3,4-二甲基吡咯-2-甲酰肼缩PMBP的镧系(Ce、Pr、Nd)配合物的合成、结构及荧光性质
2016-12-05常慧琴吴伟娜
常慧琴 陈 亮 吴伟娜*, 王 元
(1河南理工大学化学化工学院,焦作454000) (2河南理工大学物理与电子信息学院,焦作454000)
3,4-二甲基吡咯-2-甲酰肼缩PMBP的镧系(Ce、Pr、Nd)配合物的合成、结构及荧光性质
常慧琴1陈亮*,2吴伟娜*,1王元1
(1河南理工大学化学化工学院,焦作454000) (2河南理工大学物理与电子信息学院,焦作454000)
合成并通过单晶衍射表征了3个稀土配合物[LnL(NO3)3(H2O)][LnL(NO3)3(CH3CN)]·2CH3CN(Ln=Ce(1),Pr(2),Nd(3),L=3,4-二甲基吡咯-2-甲酰肼缩PMBP)。3个配合物同构,属于单斜晶系,P21/n空间群,晶胞参数分别为:1:a=1.758 83(9)nm,b= 1.460 61(8)nm,c=2.626 28(14)nm,β=97.800 0(10)°;2:a=1.756 39(15)nm,b=1.459 97(11)nm,c=2.623 1(2)nm,β=97.771(2)°;3:a=1.758 7(2)nm,b=1.463 87(17)nm,c=2.626 3(3)nm,β=97.760(2)°。每个配合物包含2个不同的中性配合物分子。配合物中每个十配位的Ln(Ⅱ)离子与来自酰腙配体L的2个氧原子和1个氮原子,3个双齿配位硝酸根及1个溶剂分子配位,采取双帽四方反棱柱配位构型。乙腈溶液中,配合物1~3具有与配体完全不同的荧光发射,可能由配位荧光增强效应导致。
吡咯;酰腙;稀土配合物;荧光;晶体结构
Recently,Schiff base complexes have drawn attention in biochemistry and biomedicine because of their unique properties[1-3].Among them,the hydrazone derivatives of 1-phenyl-3-methyl-4-benzoyl-5-pyrazolone(PMBP)and their metal complexes have been widely investigated due to their high biological and pharmaceuticalactivities[4-8].In particular,the transition metal complexes of the hydrazone ligand L(Scheme 1),which is derived from 3,4-dimethylpyrrole-2-carbohydrazide and PMBP,have been reported to show excellent antioxidative activities[7].However,to the bestof our knowledge,studies on the lanthanidemetal complexesofPMBPhydrazone derivativesare relatively few[4,8].Therefore,three lanthanide complexeswith the acylhydrazone ligand L have been synthesized and structuraldetermined bysingle-crystalX-raydiffraction. In addition,the fluorescencepropertiesofallcompounds in CH3CN solution were discussed in detail.

Scheme 1 Synthesis route of the ligand L
1 Experimental
1.1Materials and measurements
Solvents and startingmaterials for synthesis were purchased commercially and used as received. Elemental analysis was carried out on an Elemental Vario EL analyzer.The IR spectra(ν=4 000~400 cm-1) were determined by the KBr pressed disc method on a Bruker V70 FT-IR spectrophotometer.The UV spectra were recorded on a Purkinje General TU-1800 spectrophotometer.Fluorescence spectra were determined on a Varian CARY Eclipse spectrophotometer,in the measurements of emission and excitation spectra the passwidth is 5 nm.
1.2Preparations of complexes 1~3
The acylhydrazone ligand L was synthesized according to the literature method[7].The complexes 1~3 were generated by reaction of L(5mmol)with equimolar of corresponding lanthanide nitrates in acetonitrile solution(10 mL).Crystals suitable for X-ray diffraction analysis were obtained by evaporating the reaction solutions at room temperature.
1:Colorless blocks.Anal.Calcd.for C54H57N19O23Ce2(%):C,40.03;H,3.55;N,16.42.Found(%):C, 39.89;H,3.69;N,16.30.FT-IR(cm-1):ν(O-H)3 431, ν(C≡N)2 302,ν(C=O,hydrazone)1 622,ν(C=O, pyrazolone)1600,ν(C=N)1 544,ν1(NO3)1 500,ν4(NO3) 1 282,ρ(O-H)888.
