两种1,2,3-三唑衍生物的配合物合成策略:晶体结构和表面分析
2016-12-05周士艳陈金梅左泽浩
冯 超 张 舵 周士艳 陈金梅 左泽浩 赵 红
(东南大学化学化工学院,南京211189)
两种1,2,3-三唑衍生物的配合物合成策略:晶体结构和表面分析
冯超张舵周士艳陈金梅左泽浩赵红*
(东南大学化学化工学院,南京211189)
在不同反应条件下反应得到了两种1,2,3-三唑衍生物的配合物[Co(H2O)6][Co(L1)3]2·4H2O(1)和Cu(L2)2(2)(HL1=5-methyl-1-phenyl-1H-1,2,3-triazole-4-carboxylic acid;HL2=1-(4-iodophenyl)-5-methyl-1H-1,2,3-triazole-4-carboxylic acid)。通过X射线单晶衍射和红外光谱确定了晶体结构,同时对配合物1和2进行了表面作用分析(Hirshfeld surface analysis),在二维指纹图谱中可以清楚的看到配合物中的主要分子间作用。
1H-1,2,3-三唑衍生物;晶体结构;表面分析;二维指纹区
0 Introduction
The construction of coordination compounds with intriguing structuralmotifsand functionalbehaviorshas attracted considerable attention for chemists[1-5].During the past years,a substantial amount ofwork based on coordination assembled systems has emerged,which enrich the inorganic-organic hybrid materials with novel networks and properties[6-11].From the standpoint of synthetic methodology,the access to such multicomponent supramolecular systems mainly depends upon the selection of appropriate chemical building blocks.Meanwhile,1-substituted-1,2,3-triazole-4-carboxylic acid have played as good building blocks in constructing supramolecular architectures[12-14],and our group have chosen a pyridyl conjugated 1,2,3-triazole ligand 5-methyl-1-(pyridine-3-yl)-1H-1,2,3-triazole-4-carboxylic acid asorganic ligand and resulted series ofnew coordination polymers previously[15].Due to the flexible nature of the carboxyl group,1-substituted-1,2,3-triazole-4-carboxylic acid ligands may exhibit kinds of conformations in different complexes to meet the requirements of coordination geometry.Besides, the self-assembly processes are usually influenced by many other factors such as the coordination geometry of metal ion,temperature,counter anion,pH value, and solventmedium[16-20].
As our ongoing task,studying the influences of substituent triazole ligands,herein,we synthesized two kinds of 1-substituted-1,2,3-triazole-4-carboxylic acid according to different design concepts.Firstly,the phenyl was introduced to the 1-position of triazole acid,and 5-methyl-1-phenyl-1H-1,2,3-triazole-4-carboxylic acid(HL1)was obtained.In order to investigate the influence of the electron-withdrawing group on coordination ability,the iodo-group was decorated to the benzene ring,which resulted in 1-(4-iodophenyl)-5-methyl-1H-1,2,3-triazole-4-carboxylic acid(HL2).We also synthesized two new complexes [Co(H2O)6][Co(L1)3]2·4H2O(1),Cu(L2)2(2).For the purpose of investigating the intermolecular interactions of complexes,we also study the Hirshfeld surface analysis for 1 and 2.

