由氢键连接的三个金属-有机网状化合物的合成、结构及半导体性质
2016-12-05陈飞剑林清芳王添艳沈福志韦正友梁丽丽
陈飞剑 林清芳 王添艳 沈福志 韦正友 梁丽丽
(蚌埠医学院化学教研室,蚌埠233030)
由氢键连接的三个金属-有机网状化合物的合成、结构及半导体性质
陈飞剑林清芳王添艳沈福志韦正友梁丽丽*
(蚌埠医学院化学教研室,蚌埠233030)
以2,4,6-三(1-吡唑基)-1,3,5-三嗪(TPTz)与不同金属离子进行溶剂热反应,得到了3个氢键连接的金属-有机网状化合物。实验发现TPTz的水解产物6-(1-吡唑基)-1,3,5-三嗪-2,4-二酚(H2L)在反应中起到了实际的配位作用。单晶结构分析表明,它们是同构化合物,分子式为[M(HL)2]·2H2O(M=Zn,1;Co,2;Mn,3)。每个中心金属原子分别与2个吡唑基上的N、2个吡嗪环上的N和2个水分子中的O形成六配位的结构。2个HL-与1个中心金属配位形成一个零维的金属-有机配合物小分子,这些小分子通过氢键连接进一步拓展为二维层状结构。紫外-可见漫反射(UV-Vis DRS)分析结果表明,这3种化合物都是宽系半导体材料,其带隙宽度分别为3.80(Zn),3.30(Co),3.27(Mn)eV,其半导体性质同中心金属原子表现出明显的相关性。
金属-有机网状化合物;氢键;2,4,6-三(1-吡唑基)-1,3,5-三嗪
0 Introduction
During the past two decades,metal-organic frameworks(MOFs)have attracted more and more attention not only because of their high porosity and regularity structures but also due to that they usually showed versatile excellent properties such as in catalysis[1], gas adsorption and separation[2],chemical sensor[3], magnetism[4]and luminescence[5].Among which,it is recently clearly pointed out that MOFs can take potential important usage as semiconductors[6].The most-studied such MOFs were focused on materials assembled by carboxylates and d10metals,MOF-5 is a typical example,it is a wide band-gap semiconductor with a band gap of 3.4 eV which was estimated by its UV-Vis diffuse reflectance spectrum[6a](usuallymaterialswith a band-gap over 2.0 eV were defined aswide band semiconductors,such as GaN,Eg=3.4 eV,SiC, Eg=2.86 eV and ZnO,Eg=3.4 eV).Thesematerials are widely used in ultraviolet optoelectronic devices,field emission devices and electronic devices undergo conditions of high temperature,high frequency and high power[7].
Although basically proposed during the same period around the early 1990s with MOFs,research on the hydrogen-bonded organic frameworks(HOFs) has been significantly lagging behind,as their hydrogen-bonding interactions are typically too weak to stabilize the framework,thus is more fragile and usually without any permanent porosities[8].Recently, there has been a renewed interest in the exploration of porous HOFs,since some progress has been made to stabilize HOFs and thus to establish permanent porosities,which exemplifying the bright promise of HOFs as new category of functional materials[9]. However,as to our knowledge,most research of their functional properties are focused on their gas separation behavior,semiconductor properties of HOFs are still rarely mentioned in the literature[10]. Thus hydrogen-bonded metal-organic networks which simultaneously possess two distinct properties of MOFs and HOFs,is still out of the public attention[11].
For a category of materials,taking potential usage as semiconductors,flexible modulating of their band-gap and thus with different semiconductor properties should be necessary[12].Both experimental and theoretical studies showed that for MOFs assembled by carboxylates,their unique structures together with the organic ligands play important roles in their bandgap values,while isomorphous complexeswith different center metal ions just show tiny difference[7,13].On tuning of their semiconductor properties,the common experimentalmethod involved:(1)To regulate the size of their secondary building units(SBUs),since enlargement of SBU brings with a decrement on their band gap values[14];(2)to modify various substituents or change the size on the ligands,one can also tune their band-gap[12-13].
In conflictwith that of MOFs formed by carboxylates,herein,we will show that by using a N-donor ligand,the center metal ions of three isomorphous hydrogen-bonded metal-organic networks make great impact on their semiconductor properties,which has guiding significance for the synthesis of some novel compoundswith tunable semiconductor properties.
