
2016-12-06陈剑王章玮张晓山秦普丰陆海军
陈剑,王章玮,张晓山,秦普丰,陆海军
1.中国科学院生态环境研究中心大气环境科学实验室,北京100085
2.中国科学院大学资源与环境学院,北京100049
3.湖南农业大学资源环境学院,长沙410128
大气汞浓度升高对水稻叶片生理效应的影响研究
陈剑1,2,王章玮1,*,张晓山1,秦普丰3,陆海军3
1.中国科学院生态环境研究中心大气环境科学实验室,北京100085
2.中国科学院大学资源与环境学院,北京100049
3.湖南农业大学资源环境学院,长沙410128
采用大田开顶式气室熏气实验,研究大气汞浓度升高对水稻叶片气体交换参数、脯氨酸、丙二醛的积累以及超氧化物歧化酶活性的影响。实验结果显示,水稻叶片净光合速率(Pn)和气孔导度(Gs)随大气汞浓度的升高均较对照略微下降,表明大气汞浓度的升高对水稻光合作用和气孔开放程度有一定影响;扬花期水稻胞间CO2浓度(Ci)随大气汞浓度的升高明显降低(P<0.05)表明Pn的略微下降属于气孔限制,同时蒸腾速率(Tr)显著增加(P<0.01)表明大气汞对水稻的蒸腾生理功能有一定的影响。乳熟期水稻叶片气体交换参数与大气汞浓度无显著差异(P>0.05),且各指标均低于扬花期。水稻叶片脯氨酸(Pro)含量在拔节期随大气汞浓度的升高而显著增加(P<0.05),扬花期先升高后下降,在45 ng·m-3时达到最大,成熟期无显著差异(P>0.05);水稻叶片丙二醛(MDA)含量在拔节期先升高后下降,45 ng·m-3时达到最大,扬花期和成熟期均无显著差异(P>0.05);水稻叶片超氧化物歧化酶(SOD)活性在拔节期先升高后下降,15 ng·m-3时达到最大,扬花期无显著差异(P>0.05)。以上结果表明大气汞浓度的升高可以引起水稻叶片膜脂过氧化以及脯氨酸和丙二醛含量的积累,且随着体内Pro、MDA和SOD对大气汞胁迫的协同反应,水稻对逆境的适应能力增强,对汞胁迫产生了耐受性。
气态单质汞;气体交换参数;脯氨酸;丙二醛;超氧化物歧化酶;开顶式气室
自工业革命以来,人类活动将数千吨的汞以气态元素汞(gaseous elemental mercury,GEM)的形式排放到大气中[15],随大气传输扩散并通过干湿过程沉降到土壤、植物表面,进而对生物体产生毒害作用,据报道,当前大气汞的沉降速率是工业革命前的3.4倍[16]。目前国内外大量工作关注于水培或土培实验中Hg2+对植物根、叶及幼苗的生理生化影响研究[2,4,6,17],而大气汞对植物生理的直接影响研究相对缺乏。大量研究表明,植物叶片中汞的含量与大气汞浓度显著正相关[18-21],因此本文通过大田开顶式气室熏气实验,研究了大气汞浓度升高对水稻叶片气体交换参数、脯氨酸、丙二醛的积累以及超氧化物歧化酶活性的影响。
1 材料与方法(Materials and methods)
1.1 开顶式气室(OTCs)熏气实验设计
实验地点位于湖南农业大学农资系实验基地(28.28°N,113.01°E),实验田面积30 m×10 m,该地区属于典型亚热带季风湿润气候区,季节变化明显,年均气温17.2℃,供试水稻为该地区广泛播种的中青优2号。
开顶式气室为Heagle型[22],主要由气室主体、GEM生成系统和布气系统三部分组成,可以为植物提供比较接近自然的生长环境。气室主体为长1.5 m,宽1.4 m,高1 m(地面以上部分)的长方体,顶部架设一个收缩角度为45°的平截头体[23],总体积约为2.835 m3。气室骨架由PVC管连接构成,四面紧密覆盖0.08 mm厚的透明聚氯乙烯塑料薄膜。在一根上部直径2 cm、长40 cm,下部直径4 cm、长10 cm的玻璃管底部加入少量液态元素汞(liquid elemental mercury,LEM)没入恒温槽液面以下,设定恒温槽温度在20℃左右,为GEM均匀稳定的产生提供接近恒温且低于环境温度的条件。产生的GEM由一定流量的载气(高纯氮气)通过内径2 mm的聚四氟塑料管带出玻璃管并引至田间,与鼓风机产生的气流相混合,再由密布小孔的PVC管从底部通入气室[24]。
根据城市大气平均汞含量及实验区近地表大气背景汞浓度,本实验共设4组汞浓度水平,分别为(5 ±2)ng·m-3(CK)、(15~20)ng·m-3、(45~50)ng·m-3和(90~100)ng·m-3,每个水平3个重复。为避免相互遮荫,各气室之间留有3 m的间距。气室内汞浓度通过浮子流量计调节载气流速来控制,每50 s左右气室由离心鼓风机(690 m3·h-1)完成1次彻底换气。从2013-08-31正式开始熏气,到2013-11-15结束熏气,24 h连续供气,气室内汞浓度由RA-915+赛曼原子吸收汞分析仪(Lumex Inc.,Russia)在线监测。
1.2 叶片生理指标的测定
1.2.1 气体交换参数的测定
利用LI-6400便携式光合仪测定系统(LI-6400, LICOR Inc.,USA)测定不同汞浓度水平熏气实验中水稻叶片净光合速率(Pn)、气孔导度(Gs)、胞间CO2浓度(Ci)和蒸腾速率(Tr)4个气体交换参数[25-26]。实验表明植物叶片Pn一般在上午9:00-11:00达到最大值[27-28],因此本研究分别选择扬花期和乳熟期天气状况基本一致的2 d,在上午10:00-12:00对水稻叶片气体交换参数进行测定,仪器CO2浓度和流速分别设定为400μmol·mol-1和500μmol·s-1,每个处理选择4片完整无缺的剑叶进行测定。