2:Colorlessblocks.Anal.Calcd.forC54H57N19O23Pr2(%):C,39.99;H,3.54;N,16.41.Found(%):C,39.78; H,3.71;N,16.22.FT-IR(cm-1):ν(O-H)3 436,ν(C≡N)2 301,ν(C=O)1 622,ν(C=O,pyrazolone)1 600, ν(C=N)1 543,ν1(NO3)1 501,ν4(NO3)1 282,ρ(O-H) 887.
3:Colorless blocks.Anal.Calcd.for C54H57N19O23Nd2(%):C,39.82;H,3.53;N,16.34.Found(%):C, 39.69;H,3.79;N,16.19.FT-IR(cm-1):ν(O-H)3 432, ν(C≡N)2 301,ν(C=O)1 624,ν(C=O,pyrazolone) 1 600,ν(C=N)1 542,ν1(NO3)1 500,ν4(NO3)1 284, ρ(O-H)887.
1.3X-ray crystallography
The X-ray diffraction measurement for complexes 1~3 was performed on a Bruker SMART APEXⅡCCD diffractometer equipped with a graphite monochromatized Mo Kαradiation(λ=0.071 073 nm) by usingφ-ωscan mode.Semi-empirical absorption correction was applied to the intensity data using the SADABS program[9].The structures were solved by direct methods and refined by fullmatrixleast-square on F2using the SHELXTL-97 program[10].All non-hydrogen atoms were refined anisotropically.The H atoms for watermolecules are located from difference Fouriermap and refined with restraints in bond length and thermal parameters.All the other H atoms were positioned geometrically and refined using a riding model.Details of the crystalparameters,data collection and refinements for complexes 1~3 are summarized in Table1.
CCDC:1439568,1;1439569,2;1439570,3.
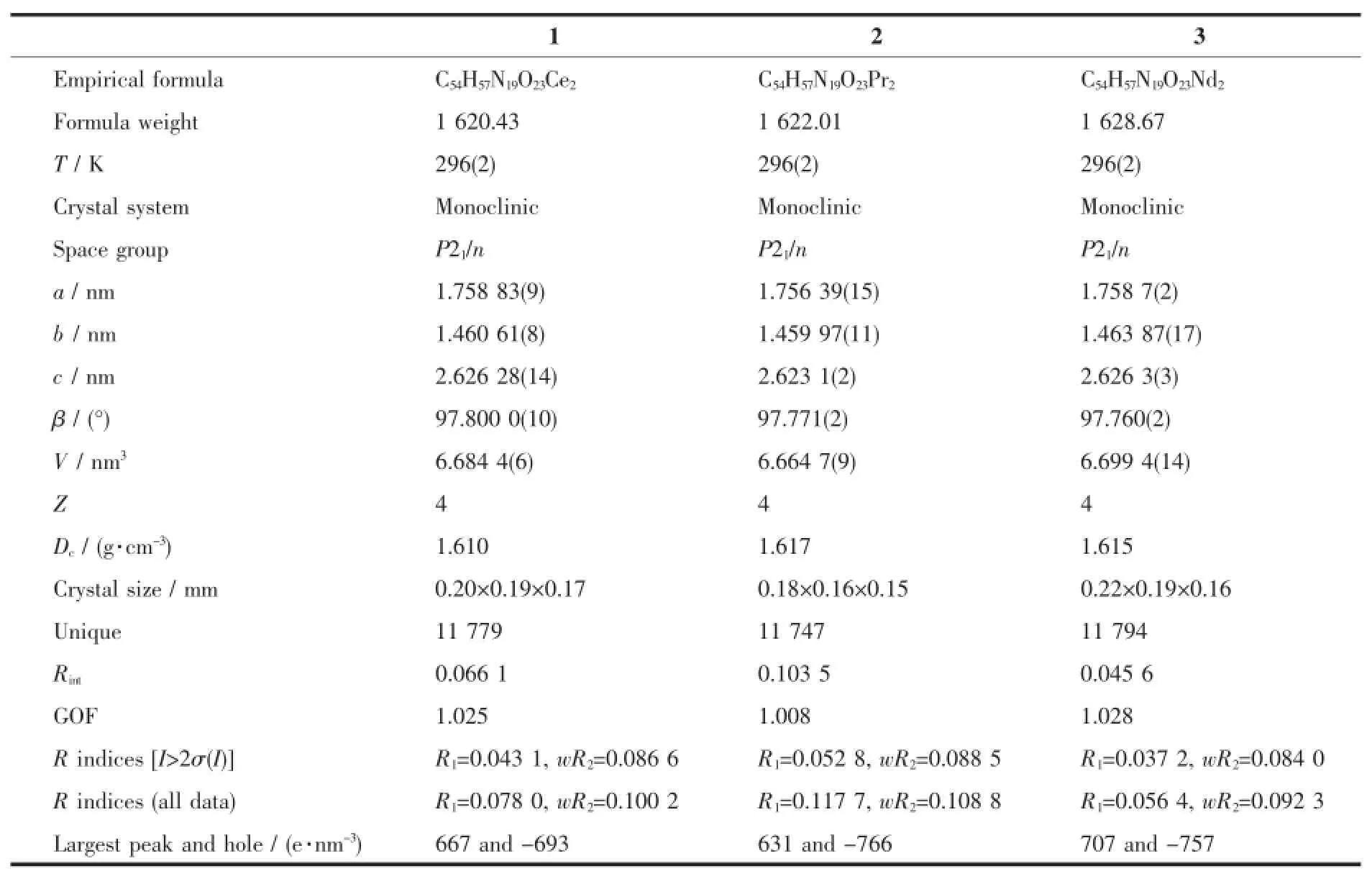