1 Experimental
1.1M aterials and methods
Elemental analyses of C,H,N were performed on an Elementar Vario MICRO Elemental Analyzer. Fourier Transform Infrared(FT-IR)spectra were obtained on a Bruker Vector 22 FT-IR spectrophotometerby using KBrpellets.Thermogravimetric analyses (TGA)were performed on a Perkin-Elmer thermal analyzer under nitrogen with a heating rate of 10℃· min-1.Powder X-ray diffraction(PXRD)patterns were collected in the 2θrange of 5°~50°on a Bruker D8 advance instrument using Cu Kα(λ=0.154 178 nm) radiation at room temperature.Ultraviolet visible diffused reflection spectra(UV-Vis DRS)were recorded on Varian Cary 5000 UV-Vis-NIR,corrected by BaSO4.Reagents and solvents were commercially available and used as received without further purification.The ligand 2,4,6-tris(pyrezole-1-yl)-1,3,5-s-triazine(TPTz)was prepared according to the reported procedure[15].
1.2Synthesis of com pounds 1~3
Compounds 1~3 was synthesized by a solvothermal reaction of TPTz with Zn(NO3)2·6H2O,Co(NO3)2· 6H2O and Mn(NO3)2·6H2O in the mixture of N,N-dimethyl formamide(DMF)and H2O solvent,respectively.Detailswere shown below.
Synthesis of[Zn(HL)2]·2H2O(1):Zn(NO3)2·6H2O (59.4 mg,0.2 mmol)and TPTz(27.9 mg,0.1 mmol) were dissolved in themixture of DMF(3mL)and H2O (1 mL),then the solution was transferred into a vial, sealed and heated at 100℃for 2 days.The vialwas then allowed to cool down to room temperature. Colorless crystals(33 mg)were obtained by filtration and washed with DMF and ether,respectively.Yield: 73%(based on TPTz).Anal.Calcd.for C12H12N10O6Zn (%):C,31.49;H,2.64;N,30.60;Found(%):C,31.60; H,2.14;N,30.68.FTIR(KBr,cm-1):3 310(w),3 160(s), 3040(s),2 810(s),1700(w),1 480(s),1390(s),1210(m), 1 090(m),1 050(s),970(m),924(s),795(m),640(s), 471(m).
Synthesis of[Co(HL)2]·2H2O(2):Orange rhombic crystals of 2 were obtained following the same procedure as 1 with the replacement of Zn(NO3)26H2O by Co(NO3)2·6H2O.Yield:83%(based on TPTz). Anal.Calcd.for C12H12N10O6Co(%):C,31.94;H,2.68; N,31.04;Found(%):C,32.01;H,2.18;N,31.24. FTIR(KBr,cm-1):3 300(w),3 160(s),3 030(s),2 800(s), 1 700(w),1 560(s),1 480(m),1 390(m),1 210(m),1 090 (s),1 050(m),966(m),922(s),796(s),644(s),471(m).
Synthesis of[Mn(HL)2]·2H2O(3):The preparation of 3 was similar to that of 1,but Zn(NO3)2·6H2O(59.4 mg,0.2mmol)was substituted by Mn(NO3)2·6H2O (50.2 mg,0.2 mmol),colorless crystals of 3 were isolated with a yield of 65%(based on TPTz).Anal. Calcd.for C12H12N10O6Mn(%):C,32.23;H,2.70;N, 31.32%;Found(%):C,31.72;H,2.26;N,30.74.FTIR (KBr,cm-1):3280(w),3160(s),3040(s),2810(s),1700 (w),1 560(s),1 480(m),1 400(m),1 210(m),1 090(s), 1 050(m),970(m),924(s),872(s),653(s),471(m).
1.3Single-crystal X-ray crystallography
The suitable crystals of compounds 1~3 were selected for single-crystal X-ray diffraction.The data collections were carried out on a Bruker Smart APEXⅡCCD diffractometer at 296 K,using graphitemonochromatic Mo Kαradiation(λ=0.071 073 nm). Data reductions and absorption corrections were performed using the SAINT and SADABS programs[16], respectively.The structures were solved by direct methods using the SHELXS-97 program and refined with full-matrix leastsquares on F2using the SHELXL -97 program[17].All non-hydrogen atoms were refined anisotropically,and the hydrogen atoms were placed in geometrically calculated positions and refined using the riding model.Details of the crystal parameters, data collection and refinements for compounds 1~3 are summarized in Table1.Selected bond lengths and angles are shown in Table2.