2.3.2推进农作物秸秆资源化利用 指导长江经济带以县为单元编制全量化利用实施方案,提高秸秆综合利用的区域统筹水平。坚持农用为主、五料并举,积极推广深翻还田、捡拾打捆、秸秆离田多元利用等技术,指导创设秸秆还田离田利用政策机制,培育秸秆资源化利用产业化龙头企业,推进秸秆产业化发展。
1.2.2 抗逆指标的测定
采集水稻拔节期、扬花期和成熟期的叶片鲜样,用便携式冰箱迅速带回实验室,并先后用自来水和去离子水冲洗干净。叶片游离脯氨酸(Pro)和丙二醛(MDA)含量的测定参照朱广廉[29]、陈建勋[30]的方法,脯氨酸含量采用磺基水杨酸提取,酸性茚三酮染色的方法进行测定;丙二醛含量采用硫代巴比妥酸(TBA)法测定;叶片总超氧化物歧化酶(T-SOD)活性采用黄嘌呤自氧化法(羟胺法)测定[31]。
2 结果(Results)
2.1 大气汞对水稻叶片气体交换参数的影响
实验结果表明,在3个熏气汞浓度下,扬花期水稻叶片净光合速率(Pn)和气孔导度(Gs)均比对照略低(图1A、B),Pn在15和45 ng·m-3的大气汞浓度下显著低于对照(P<0.05),但Gs在不同大气汞浓度下无显著差异(P>0.05),说明在实验处理水平下的大气汞对水稻叶片的气孔开放程度无明显影响,对光合作用有一定影响;胞间CO2浓度(Ci)随大气汞浓度的升高有明显降低的趋势(图1C,P<0.05);蒸腾速率(Tr)随大气汞浓度升高而显著增加(图1D,P<0.01)。乳熟期水稻叶片Pn在45 ng·m-3的大气汞浓度下显著低于对照,Gs、Ci和Tr与大气汞浓度均无显著差异(图1A、B、C、D,P>0.05);同时,除Ci与扬花期无显著差异外,乳熟期Pn、Gs和Tr在4个大气汞浓度水平下明显低于扬花期,表明乳熟期水稻叶片的光合作用和蒸腾作用较扬花期弱,且大气汞对乳熟期水稻叶片气体交换参数均无明显影响。
2.2 大气汞对水稻叶片脯氨酸含量的影响
拔节期水稻叶片中脯氨酸含量随大气汞浓度的升高而显著增加(图2,P<0.05),扬花期和成熟期水稻叶片中游离脯氨酸含量随大气汞浓度的升高均有增加的趋势,表明大气汞浓度的升高会胁迫水稻叶片产生并积累大量的游离脯氨酸。大气汞浓度在5、15和45 ng·m-3时,扬花期叶片中脯氨酸含量明显高于拔节期和成熟期,在90 ng·m-3的大气汞浓度下,叶片中脯氨酸含量关系为:成熟期>扬花期>拔节期,表明环境浓度下的大气汞对水稻扬花期叶片中游离脯氨酸的积累影响更大,而在高汞浓度下,汞在叶片中随生长时期的延长而富集对脯氨酸的积累影响更大。
2.3 大气汞对水稻叶片丙二醛含量的影响
水稻叶片中丙二醛(MDA)含量在各生长时期随着大气汞浓度的升高无显著差异(图3,P>0.05),但在拔节期和成熟期会随大气汞浓度从5 ng·m-3升高到45 ng·m-3而不断增加,在90 ng·m-3时又下降,表明大气汞浓度的升高会胁迫叶片产生过多的丙二醛。在4个大气汞浓度水平下,不同时期叶片中MDA含量关系均为:拔节期>成熟期>扬花期,拔节期明显最高,且在15和45 ng·m-3时,叶片内MDA含量显著高于对照(P<0.05),表明大气汞对水稻拔节期叶片中丙二醛含量的影响最大。
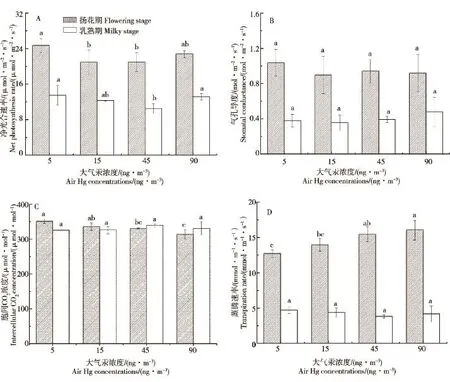
图1 大气汞对水稻叶片气体交换参数的影响Fig.1 Effects of air Hg to gas exchange parameters of rice foliage
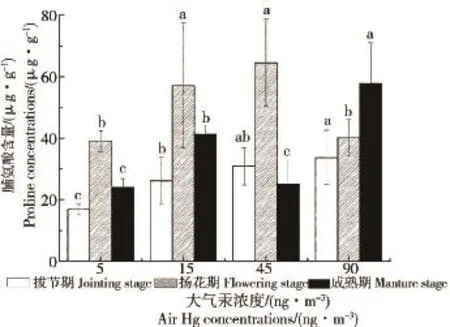
图2 大气汞对水稻叶片脯氨酸含量的影响Fig.2 Effects of air Hg to proline of rice foliage

图3 大气汞对水稻叶片丙二醛(MDA)含量的影响Fig.3 Effects of air Hg to malonaldehyde(MDA)of rice foliage
2.4 大气汞对水稻叶片超氧化物歧化酶活性的影响
水稻叶片中总超氧化物歧化酶(T-SOD)的活性在拔节期和扬花期随着大气汞浓度的升高均无显著差异(图4,P>0.05),但在15 ng·m-3时拔节期叶片中SOD活性显著高于其他汞浓度下(P<0.05);在5、15和45 ng·m-3时,拔节期叶片中SOD活性均较扬花期高,而在90 ng·m-3时,扬花期较拔节期略高。
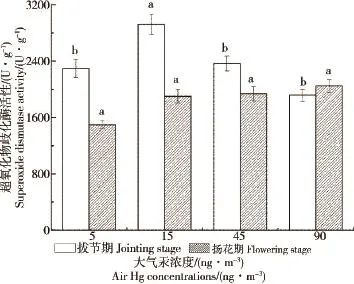
图4 大气汞对水稻叶片超氧化物歧化酶活性(SOD)的影响Fig.4 Effects of air Hg to superoxide dismutase(SOD) of rice foliage
3 讨论(Discussion)
大量实验结果表明,汞影响植物的光合作用,影响光反应和暗反应[32-33],但也有一些实验结果表明,水培实验中汞浓度低于10mg·L-1时,汞对植物的光合作用影响较小甚至无影响[32,34-37]。Ericksen和Gustin[34]的实验结果表明,不同浓度的大气汞(2.4、11和30 ng·m-3)熏蒸对杨树叶的Pn和Gs均无显著影响,Niu等[38]的实验结果也表明,环境浓度的大气汞(2、10、20和50 ng·m-3)对玉米叶片的气体交换参数基本没有影响。Ci的变化是分析植物气孔与非气孔限制的基础,作为光合过程中CO2的中介,一方面受到作为源的外界CO2浓度和气孔导度的影响,另一方面又受叶片光合消耗的影响[26,39],而非气孔限制常用于解释胞内恒定或者增加的Ci[40-41],本实验中扬花期Ci随着大气汞浓度的升高明显下降,且Pn和Gs均有一定程度的降低,说明水稻扬花期属于气孔限制。王孟本等[42]对河北杨和柠条的研究表明,在土壤水分充足的条件下,若树木根系吸水力依然较强,而其蒸腾生理调控力却大为减弱,蒸腾速率就会异常增大,本实验中随着大气汞浓度的升高,水稻叶片光合作用和气孔导度均无显著变化,而蒸腾速率却线性升高,说明大气汞影响了水稻叶片的蒸腾生理功能。