Table1 Selected crystallographic data for com p lexes 1~3
2 Results and discussion
2.1Crystal structures of the complexes
All three complexes are isostructural(Fig.1a~c) and crystallize in the monoclinic,space group P21/n. Thus,the complex 1 is discussed in detail for an example.As shown in Fig.1a,the asymmetric unit of 1 contains two kinds of independent neutral complex molecules with similar structures,together with two lattice CH3CNmolecules.Each Ce(Ⅱ)ion in complex 1 is ten-coordinated and surrounded by one tridentate ligand L with NO2donor set,three bidentate nitrate anions and one coordinated solventmolecule(CH3CN for Ce1,while water for Ce2),thus giving a bicapped square antiprism coordination geometry(Fig.1d).The Ce-O/N bond lengths are in the range of 0.236 0(3)~0.274 4(4)nm(Table2),comparable to the Ce(Ⅱ) complexeswith similar donor sets[11].In complexes 1~3,the Ln-O/N bond lengths decrease gradually with the increase of the atomic number of Ln(Ⅱ)ions, which is consistent with the effect of lanthanide contraction.In the crystal of each complex(Fig.1e,1 for example),intermolecular O-H…O(O23-H23A…O6i),O-H…N(O23-H23B…N18)and N-H…O(N6-H6…O5i,N1-H1…O22ii,N9-H9…O12iiiand N4-H4…O14)hydrogen bonds are helpful to construct the 2D supramolecular network parallel to(110)plane (Symmetry codes:i-x+1/2,y-1/2,-z+1/2;ii-x+1/2, y+1/2,-z+1/2;iiix+1,y,z).