Scheme 1 1H-1,2,3-triazole acid derivatives
1 Experimental
1.1Materials and measurements
All reagents and solvents were obtained from commercial sources and used without further purification.The ligandswere synthesized according to literatures[21].Elemental analyses of carbon,hydrogen, and nitrogen were carried out with a Perkin-Elmer 240Celementalanalyzer.IR spectrawereobtained with KBr pellets from 4 000 to400 cm-1usinga Nicolet5700 spectrophotometer.Crystal structures were determined with Rigaku SCXminidiffractometer.
1.2Preparations
1.2.1[Co(H2O)6][Co(L1)3]2·4H2O(1)
A solution of CoCl2·6H2O(0.023 8 g,0.1 mmol) in water(8 m L)was added into a solution of HL1(0.040 6 g,0.2 mmol)in anhydrous ethanol(8 mL), then the mixture was left at room temperature.After one week,red block crystals were obtained and dried in air.Yield:42%(based on CoCl2·6H2O).Anal. Calcd.for C60H68Co3N18O22(%):C,45.86;H,4.33;N, 16.05.Found(%):C,45.78;H,4.41;N,16.01.IR (KBr,cm-1):3 440(w),1 618(s),1 600(vs),1 582 (s),1 563(m),1 485(m),1 403(w),1 365(m),1 323 (s),1 300(m),1 241(m),1 133(m),830(m),766(m).
1.2.2 Cu(L2)2(2)
The synthesis of complex 2 is similar with 1,just using CuCl2·2H2O(0.017 2 g,0.1 mmol)and HL2(0.065 6 g,0.2mmol)instead of CoCl2·6H2O and HL1. Yield:46%(based on CuCl2·2H2O).Anal.Calcd.for C40H28Cu2I2N12O8(%):C,33.35;H,1.95;N,11.67. Found(%):C,33.12;H,2.02;N,11.71.IR(KBr; cm-1):3 440(w),1 621(s),1 605(s),1 492(m),1 421 (s),1 370(m),1 303(s),1 309(m),1 240(m),1 136(m), 1 018(w),830(m),and 755(m).
1.3Crystal structure determ ination
Single-crystal X-ray diffraction measurements for complexes were carried out using a Rigaku SCX mini diffractometer with Mo Kαradiation(λ=0.071 073 nm).The crystal size is 0.23 mm×0.21 mm×0.20 mmfor 1 and 0.25mm×0.23mm×0.20 mm for 2.The structure was solved by directmethodswith SHELXS-97 and refined by full-matrix least-squares on F2with SHELXL-97[22].All non-hydrogen atoms were refined with anisotropic displacement parameters.Hydrogen atoms were added theoretically and refined with riding model and fixed isotropic thermal parameters.The crystallographic parameters and structural determination of 1 and 2 are summarized in Table1. Selected bond lengths and bond angles of 1 and 2 are listed in Table2.
CCDC:1400174,1;1400175,2.

Table1 C rystallographic and refinem ent data for 1 and 2

Table2 Selected bond lengths(nm)and angles(°)for 1 and 2

Continued Table2
2.2Com putational details
Molecular Hirshfeld surface calculations were performed by using the CrystalExplorer program[23]. The principles of Hirshfeld surfaces were reported in the literature[23].When the cif files of 1 and 2 were read into the CrystalExplorer program for analysis,all bond lengths to hydrogen were automatically modified to typical standard neutron values(C-H 0.108 3 nm and N-H 0.100 9 nm).In this study,all the Hirshfeld surfaces were generated using a standard(high) surface resolution.The 2D fingerprint plots were displayed by using the standard 0.06~0.26 nm view with the deand didistance scales displayed on the graph axes.
2 Results and discussion
2.1Crystal structural description
X-ray single crystal diffraction confirms the complex 1 crystallizes in triclinic system with P1 space group.The asymmetric unit of 1 consists of a [Co(L1)3]-anion,half[Co(H2O)6]2+counter cation and two lattice H2O molecules as shown in Fig.1.The Co(Ⅱ)cation of the[Co(L1)3]-anion is coordinated to three HL1ligands.Each ligand coordinated to the Co(Ⅱ)cation adopts the coordination mode of bidentate chelating.The coordination geometry around the Co(Ⅱ)is best described as a distorted octahedral geometry. The[Co(H2O)6]2+unit in 1 plays as a counter ion to balance the charge on the[Co(L1)3]-anion.The Co2 is surrounded by six water molecules,with the distance of Co2-O from 0.205 6(3)to 0.210 2(3)nm.It gives a nearly standard octahedral geometry with water molecules on six vertexes.This similar counter ion is involved in many documents[24-25].It is surprisingly found that O atoms of[Co(H2O)6]2+are only electron donors,not acceptors.Hydrogen bonds of O7-H7B…O4i,O8-H8B…O3iiand O9-H9B···O1iii(Symmetry codes:i1-x,2-y,1-z,iix,y,-1+z,iii-x+1,-y+2, -z+1)between the discrete[Co(L1)3]-anions(A)and [Co(H2O)6]2+cations(B)form an[A-B-A]terminal. Each Crystallographic independent lattice water molecule(C)connected the[A-B-A]terminals into zigzag chains-[A-B-A]-[C]-[A-B-A]-through O-H…O hydrogen bonds(Fig.2,Table3),thus the supramolecular architecture of 1 is constructed.