脯氨酸(Pro)是植物蛋白质的组分之一,可以游离状态广泛存在于植物体中,在逆境(旱、盐碱、热、冷、冻等)胁迫条件下,许多植物体内脯氨酸含量显著增加,在一定程度上反映了植物的抗逆性。实验表明,重金属胁迫下植物体内 Pro浓度会增加[11-12,43]。刘玲等[44]的砂培实验结果表明,在低汞浓度、短时间内,玉米体内Pro含量有上升趋势,在高汞浓度、长时间内,玉米体内Pro含量会严重下降,且Pro对汞的胁迫非常敏感;Niu等[38]对玉米的研究结果表明,大气汞浓度与Pro含量之间无显著相关性(P>0.05),但在大气汞浓度为20和50 ng·m-3时Pro含量显著高于对照(P<0.05)。本实验中,在15和45 ng·m-3大气汞浓度下扬花期叶片脯氨酸含量显著较高,而在90 ng·m-3时成熟期叶片脯氨酸含量显著较高,表明随着大气汞浓度的升高,水稻叶片脯氨酸含量随着大气汞浓度的升高均有不同程度的增加。
在植物器官衰老或在逆境条件下,细胞内会产生大量的活性自由基,并与脂质发生过氧化反应,最终产物即为丙二醛(MDA),通常利用它作为脂质过氧化指标,表示细胞膜脂过氧化程度和植物对逆境条件反应的强弱。大量实验结果表明,重金属胁迫下植物体内丙二醛的含量会增加[6,45-46]。Cho和Park[6]报道西红柿叶中MDA水平随叶汞浓度的增加而增加,Moreno-Jimeˊnez等[47]对2种野生植物的研究结果也表明叶中MDA含量与叶汞浓度显著正相关(P<0.05);而Niu等[38]对玉米的研究结果表明,大气汞浓度与MDA含量之间无显著相关性(P>0.05),仅在大气汞浓度为20和50 ng·m-3时MDA含量显著高于对照(P<0.05)。本实验中,高浓度的大气汞熏气显著增加了丙二醛的含量,表明会引起植物叶的膜脂过氧化。
超氧化物歧化酶(SOD)是机体内天然存在的超氧自由基清除因子,是生物体内清除自由基的首要物质,与体内的过氧化氢酶(CAT)和过氧化物酶(POD)组成了一个完整的防氧化链条。大量实验结果表明重金属胁迫下植物体内SOD活性会发生变化[12,45,48-49]。马成仓[44]用不同浓度的HgCl2溶液灌溉油菜结果显示,油菜叶细胞内SOD活性在0.5mg·L-1的汞浓度下无明显变化,(1~10)mg·L-1的汞浓度下逐渐升高,50mg·L-1时持续下降;陆海燕等[12]的研究结果显示,随着溶液中Cd2+浓度的增加,芦苇叶片中SOD活性呈先上升后下降的趋势;施国新等[49]的研究也表明,随着汞浓度的升高,满江红叶片SOD活性逐渐增强,当浓度超过一定范围时,SOD活性则开始降低。本实验中,拔节期水稻叶片SOD活性随大气汞浓度的升高先上升后下降,15 ng·m-3时达到最大,表明水稻体内氧自由基清除能力较强,水稻耐汞性较好。
综上分析,水稻叶片中Pro、MDA含量和SOD活性随着大气汞浓度的升高变化不同。在拔节期, Pro含量随大气汞浓度升高呈线性增加,MDA含量先急剧增加然后降低,在45 ng·m-3的汞浓度下达到最大,SOD活性先增大,在15 ng·m-3的汞浓度下达到最大后又开始下降;在扬花期,Pro含量随大气汞浓度的升高先增加后下降,且明显比拔节期高, MDA含量和SOD活性无显著变化,且较拔节期低。出现这种情况的可能原因是,汞胁迫下水稻叶片细胞膜脂出现过氧化,体内SOD活性被激发,同时Pro和MDA含量增加,Pro具有减少膜脂过氧化和稳定细胞膜结构及生物大分子的作用[50],因此对SOD这种生物蛋白大分子具有一定的保护作用,对其他膜结构也有一定的保护作用,Pro的这种双重作用机制,使得SOD活性在迅速升高后又恢复到正常水平,并随着Pro的不断增加,MDA含量也开始下降;随着生长期的延长,汞胁迫使水稻体内Pro含量升高,同时对逆境适应能力增强,对汞污染产生了耐受性,体内Pro含量开始降低。这与陆海燕等[12]在镉污染下对芦苇叶片丙二醛、脯氨酸及SOD保护酶反应的研究结果一致。因此,水稻叶片中Pro、MDA和SOD对大气汞浓度升高有协同反应。
[1]沈盎绿.Hg2+对细叶蜈蚣草的毒害效应[D].西南农业大学,2004
[2]詹嘉红,蓝宗辉.汞对水稻幼苗部分生理生化指标的影响[J].生物技术,2007,17(3):76-77 Zhan J H,Lan Z H.Effects of mercury on some of physiological indicators of rice seedlings[J].Chinese Journal of Biotechnology,2007,17(3):76-77(in Chinese)
[3]Patra M,Sharma A.Mercury toxicity in plants[J].The Botanical Review,2000,66(3):379-422
[4]Lu C M,Chau C W,Zhang J H.Acute toxicity of excess mercury on the photosynthetic performance of cyanobacterium,S.platensis--assessment by chlorophyll fluorescence analysis[J].Chemosphere,2000,41(1-2):191-196