2.2IR spectra
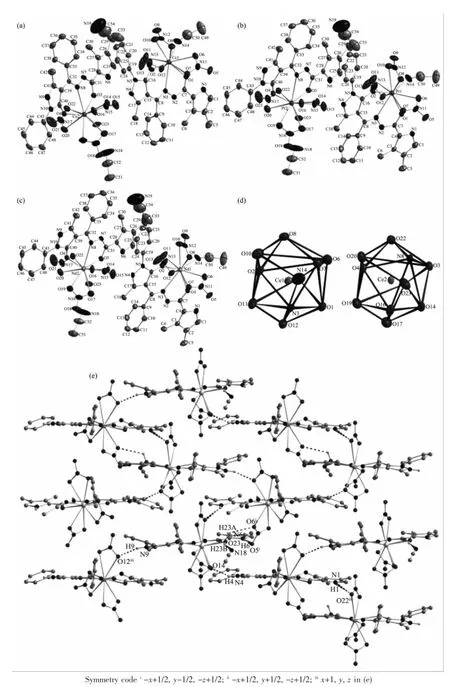
Fig.1 Molecular structures of comp lexes 1~3(a~c)shown with 30%probability displacement ellipsoids;(d)Coordination geometry of the center Ce(Ⅱ)ion in complex 1 with atoms shown with 30%probability displacementellipsoids; (e)Extended 2D supramolecular network parallel to(110)plane formed by hydrogen bonds in the crystal of 1

Table2 Selected bond lengths(nm)in com p lexes 1~3
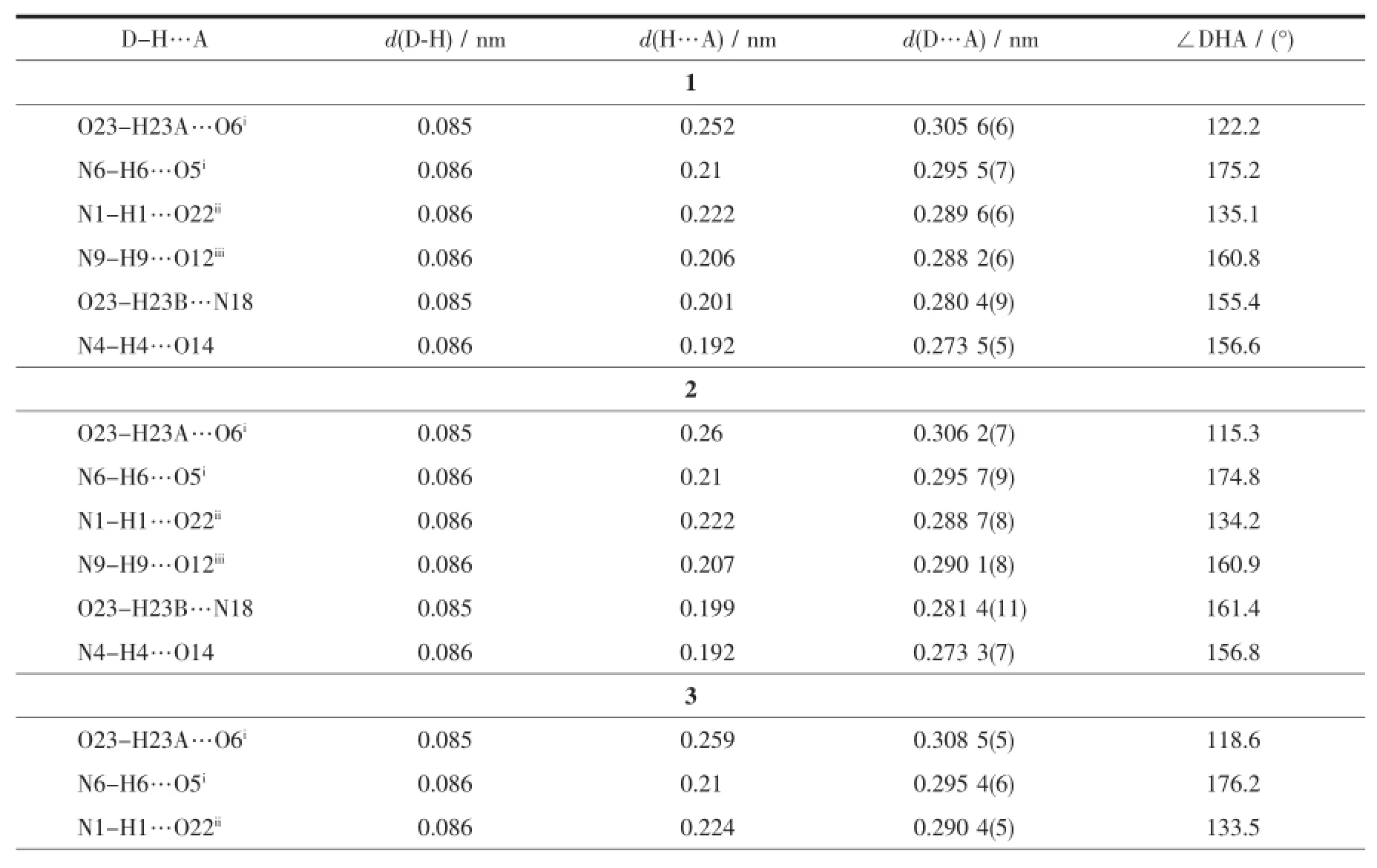
Table3 Hydrogen bonds information in com p lexes 1~3
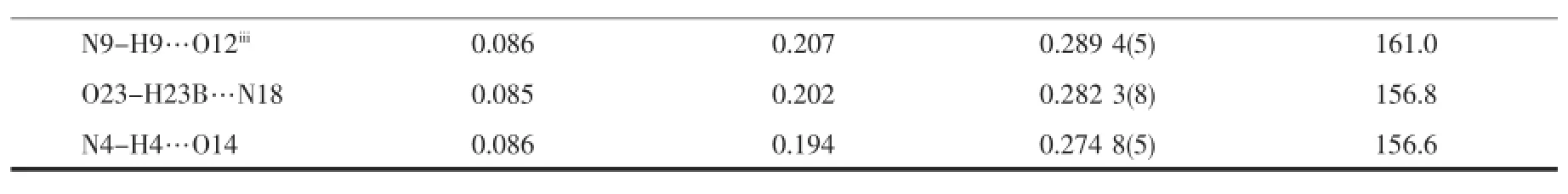
Continued Table3
The spectral regions for all the complexes are more or less similar due to the similarity in coordination modes of the ligand with the metal centre.The free ligand L exhibit three absorption bands at 1 632,1 606 and 1 585 cm-1,assigned to ν(C=O,hydrazone),ν(C=O,pyrazolone)andν(C=N) respectively[3,7].However,in the complexes,such bands shift to lower frequency,indicating that the oxygen atoms of both carbonyl groups and imine nitrogen atom take part in coordination to the central Ln(Ⅱ)ion[2-3].The peak atabout2 300 cm-1of three complexes should be assigned to theν(C≡N)vibration,clearly showing the existence of CH3CNmolecules.Theν(O-H) bands atabout3 430 cm-1andmedium intensity bands atabout880 cm-1show that thereare some coordinative watermolecules in all complexes[12].Additionally,the general pattern of the IR spectroscopy supports the normal coordination of the bidentate nitrate group[13].It is in accordance with the X-ray diffraction analysis result.