Fig.1 Asymmetric unit of complex 1 with thermal ellipse at the 30%probability level

Table3 Geom etrical parameters of hydrogen bonds in 1
The crystal structure of 2 shows that each unit contains two(L2)-anions and one Cu.The local coordination geometry around Cu can be described as a slightly distorted quadrilateral(Fig.3).The Cu coordinates to two ligands with a Cu-O distance of 0.193 5(3)~0.196 0(3)nm and two triazole nitrogensof two(L2)-ligands in a trans fashion with Cu-N distance 0.196 6(3)~0.197 8(3)nm.The O-Cu1-O1 angle is 174.85(14)°,whereas the N-Cu-O angles around the Cu center range from 82.03(13)°to 97.43(13)°.In 2, the(L2)-is bidentate with one oxygen of carboxylate and one nitrogen of 1,2,3-triazole ring chelating to Cu, resulting in the formation of a stable five-numbered ring(Cu1-O1-C1-C2-N1)and it takes the coordination mode as bidentate chelating.In the crystal packing of 2,the coordinated O3 of carboxylate forms a strong contact(0.373 53(19)nm)with the iodine,resulting in a 1D zigzag chain,and adjacent chains formed into a 2D layer by C19…O2 contacts(Fig.4).In the 2D network,the Cu…Cu distances through intermolecular contacts are 1.239 1 and 1.354 4 nm,respectively.In addition,there also exists C-I…πstacking interactionswith a distance of 0.3858(2)nm.
2.2Hirshfeld surface analysis for 1 and 2

Fig.2 1D zigzag chain formed by hydrogen bonds

Fig.3 Molecular structure of 2 with thermal ellipse at the 30%probability level
In order to compare the interactions in the crystal structures of compounds 1 and 2,the Hirshfeld surface analysis and the two-dimensional(2D) fingerprint plots generated,based on the deand didistances(deand diare the distances from the Hirshfeld surface to the nearest atom outside and inside the surface,respectively),were carried out using CrystalExplorer 2.0.This analysis shows that in both compounds,the intermolecular H…H contacts have a major contribution to the crystal packing(Fig. 5).These contacts comprise 37.6%and 18.8%of the total Hirshfeld surfaces of molecules 1 and 2, respectively.
The shortest contacts of this type show up in the fingerprint plots as characteristic spikes.The structures are also dominated by O…H/H…O contacts which comprise 26.7%and 12.9%of total Hirshfeld surface areas as sharp antennas.Compound 1 occupiesmore proportion of C…H contacts(18.0%)than 2,which contains only 16.2%.The structure of 1 is also dominated by N…H/H…N contacts which comprise 10.6%of total Hirshfeld surface areas. Compared with 1,the contacts of I…H,I…O and I… N in 2 comprise 12.9%,6.4%and 5.2%,respectively.