[5]王琳,王林嵩,王丽,等.Hg2+胁迫对小麦幼苗POD、CAT和SOD同工酶的影响[J].安徽农业科学,2008,36 (35):15326-15328,15338 Wang L,Wang L H,Wang L,et al.Effect of Hg2+on isozymes of peroxidase,catalaseand and superoxide diamutase in wheat seedling[J].Journal of Anhui Agricultural Sciences,2008,36(35):15326-15328,15338(in Chinese)
[6]Cho U H,Park J O.Mercury-induced oxidative stress in tomato seedlings[J].Plant Science,2000,156(1):1-9
[7]Han Y,Xuan W,Yu T,et al.Exogenoushematin alleviates mercury‐ induced oxidative damage in the roots of medicago sativa[J].Journal of Integrative Plant Biology, 2007,49(12):1703-1713
[8]Clijsters H,VanAssche F.Inhibition of photosynthesis by heavy metals[J].Photosynthesis Research,1985,7(1):31-40
[9]Laliberté G,Hellebust J A.Regulation of proline content ofChlorella autotrophicain response to changes in salinity[J].Canadian Journal of Botany,1989,67(7):1959-1965
[10]Zengin F K,Munzuroglu O.Effects of some heavy metals on content of chlorophyll,proline and some antioxidant chemicals in bean(Phaseolus vulgarisL.)seedlings[J]. Acta Biologica Cracoviensia Series Botanica,2005,47(2): 157-164
[11]Zhang L P,Mehta S K,Liu Z P,et al.Copper-induced proline synthesis is associated with nitric oxide generation inChlamydomonas reinhardtii[J].Plant and Cell physiology,2008,49(3):411-419
[12]陆海燕,刘志辉,吕光辉.镉污染下芦苇叶片丙二醛、脯氨酸及SOD保护酶反应研究[J].干旱区资源与环境,2013,27(8):171-175 Lu H Y,Liu Z H,Lv G H.Reaction of MDA,proline and SOD under Cd stress in mixture ofPhragmites australis's stems and leaves[J].Journal of Arid Land Resources and Environment,2013,27(8):171-175(in Chinese)
[13]Janero D R.Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury[J].Free Radical Biology and Medicine,1990,9(6):515-540
[14]刘惠芬,高大翔,马勇.汞胁迫对水稻生长及幼苗生理生化的影响[C].第二届全国农业环境科学学术研讨会论文集,2007,7:16-19 Liu H F,Gao D X,Ma Y.Effects of Hg stress on growth and physiological and biochemical characteristics of rice seedlings[C].The Second National Agricultural Academic Conference on Environmental Sciences,2007,7:16-19 (in Chinese)
[15]Pirrone N,Cinnirella S,Feng X B,et al.Global mercury emissions to the atmosphere from anthropogenic and natural sources[J].Atmospheric Chemistry and Physics, 2010,10(13):5951-5964
[16]Swain E B,Engstrom D R,Brigham M E,et al.Increasing rates of atmospheric mercury deposition in midcontinental North America[J].Science,1992,257(5071):784-787
[17]瞿爱权,东惠如,李俊国.汞对水稻、油菜影响的研究初报[J].环境科学,1980,1(6):50-52