2.3UV spectra
The UV spectra of the ligand L and complexes 1~3 in CH3CN solution(Concentration:1×10-5mol·L-1) were measured at room temperature(Fig.2).The spectra of free ligand L features two main bands located around 304(ε=69 415 L·mol-1·cm-1)and 358 nm(ε=46 255 L·mol-1·cm-1),which could be assigned to characteristicπ-π*transitions centered on pyrrole ring and imine unit,respectively[3,13].Similar bonds can be observed in the spectra of 1(308(ε=171 806 L·mol-1·cm-1)and 369(ε=253 409 L·mol-1·cm-1) nm),2(309(ε=150 562 L·mol-1·cm-1)and 369(ε= 240 918 L·mol-1·cm-1)nm)and 3(309(ε=90 659 L· mol-1·cm-1)and 369(ε=190 224 L·mol-1·cm-1)nm), respectively.However,the hyperchromicities and red shifts indicate that the ligand L takes part in the coordination in all complexes.
2.4Fluorescence spectra
The fluorescence spectra of the ligand L and complexes 1~3 have been studied in CH3CN solution (Concentration:1×10-5mol·L-1)at room temperature. The results show that the emission spectra of the ligand L exhibitonly one broad peak at 475 nm when excited at 380 nm,while the three complexes show one main peak at 415 nm when excited at 335 nm (Fig.3).Obviously,the fluorescence intensity of the complex ismuch higher than that of the ligand L.The behavior of Ln(Ⅱ)ion coordinated to the ligand is regarded as that of emissive species resulted from a CHEF(chelation enhancement of the fluorescence emission)effect[14].