CCDC:904985,1;904986,2;904987,3.

Table1 C rystallographic data for com pounds 1~3
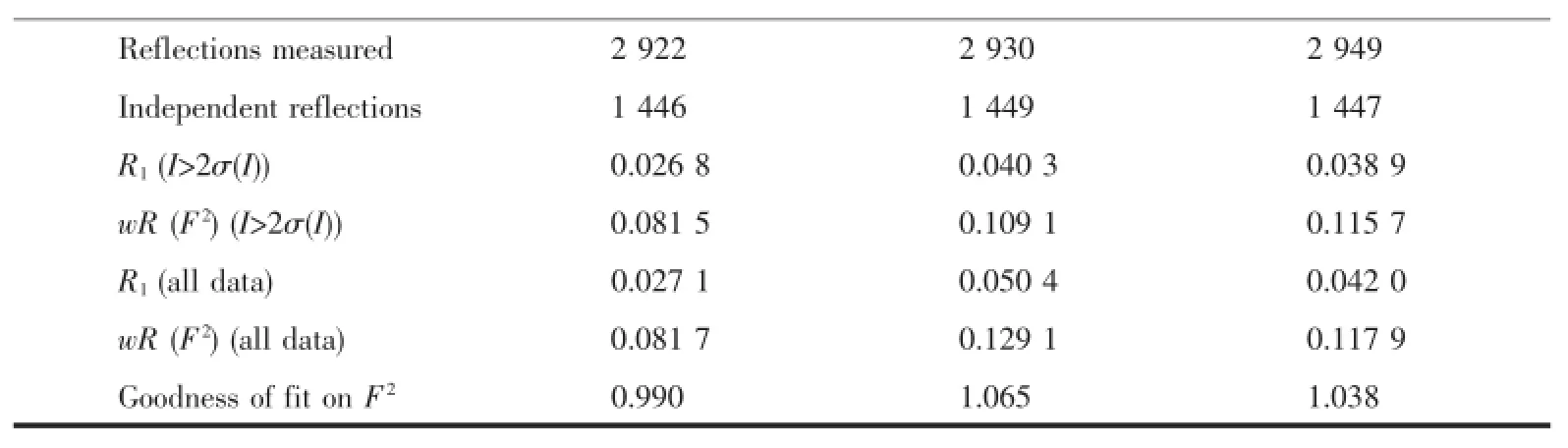
Continued Table1
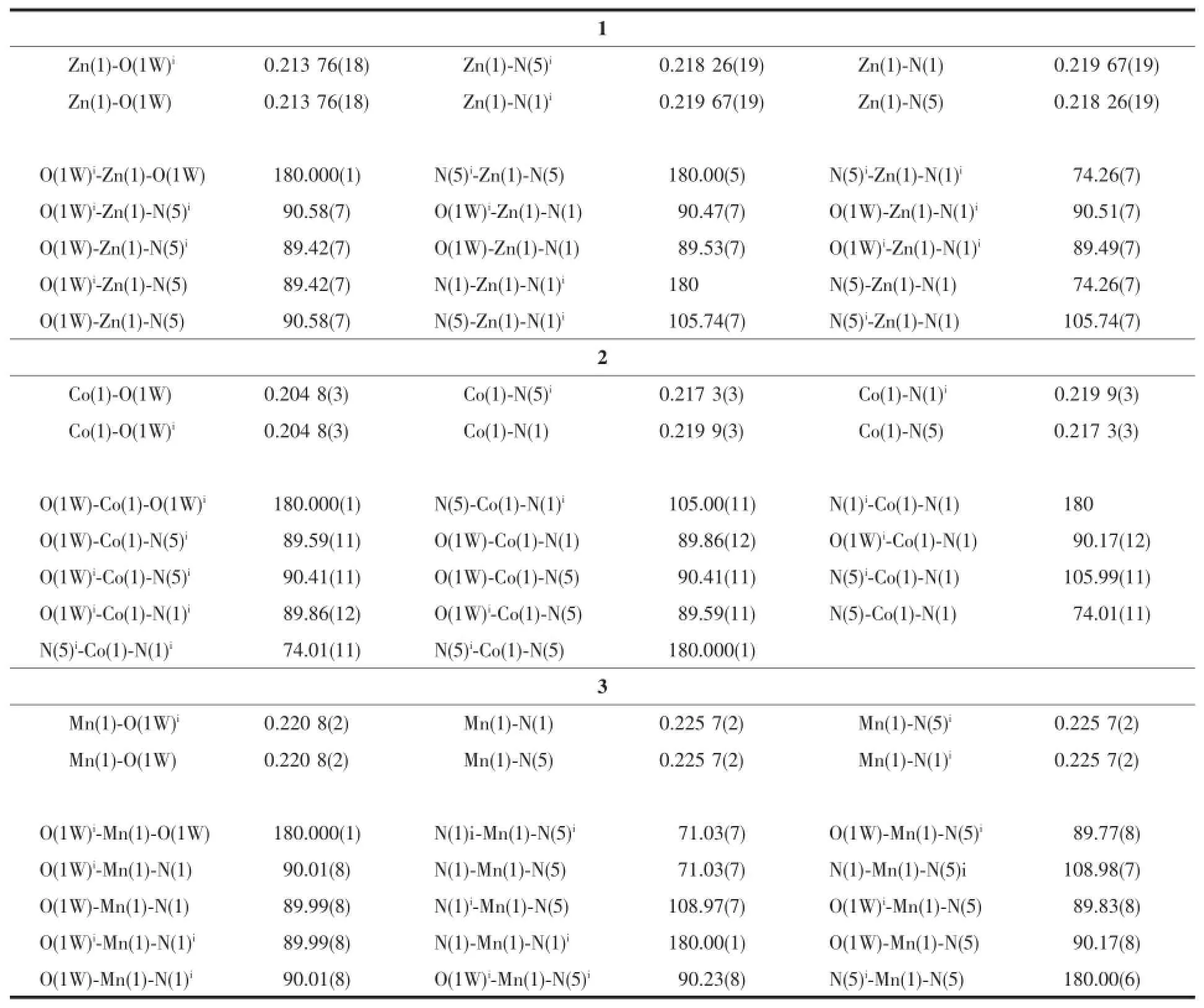
Table2 Selected bond lengths(nm)and angles(°)of com pounds 1~3
2 Results and discussion
2.1Crystal structures of compounds 1~3
Single-crystal X-ray diffraction analysis reveal that 1,2 and 3 are isomorphous,so compound 1 is chosen to describe the crystal structure.Theminimum asymmetric unitof 1 is shown in Fig.1,from which we can see that TPTz was hydrolyzed in the coordination reaction,with two pyrazolyl substituted by oxygen atoms,while the remaining unhydrolyzed pyrazolyl coordinates with metal ions.Hydrolysis of triazine ligands was previously reported by Manzur et al.[18], where Cu2+can catalyze TPTz hydrolyzed into the same productaswegot inmethanolsystem,while Zhou et al.found that in methanol system TPTz hydrolyzed to 2,4, 6-trimethoxy-1,3,5-triazine catalyzed by Zn2+,elsewhere Hg2+can′t catalyze the hydrolysis process of TPTz[19].

Fig.1 ORTEP drawings of theminimum asymmetric unit of 1 with the thermal ellipsoids drawn at 50% probability
As shown in Fig.2,it is clear to see that the center Zn atom is six-coordinated by two N atoms from pyrazolyl,two N atoms from triazine ring and two O atoms from water,respectively.While two HL-ligands coordinate to one center metal forming a simple zero-dimensional compound.
PLATON simulation shows that there are plenty of hydrogen bonds in compound 1,as shown in Fig.3, H1Wiand H1Wiiformed hydrogen bonds with N3 and O2 atoms of the adjacent compound,respectively. While H6iiiand O1 formed intramolecular hydrogen bond in the complex.The hydrogen bond lengths and bond angles are listed in Table3.The hydrogen bond linked the simple zero-dimensional complex,and then extended to a two-dimensional layer system along the c-axis(Fig.4).As viewed along the b-axis orientation,the layer is isolated by an interval of 0.245 nm, without formation of any hydrogen bond.