Fig.4 2D layer constructed through intermolecular interactions
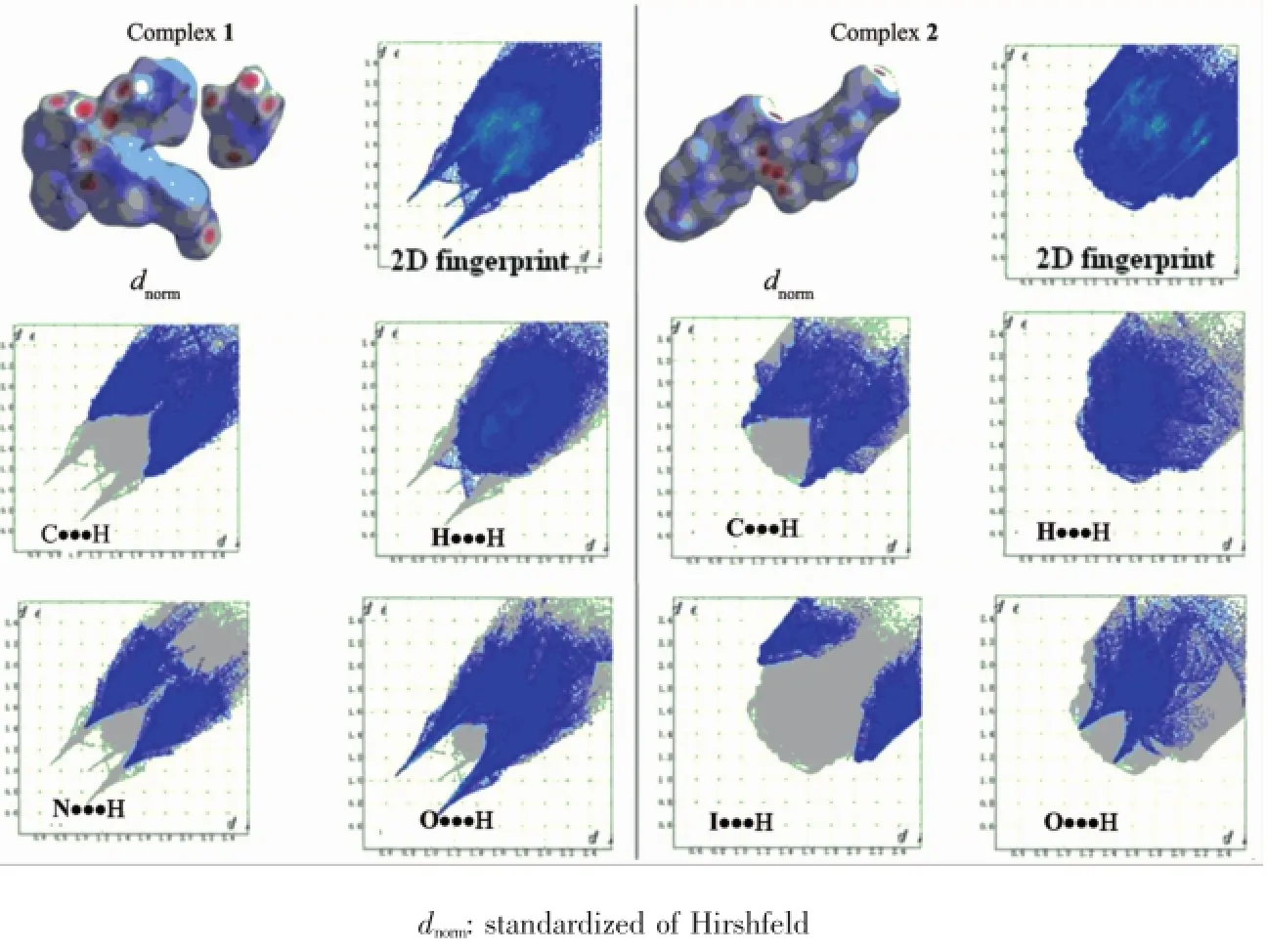
Fig.5 Hirshfeld surface and 2D fingerprint plots for 1 and 2