[18]Ericksen J,Gustin M,Schorran D,et al.Accumulation of atmospheric mercury in forest foliage[J].Atmospheric Environment,2003,37(12):1613-1622
[19]Millhollen A G,Gustin M S,Obrist D.Foliar mercury accumulation and exchange for three tree species[J].Environmental Science&Technology,2006,40(19):6001-6006
[20]Niu Z C,Zhang X S,Wang Z W,et al.Field controlled experiments of mercury accumulation in crops from air and soil[J].Environmental Pollution,2011,159(10): 2684-2689
[21]Niu Z C,Zhang X S,Wang S,et al.The linear accumulation of atmospheric mercury by vegetable and grass leaves:Potential biomonitors for atmospheric mercury pollution[J].Environmental Science and Pollution Research, 2013,20(9):6337-6343
[22]Heagle A S,Body D E,Heck W W.An open-top field chamber to assess the impact of air pollution on plants[J]. Journal of Environmental Quality,1973,2(3):365-368
[23]王春乙.OTC-1型开顶式气室的结构和性能与国内外同类气室的比较[J].环境科学进展,1996,4(1):50-57 Wang C Y.The structure and function comparison between OTC-1 open top chamber with the similar one in home and overseas[J].Chinese Journal of Advances in Environmental Sciences,1996,4(1):50-57(in Chinese)
[24]Mandl R,Weinstein L,McCune D,et al.A cylindrical, open-top chamber for the exposure of plants to air pollutants in the field[J].Journal of Environmental Quality, 1973,2(3):371-376
[25]王建林,于贵瑞,王伯伦,等.北方粳稻光合速率,气孔导度对光强和 CO2浓度的响应[J].植物生态学报, 2005,29(1):16-25 Wang J L,Yu G R,Wang B L,et al.Response of photosynthetic rate and stomatal conductance of rice to light intensity and CO2concentration in northern china[J].Chinese Journal of Acta Phytoecologica Sinica,2005,29(1): 16-25(in Chinese)
[26]徐俊增,彭世彰,魏征,等.节水灌溉水稻叶片胞间CO2浓度及气孔与非气孔限制[J].农业工程学报,2010, 26(7):76-80 Xu J Z,Peng S Z,Wei Z,et al.Intercellular CO2concentration and stomatal or non-stomatal limitation of rice under water saving irrigation[J].Transactions of the CSAE, 2010,26(7):76-80(in Chinese)
[27]He J,Austin P T,Nichols M A,et al.Elevated root-zone CO2protects lettuce plants from midday depression of photosynthesis[J].Environmental and Experimental Botany,2007,61(1):94-101
[28]Wang J,Yu Q,Li J,et al.Simulation of diurnal variations of CO2,water and heat fluxes over winter wheat with a model coupled photosynthesis and transpiration[J].Agricultural and Forest Meteorology,2006,137(3):194-219
[29]朱广廉,钟诲文,张爱琴.植物生理学实验[M].北京:北京大学出版社,1990:245-252
[30]陈建勋,王晓峰.植物生理学实验指导(第二版)[M].广州:华南理工大学出版社,2002:66-74