Fig.2 Zero-dimensional complex formed by HL-and the metal ion
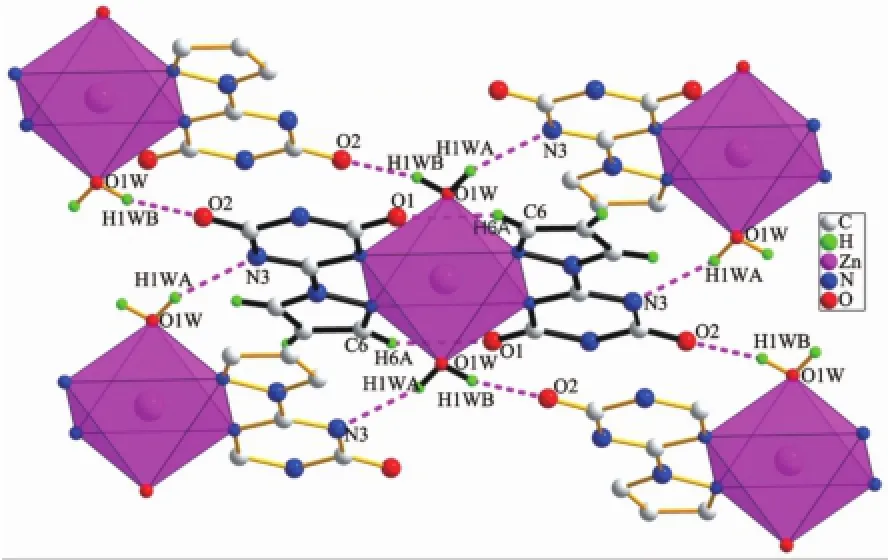
Fig.3 Hydrogen bonds formed in compound 1

Table3 Hydrogen bond lengths(nm)and bond angles(°)in com pound 1

Fig.4 Layers formed by hydrogen bonds viewed along the c-axis orientation(a)and b-axis orientation(b)
2.2PXRD and therm ogravimetric(TG)analysis The powder X-ray diffraction(PXRD)pattern calculated from the single-crystal structure data of compound 1 is in good agreementwith those observed for the as-synthesized compounds 1~3,as shown in Fig.5.

Fig.5 Simulated PXRD pattern of compound 1 and experimental patterns of 1~3
To study the thermal stability of compounds 1~3, thermogravimetric analyses(TGA)were carried out. As shown in Fig.6,compound 2 exhibits obviously weight loss at about 220℃,while it appears at 260℃for 1 and 3,which indicates that compounds 1 and 3 are much more stable than 2.This difference may be due to their different centermetal ions.From 260 to 325℃,there was a distinct platform in compound 1,with a weight loss of 7.75%corresponding to the loss of the coordinated water molecules(Calcd. 7.91%),which was not too clear in the curves of 2 and 3.After that,the continuous weight loss without any platform indicates that the ligand decomposed gradually as the temperature increasing.

Fig.6 TG curves of compounds 1~3
2.3UV-Vis DRS analysis and sem i-conductive properties
The solid UV-Vis DRS spectra(Fig.7a)clearly shows that themaximum absorption peaksofcompounds 1~3 locate at 280,290 and 320 nm,respectively. Besides,there was a broad absorption peak around 470 nm for compound 2 which attributed to the transition energy decrease ofπ-π*following the coordination bond formation between Co ion and electro-donating N atom[5b].

Fig.7 UV-Vis DRSspectra of compounds 1~3(a)and band gaps of 1~3(b)
The absorption peak falls sharply from 300 to 400 nm,which implies that the materials may have semiconductor properties.The band gap of material can be calculated from its UV-Vis DRS spectrum by Kubelka-Munk method[13]:(αhν)2=A(hν-Eg),where h is Planck constant;νis the frequency;αis the absorption coefficient;A is the proportionality constant;Egis the band gap.As shown in Fig.7b,the band gap of compound 1 is 3.80 eV,while that for 2 and 3 to be3.30 and 3.27 eV,respectively.In consideration that 1~3 are isomorphous,it is clear to see that semiconductor properties of these complexes formed from N-donor ligand have important relationship with the center metal ions which is different from the carboxylates.Although themechanism is still unclear, there is guiding significance for the synthesis of some novel coordination compounds based on N-donor ligandswith tunable semiconductor properties.