Fig.6 Hirshfeld surface calculations for complex 1 and 2
By comparison in Fig.6,compounds 1 and 2 have different cases about Hirshfeld surfaces.Two major differences are as follows.On the one hand,thedistributions of hydrogen bonds indicate that although both of 1 and 2 have H…H and O…H/H…O contacts,the interactionsmake bigger contributions to the whole Hirshfeld surface in 1.This may be raised from differentmolecular structures of both compounds, while 1 contains a counter cation and free water molecules,but 2 is very clear.On the other hand,2 has I…X(X=H,O,and N)interactions.The reason for this phenomenon is still the special conformation in crystal structure of 2.
This phenomenon indicates that through proper design principles,it can be possible to exploit the presumably weak interactions in the design of supramolecular architectures.The Hirshfeld surfaces certainly allow a much more detailed scrutiny by displaying all the intermolecular interactions within the crystal and this methodology has very important promise in crystal engineering.
3 Conclusions
In summary,we have synthesized and structurally characterized two complexes with 1,2,3-triazole derivatives by rational designing.We further investigated the Hirshfeld surfaceofcomplexes 1 and 2, and surprisingly find that the main intermolecular interactions in the two complexes are O…H and H…H contacts.Moreover,higher dimensional supramolecular networks can further be achieved via hydrogen bonds and intermolecular close packing interactions. Accordingly,these resultsmay offer new insights into the design and assembly of such supramolecular crystals,and we believe that it could make a contribution to the strategy ofcrystalengineering.
Acknow ledg ments:We gratefully acknowledge the financial support of the Fundamental Research Funds for Central Universities(Grant No.3207045420)and the financial support from Jiangsu Ainaji Neoenergy Science&Technology Co.,Ltd(Grant No.8507040091).
References:
[1]Zhu Q L,Xu Q.Chem.Soc.Rev.,2014,43(16):5468-5512
[2]Du M,Chen M,Wang X,et al.Inorg.Chem.,2014,53(14): 7074-7076
[3]Mondal SS,Bhunia A,Kelling A,et al.J.Am.Chem.Soc., 2014,136(1):44-47
[4]Suh M P,Park H J,Prasad T K,et al.Chem.Rev., 2012,112(2),782-835
[5]Cui Y,Yue Y,Qian G,et al.Chem.Rev.,2012,112(2): 1126-1162
[6]EddaoudiM,Sava D F,Eubank JF,et al.Chem.Soc.Rev., 2015,44(1):228-249
[7]DVries R F,Iglesias M,Snejko N,et al.Inorg.Chem.,2012, 51(21):11349-11355
[8]Du M,Li C P,Chen M,et al.J.Am.Chem.Soc.,2014,136 (31):10906-10909
[9]Wang C,Liu D,Lin W.J.Am.Chem.Soc.,2013,135(36): 13222-13234
[10]Horike S,Umeyama D,Kitagawa S.Acc.Chem.Res.,2013, 46(11):2376-2384
[11]You W,Guo JH,Li C P,et al.Polyhedron,2015,91:104-109
[12]Zhao H,Zhou SY,Feng C,et al.Inorg.Chim.Acta,2014, 421:169-175
[13]Zhou S Y,Qu Z R,Ma H J,et al.J.Inorg.Organomet. Polym.,2014,24(3):656-663
[14]Feng C,Gao G Y,Qu Z R,et al.J.Inorg.Organomet. Polym.,2015,25(5):1233-1238
[15]Hong JL,Qu ZR,Ma H J,et al.Bull.Korean Chem.Soc., 2014,35(5):1495-1500
[16]Wang X Y,Wang L,Wang Z M,et al.J.Am.Chem.Soc., 2006,128(3):674-675
[17]Khavasi H R,Sadegh B M M.Inorg.Chem.,2010,49(12): 5356-5358
[18]Fang S M,Zhang Q,Hu M,et al.Inorg.Chem.,2010,49 (20):9617-9626
[19]Long LS.CrystEngComm,2010,12(5):1354-1365
[20]Chen S C,Zhang Z H,Huang K L,et al.Cryst.Growth Des.,2008,8(9):3437-3445
[21](a)Zhao H,Chen JM,Lin J R,et al.J.Coord.Chem., 2011,64(15):2735-2745
(b)Wang G G,Zhao H.Acta Crystallogr.Sect.E,2010,E66: o3001
[22]Sheldrick G M.SHELXL-97,Program for X-ray Crystal Structure Refinement,University of Göttingen,Göttingen, Germany,1997.
[23]Wolff S K,Grimwood D J,McKinnon J J,et al. CrystalExplorer 2.0,University ofWestern Australia,Perth, Australia,2007.
[24]Worl S,Hellwinkel D,Pritzkow H,et al.Dalton Trans., 2004:2750-2757
[25]Shiu K B,Yen C H,Liao F L,et al.Acta Crystallogr.Sect. E,2004,E60:m35
A Synthetic Strategy for Two Com p lexesw ith 1,2,3-Triazole Derivatives:Crystal Structures and Hirshfeld Surface Analysis
FENG Chao ZHANG Duo ZHOU Shi-Yan CHEN Jin-Mei ZUO Ze-Hao ZHAO Hong*
(School of Chemistry and Chemical Engineering,Southeast University,Nanjing 211189,China)
Two new complexes[Co(H2O)6][Co(L1)3]2·4H2O(1)and Cu(L2)2(2)were synthesized by 1H-1,2,3-triazole acid derivatives under various reaction conditions(HL1=5-methyl-1-phenyl-1H-1,2,3-triazole-4-carboxylic acid; HL2=1-(4-iodophenyl)-5-methyl-1H-1,2,3-triazole-4-carboxylic acid).The crystal structures have been established by single-crystal X-ray diffraction,and characterized by FTIR.Hirshfeld surface analysis for complexes 1 and 2 indicate that the 2D fingerprint plots obviously show the main intermolecular interactions in both complexes. CCDC:1400174,1;1400175,2.
1H-1,2,3-triazole acid derivatives;crystal structure;Hirshfeld surface analysis;2D fingerprint plots
O614.81+2;O614.121
A
1001-4861(2016)07-1215-08
10.11862/CJIC.2016.147
2015-12-12。收修改稿日期:2016-05-02。
*通信联系人。E-mail:zhaohong@seu.edu.cn;Tel/Fax:+86 25 52090619