Fig.2 UV spectra of the ligand L(a),1(b),2(c)and 3(d)in CH3CN solution at room temperature

Fig.3 Fluorescence emission spectra of the ligand L(a), 1(b),2(c)and 3(d)in CH3CN solution at room temperature
References:
[1]Jia Y,Li J.Chem.Rev.,2015,115:1597-1621
[2]CHEN Yan-Min(陈延民),XIE Qin-Fan(解庆范),LIU Jin-Hua(刘金花),et al.Chinese J.Inorg.Chem.(无机化学学报),2015,31:74-80
[3]LIXiao-Jing(李晓静),CAIHong-Xin(蔡红新),WUWei-Na (吴伟娜),et al.Chinese J.Inorg.Chem.(无机化学学报), 2015,31:1661-1666
[4]Yang Z Y,Wang B D,Li Y H.J.Organomet.Chem.,2006, 691:4159-4166
[5]Li Y,Zhao J,He C C,et al.J.Inorg.Biochem.,2015,150: 28-37
[6]Yang Y,Zhang L,Liu L,et al.Inorg.Chim.Acta,2007,360: 2638-2646
[7]Wang Q,Wang Y,Yang Z Y.Chem.Pharm.Bull.,2008,56: 1018-1021
[8]Yang Z Y,Yang R D,Li F S,et al.Polyhedron,2000,19: 2599-2604
[9]Sheldrick G M.SADABS,University of Göttingen,Germany, 1996.
[10]Sheldrick G M.SHELX-97,Program for the Solution and the Refinementof Crystal Structures,University ofGöttingen, Germany,1997.
[11]ZHANG Zhao-Po(张照坡),WANG Yuan(王元),WUWei-Na (吴伟娜),et al.Chinese J.Inorg.Chem.(无机化学学报), 2013,29:2239-2244
[12]Wang Y,Yang Z Y.Transition Met.Chem.,2005,30:902-906
[13]Song X Q,Zang Z P,Liu W S,et al.J.Solid State Chem., 2009,182:841-848
[14]Vicente M,Bastida R,Lodeiro C,et al.Inorg.Chem.,2003, 42:6768-6779
Lanthanide Com plexes(Ce,Pr,Nd)w ith an Acylhydrazone Derived from 3,4-Dimethylpyrrole-2-carbohydrazide and PMBP:Syntheses, Crystal structuresand Fluorescence Properties
CHANG Hui-Qin1CHEN Liang*,2WUWei-Na*,1WANG Yuan1
(1School of Chem istry and Chemical Engineering,Henan Polytechnic University,Jiaozuo,Henan 454000,China) (2School of Physics and Electronic Information Engineering,Henan Polytechnic University,Jiaozuo,Henan 454000,China)
Three lanthanide(Ⅱ)complexes,[LnL(NO3)3(H2O)][LnL(NO3)3(CH3CN)]·2CH3CN(Ln=Ce(1),Pr(2)and Nd(3))based on L(L is derived from 3,4-dimethylpyrrole-2-carbohydrazide and PMBP)have been synthesized and characterized by elemental analyses,IR spectra and X-ray diffraction analyses.The results reveal that three complexesare isostructuraland crystallize in themonoclinic,spacegroup P21/n(a=1.758 83(9)nm,b=1.460 61(8) nm,c=2.626 28(14)nm,β=97.800 0(10)°for 1;a=1.756 39(15)nm,b=1.459 97(11)nm,c=2.623 1(2)nm,β= 97.771(2)°for 2;a=1.758 7(2)nm,b=1.463 87(17)nm,c=2.626 3(3)nm,β=97.760(2)°for 3).Each of them contains two kinds of independent neutral complex molecules.Concomitantly,each Ln(Ⅱ)ion is surrounded by one tridentate ligand L with NO2donor set,three bidentate nitrate anions and one coordination solventmolecule, thus giving a bicapped square antiprism coordination geometry.In addition,in CH3CN solution,complexes 1~3 exhibit different fluorescence emission from that of the ligand L probably due to a CHEF(chelation enhancement of the fluorescence emission)effect.CCDC:1439568,1;1439569,2;1439570,3.
pyrrole;hydrazone;lanthanide complex;fluorescence;crystal structure
O614.33+2;O614.33+4;O614.33+5
A
1001-4861(2016)07-1239-07
10.11862/CJIC.2016.146
2015-12-18。收修改稿日期:2016-04-19。
国家自然科学基金(No.21001040)和河南省教育厅自然科学基金(No.12B150011,14B150029)资助项目。
*通信联系人。E-mail:cl721@hpu.edu.cn,wuwn08@hpu.edu.cn;会员登记号:S06N6704M1112。