3 Conclusions
In summary,we have successfully synthesized three isomorphous coordination compounds[M(HL)2]· 2H2O(M=Zn,1;Co,2;Mn,3)with 6-(pyrezole-1-yl)-1,3,5-triazine-2,4-diol(H2L)hydrolyzed from TPTz.Two HL-coordinate toone centermetal ion forminga simple zero-dimensional complex,which was linked by the hydrogen bonds,and then extended to a twodimensional layer system.They are all wide band semiconductors with a band-gap of 3.80(Zn),3.30 (Co)and 3.27(Mn)eV,respectively.The result shows that the center metal ions have significant effect on their semiconductor properties,which has guiding significance for the synthesis of some novel coordination compounds based on N-donor ligands with tunable semiconductor properties.
References:
[1](a)EddaoudiM,Moler DB,LiH,etal.Acc.Chem.Res.,2001, 34:319-330 (b)Janiak C.Dalton Trans.,2003:2781-2804
[2](a)Ma S,Zhou H C.Chem.Commun.,2010,46:44-53
(b)Murray L J,Dinc M,Long JR.Chem.Soc.Rev.,2009,38: 1294-1314
(c)Sum ida K,Rogow D L,Mason JA,etal.Chem.Rev.,2012, 112:724-781
[3]Kreno L E,Leong K,Farha O K,et al.Chem.Rev.,2012, 112:1105-1125
[4](a)SatoO,Tao J,Zhang Y Z,etal.Angew.Chem.Int.Ed.,2007, 46:2152-2187
(b)Kurmoo M.Chem.Soc.Rev.,2009,38:1353-1379
[5](a)Allendorf M D,Bauer CA,Bhakta R K,et al.Chem.Soc. Rev.,2009,38:1330-1352
(b)Cui Y,Yue Y,Qian G,et al.Chem.Rev.,2012,112:1126-1162
[6](a)Silva C G,Corma A,García H.J.Mater.Chem.,2010,20: 3141-3156
(b)Halder S,Layek A,Ghosh K,et al.Dalton Trans.,2015, 44:16149-16155
[7]Kuc A,Enyashin A,Seifert G.J.Phys.Chem.B,2007,111: 8179-8186
[8](a)Holman K T,Pivovar A M,Swift J A,et al.Acc.Chem. Res.,2001,34:107-118
(b)Makowski S J,Kstler P,Schnick W.Chem.Eur.J.,2012, 18:3248-3257
[9](a)Dalrymple SA,Shimizu G K H.J.Am.Chem.Soc.,2007, 129:12114-12116
(b)Yang W,Greenaway A,Lin X,et al.J.Am.Chem.Soc., 2010,132:14457-14469
(c)Luo X Z,Jia X J,Deng JH,et al.J.Am.Chem.Soc., 2013,135:11684-11687
(d)LüJ,Perez-Krap C,Suyetin M,et al.J.Am.Chem.Soc., 2014,136:12828-12831
(e)Natarajan R,Bridgland L,Sirikulkajorn A,et al.J.Am. Chem.Soc.,2013,135:16912-16925
(f)Tian J,Thallapally PK,Dalgarno S J,et al.J.Am.Chem. Soc.,2009,131:13216-13217
(g)Thallapally P K,McGrail B P,Atwood J L,et al.Chem. Mater.,2007,19:3355-3357
(h)MastalerzM,Oppel IM.Angew.Chem.Int.Ed.,2012,51: 5252-5255
(i)Dalapati S,Saha R,Jana S,et al.Angew.Chem.Int.Ed., 2012,51:12534-12537
(j)Hisaki I,Nakagawa S,Tohnai N,et al.Angew.Chem.Int. Ed.,2015,54:3008-3012
(k)Soldatov D V,Moudrakovski IL,Ripmeester JA.Angew. Chem.Int.Ed.,2004,43:6308-6311
(l)A fonso R V,Duro J,Mendes A,et al.Angew.Chem.Int. Ed.,2010,49:3034-3036
(m)Wang H,Li B,Wu H,et al.J.Am.Chem.Soc.,2015, 137:9963-9970
(n)He Y,Xiang S,Chen B.J.Am.Chem.Soc.,2011,133: 14570-14573
(o)Li P,He Y,Zhao Y,et al.Angew.Chem.Int.Ed.,2015, 54:574-577
(p)Li P,He Y,Arman H D,etal.Chem.Commun.,2014,50: 13081-13084
(q)Li P,He Y,Guang J,et al.J.Am.Chem.Soc.,2014,136: 547-549