[31]黎瑞珍,杨庆建,陈贻锐.超氧化物歧化酶(SOD)活性的测定及其应用研究[J].琼州大学学报,2005,11(5): 34-36 Li R Z,Yang Q J,Chen Y R.Study of determination of superoxide dismutase(SOD)activation and application [J].Chinese Journal of Qiongzhou University,2005,11 (5):34-36(in Chinese)
[32]Baszynski T,Krupa Z.Some aspects of heavy metals toxicity towards photosynthetic apparatus-direct and indirect effects on light and dark reactions[J].Acta Physiologiae Plantarum,1995,17(2):177-190
[33]Pisani T,Munzi S,Paoli L,et al.Physiological effects of mercury in the lichensCladonia arbusculasubsp.Mitis (Sandst.)Ruoss andPeltigera rufescens(Weiss)Humb[J]. Chemosphere,2011,82(7):1030-1037
[34]Ericksen J A,Gustin M S.Foliar exchange of mercury as a function of soil and air mercury concentrations[J].Science of the Total Environment,2004,324(1-3):271-279
[35]Bernier M,Popovic R,Carpentier R.Mercury inhibition at the donor side of photosystem II is reversed by chloride[J].FEBS Letters,1993,321(1):19-23
[36]Šeršeň F,Král'ová K,Bumbalova A.Action of mercury on the photosynthetic apparatus of spinach chloroplasts[J].Photosynthetica,1998,35(4):551-559
[37]Israr M,Sahi S,Datta R,et al.Bioaccumulation and physiological effects of mercury inSesbania drummondii[J]. Chemosphere,2006,65(4):591-598
[38]Niu Z C,Zhang X S,Wang S,et al.Field controlled experiments on the physiological responses of maize(Zea maysL.)leaves to low-level air and soil mercury exposures[J].Environmental Science and Pollution Research, 2014,21(2):1541-1547
[39]傅伟,王天铎.一个气孔对环境因子响应的机理性数学模型[J].植物生理学报,1994,20(3):277-284 Fu W,Wang T D.A mechanistic model of stomatal responses to environmental factors[J].Chinese Journal of Acta Phytophysiologica Sinica,1994,20(3):277-284(in Chinese)
[40]Farquhar G D,Sharkey T D.Stomatal conductance and photosynthesis[J].Annual Review of Plant Physiology, 1982,33(1):317-345
[41]刘俊祥,孙振元,巨关升,等.重金属Cd2+对结缕草叶片光合特性的影响[J].核农学报,2009,23(6):1050-1053 Liu J X,Sun Z Y,Ju G S,et al.Effects of Cd2+stress on photosynthetic characteristics in leaves ofZoysia japonica [J].Chinese Journal of Nuclear Agricultural Sciences, 2009,23(6):1050-1053(in Chinese)
[42]王孟本,李洪建,柴宝峰,等.树种蒸腾作用,光合作用和蒸腾效率的比较研究[J].植物生态学报,1999,23(5): 401-410 Wang M B,Li H J,Chai B F,et al.A comparison of transpiration,photosynthesis and transpiration efficiency in four tree species in loess region[J].Chinese Journal of Acta Phytoecologica Sinica,1999,23(5):401-410(in Chinese)