(r)Yang W,Li B,Wang H,et al.Cryst.Growth Des.,2015, 15:2000-2004
[10](a)Wu C,Lu C,Yang W,et al.Eur.J.Inorg.Chem.,2002:797-800 (b)Hou G,Bi L,Li B,et al.Inorg.Chem.,2010,49:6474-6483
(c)Hou Y,Song J,Bai F,et al.Inorg.Chim.Acta,2016,440: 69-76
(d)Navarro A,Paz Fernandez-Liencres M,Garcia G,et al. Phys.Chem.Chem.Phys.,2015,17:605-618
(e)Sun T,Jiao C,LiW,etal.RSCAdv.,2015,5:26410-26419
(f)Troyano J,Perles J,Amo-Ochoa P,et al.CrystEngComm, 2016,18:1809-1817
[11]Baburin I A,Blato V A,Carlucci L,et al.CrystEngComm, 2008,10:1822-1838
[12]Gascon J,Hernández-Alonso M D,Almeida A R,et al. ChemSusChem,2008,1:981-983
[13](a)Yang L M,Ravindran P,Vajeeston P,et al.RSC Adv., 2012,2:1618-1631
(b)Yang L M,Vajeeston P,Ravindran P,et al.Phys.Chem. Chem.Phys.,2011,13:10191-10203
(c)Yang L M,Ravindran P,Vajeeston P,et al.Phys.Chem. Chem.Phys.,2012,14:4713-4723
(d)Yang L M,Ravindran P,Vajeeston P,et al.J.Mater. Chem.,2012,22:16324-16335
[14]Lin C K,Zhao D,Gao W Y,et al.Inorg.Chem.,2012,51: 9039-9044
[15]Azarifar D,Zolfigol M A,Forghaniha A.Heterocycles,2004, 63:1897-1901
[16]SMARTand SADABS,Bruker AXS Inc.,Madison,Wisconsin, USA,1997.
[17]Sheldrick G M.SHELXS-97,Program for X-ray Crystal Structure Solution,University of Göttingen,Germany,1997.
[18]Manzur J,Acua C,Vega A,et al.Inorg.Chim.Acta,2011, 374:637-642
[19]Zhou H P,Gan X P,Li X L,et al.Cryst.Growth Des.,2010, 10:1767-1776
Three Hydrogen-Bonded M etal-Organic Networksw ith Tunable Sem iconductor Properties
CHEN Fei-Jian LIN Qing-Fang WANG Tian-Yan SHEN Fu-Zhi WEIZheng-You LIANG Li-Li*
(Department of Chemistry,Bengbu Medical College,Bengbu,Anhui233030,China)
Solvothermal reacition of 2,4,6-tris(pyrezole-1-yl)-1,3,5-s-triazine(TPTz)and three metal ions resulted in the formation of three hydrogen-bonded metal-organic networks.It′s found that 6-(pyrezole-1-yl)-1,3,5-triazine-2,4-diol(H2L)hydrolyzed from TPTz acts as the ligand in reality.Single-crystal structure analysis shows that the three compounds are isomorphouswith the formula of[M(HL)2]·2H2O(M=Zn,1;Co,2;Mn,3).The centermetal ion is six-coordinated by two N atoms from pyrazolyl,two N atoms from triazine ring and two O atoms from waters,respectively.Two HL-coordinate to one center metal ion forming a zero-dimensional metal-organic molecular,which was linked by the hydrogen bond,and then extended to a two-dimensional layer network.UVVis DRS analysis shows that they are allwide band semiconductorswith a band-gap of 3.80(Zn),3.30(Co),3.27 (Mn)eV,respectively,showing an obvious dependence on their centermetal ions.CCDC:904985,1;904986,2; 904987,3.
metal-organic networks;hydrogen-bond;2,4,6-tris(pyrezole-1-yl)-1,3,5-s-triazine
O614.24+1;O614.81+2;O614.71+1
A
1001-4861(2016)07-1275-08
10.11862/CJIC.2016.153
2016-02-21。收修改稿日期:2016-05-26。
安徽省高校自然科学研究重点项目(No.KJ2016A462)和蚌埠医学院自然科学基金(No.BYKC1401ZD,BYKY1433)资助。
*通信联系人。E-mail:liangjyt@163.com;会员登记号:S06N3381M1508。