[43]Radic'S,Babic'M,Škobic'D,et al.Ecotoxicological effects of aluminum and zinc on growth and antioxidants in Lemna minorL[J].Ecotoxicology and Environmental Safety,2010,73(3):336-342
[44]刘玲,杨双春,张洪林.Hg2+胁迫下玉米生理生态变化的研究[J].生态环境,2004,13(2):161-163 Liu L,Yang S C,Zhang H L.Physiological and ecological response of maize to mercury stress[J].Chinese Journal of Ecology and Environment,2004,13(2):161-163(in Chinese)
[45]马成仓.Hg对油菜叶细胞膜的损伤及细胞的自身保护作用[J].应用生态学报,1998,9(3):323-326 Ma C C.Hg harm on cell membrane of rape leaf and cell endogenous protection effect[J].Chinese Journal of Applied Ecology,1998,9(3):323-326(in Chinese)
[46]Cargnelutti D,Tabaldi L A,Spanevello R M,et al.Mercury toxicity induces oxidative stress in growing cucumber seedlings[J].Chemosphere,2006,65(6):999-1006
[47]Moreno-Jiménez E,Gamarra R,Carpena-Ruiz R,et al. Mercury bioaccumulation and phytotoxicity in two wild plant species of Almadén area[J].Chemosphere,2006,63 (11):1969-1973
[48]张利红,李培军,李雪梅,等.镉胁迫对小麦幼苗生长及生理特性的影响[J].生态学杂志,2005,24(4):458-460 Zhang L H,Li P J,Li X M,et al.Effects of cadmium stress on the growth and physiological characteristics of wheat seedlings[J].Chinese Journal of Ecology,2005,24 (4):458-460(in Chinese)
[49]Shi G X,Xu Q S,Xie K B,et al.Physiology and ultrastructure ofAzolla imbricataas affected by Hg2+and Cd2+Toxicity[J].Acta Botanica Sinica,2003,45(4):437-444
[50]蒋明义,郭绍川.氧化胁迫下稻苗体内积累的脯氨酸的抗氧化作用[J].植物生理学报,1997,23(4):347-352 Jiang M Y,Guo S C.Proline accumulation in rice seedlings exposed to oxidative stress in relation to antioxidation[J].Chinese Journal of Acta Phytophysiologica Sinica,1997,23(4):347-352(in Chinese)
Physiological Responses of Rice Leaves to the Elevated Gaseous Elemental Mercury in the Atmosphere
Chen Jian1,2,Wang Zhangwei1,*,Zhang Xiaoshan1,Qin Pufeng3,Lu Haijun3
1.Laboratory of Atmospheric Environmental Sciences,Research Center for Eco-Environmental Sciences,Chinese Academy of Sciences,Beijing 100085,China
2.College of Resources and Environment,University of Chinese Academy of Sciences,Beijing 100049,China
3.College of Resources&Environment,Hunan Agricultural University,Changsha 410128,China
27 January 2015 accepted 25 March 2015
The effects of elevated gaseous elemental mercury(GEM)on gas exchange parameters,accumulation of proline(Pro)and malondialdehyde(MDA),activity of superoxide dismutase(SOD)in rice foliage were studied with field open-top chambers(OTCs)fumigation experiment.The results showed that the net photosynthesis rate(Pn)and stomatal conductance(Gs)were less slightly in GEM treatment than those in the control,which indicating that elevated GEM had some effect on photosynthesis and stomatal openness of rice leaves.In flowering stage of rice,the distinct decrease(P<0.05)of intercellular CO2concentration(Ci)with elevated GEM indicated that stomatal limitation led to the slight decrease of Pn,and the significant increase(P<0.01)of transpiration rate(Tr)with elevated GEM showed that the physiological function of rice transpiration was effected by Hg in the atmosphere.Gas exchange parameters of rice leaves in milky stage were insignificantly difference with GEM in air(P>0.05)and lower than that in flowering stage.Proline concentrations in rice foliage were increased obviously with elevated GEM(P<0.05)in jointing stage,declined after increasing and reached to the maximum value at 45 ng·m-3in flowering stage,and it was no significant difference(P>0.05)in mature stage among four treatments.The contents of MDA in rice foliage increased first and reached the highest value at 45 ng·m-3,and then decreased in jointing stage,and it was no significant difference(P>0.05)with the increase of GEM in flowering and mature stage.The activity of SOD in rice foliage also increased first and then declined at 15 ng·m-3in jointing stage,and there was no significant difference(P>0.05)in flowering stage.These results suggested that elevated GEM in air can cause membrane lipid peroxidation and accumulation of Pro and MDA in rice foliage,furthermore the ability of adapting to adversity and the tolerance to elevated GEM for rice were enhanced with the concerted reactions in vivo among Pro,MDA and SOD on the atmospheric mercury stress.
GEM;gas exchange parameters;proline;malondialdehyde;superoxide dismutase;open-top chamber
2015-01-27 录用日期:2015-03-25
1673-5897(2016)1-133-08
X171.5
A
10.7524/AJE.1673-5897.20150127003
陈剑,王章玮,张晓山,等.大气汞浓度升高对水稻叶片生理效应的影响研究[J].生态毒理学报,2016,11(1):133-140
Chen J,Wang Z W,Zhang X S,et al.Physiological responses of rice leaves to the elevated gaseous elemental mercury in the atmosphere[J].Asian Journal of Ecotoxicology,2016,11(1):133-140(in Chinese)
国家自然科学基金项目(No.41373124,41073092);国家重大基础研究(973)计划项目(2013CB430002)
陈剑(1990-),男,硕士研究生,研究方向为大气汞循环,E-mail:chenjianev2008@126.com
),E-mail:wangzhw@rcees.ac.cn
简介:王章玮(1978-),女,博士,副研究员,主要研究方向大气汞循环。
