两个四唑配合物的原位合成、多样化配位模式和强荧光性质
2016-12-05高继兴谭育慧温和瑞唐云志
高继兴 徐 庆 谭育慧 刘 艺 温和瑞 唐云志
(江西理工大学工程研究院,赣州341000)
两个四唑配合物的原位合成、多样化配位模式和强荧光性质
高继兴徐庆谭育慧刘艺温和瑞唐云志*
(江西理工大学工程研究院,赣州341000)
在路易斯酸ZnCl2或MnSO4·7H2O作用下,通过1-甲基-1-氢-咪唑-4,5-二甲腈与NaN3水热原位合成了2个四唑配合物:{[Zn2(midt)(Hmidt)](N3)·H2O}n(1)和[Mn(m idt)2·(H2O)2]·H2O(2)(m idt=1-甲基-1-氢-咪唑-4,5-二四唑)。X射线单晶衍射表明尽管配合物1和2均结晶于同样的P1空间群,但他们有完全不同的结构。配合物1为一个有趣的二维聚合物结构,含有两个不同配位环境的锌原子和多种配位模式的midt配体,而配合物2为一个三维超分子结构,包含一个有趣的水分子链结构。固态下1和2分别在353和382 nm处显示出较强的蓝色荧光。
Sharpless反应;荧光;四唑配合物;晶体结构
The tetrazole functional group has found a wide range of applications in medicinal chemistry as metabolically stable surrogates for a carboxylic acid groups,and in materials science as high density energymaterials[1].Especially,tetrazoles have attracted increasing attention in coordination chemistry due to the excellent coordination ability of four nitrogen atoms from the functional group to act as either a multidentate or a bridging building block[2-8].
It has inspired that some new progress were made on the in situ synthesis of cycloaddition reactions recently.Our groups discovered the first pair of enantiomers ofmetal tetrazole complex([Cu(Tzmp)]n) from in situ[2+3]cycloaddition reactions of a flexible organic nitrile ligand with sodium azide in the presence of Cu2+as Lewis acid[9].Following from that,we obtained another new pair enantiomers[Mn(4-tzba) (bpy)2·H2O](bpy)·3H2O(4-tzba=4-tetrazolbenzoic acid) from[2+3]cycloaddition reaction ofbpy,4-tzba,sodium azide and manganese acetate[10].These investigations have suggested that different conditions such as different Lewis acids(metal cations),negative ions, temperaturesand pH valueshavea significantinfluence on their coordination modes of tetrazolyl groups and crystal structures[11-15].In our previous work,most reaction substitutes of the in situ metal tetrazole complexes are organic mononitrile[16-19],the metal tetrazole complexes obtained from the in situ syntheses of bisnitriles and multinitriles are relatively spare[20-21]. When the organic bisnitriles were used as substitute in[2+3]cycloaddition reactions,there are possible two tetrazolyl groups formed from in situ produced ligands, thus,they maybe exhibit diversified coordination modes and form variable structures.
As an ongoing effect to explore new type of bistetrazole complexes and analyze the crystal structures and photluminescent properties,we have attempted to construct two new tetrazole complexes, {[Zn2(midt)(Hmidt)](N3)·H2O}n(1)and[Mn(midt)2· (H2O)2]·H2O(2),by using 1-methyl-1H-imidazole-4,5-dicarbonitrile(midn)assubstitute in Sharpless reaction. To our surprise,1 contains four different types of tetrazolyl groups in terms of their coordination modes. Here we detailed their crystal structures,photoluminescent properties and PXRD.
1 Experimental
1.1Synthesis
All reagents were purchased from commercial sources and used as received.As shown in Scheme 1, hydrothermal reaction ofmidn(0.026 4 g,0.2 mmol), NaN3(0.039 0 g,0.6mmol)(Caution:Metalazidesmay be explosive),and ZnCl2(0.027 2 g,0.2 mmol)or MnSO4·7H2O(0.055 4 g,0.2mmol)in amixed solution of ethanol(0.5 mL)and water(2 mL)for 3 days give colorless block crystals of 1 or yellow block crystals of 2.For 1,Yield:0.031 1 g,49.8%based on mind. Anal.Calcd.for C12H11Cl0N23OZn2(%):C,23.09;H, 1.78;N,51.61.Found(%):C,23.12;H,1.75;N, 51.58.IR(KBr,cm-1):3 483(m),3 201(m),2 095(s), 1 648(s),1 583(m),1 492(s),1 449(m),1 380(s),1 278 (w),867(m),760(m),680(m).For 2,Yield:0.035 0 g, 62.3%.Anal.Calcd.for C12H18N20O4Mn(%):C,25.68; H,3.23;N,49.90.Found(%):C,25.71;H,3.19;N, 49.85.IR(KBr,cm-1):3 481(m),3 200(m),1 652(s), 1 582(m),1 488(s),1 439(m),1 380(s),1 250(s),967 (m),762(m),680(m).
1.2Crystallography
X-ray single-crystal diffraction data for 1 and 2 were collected on a Bruker P4 diffractometer with Mo Kαradiation(λ=0.071 073 nm)at296K using the θ-2θscan technique and corrected by Lorentz-polarization and absorption corrections[22-23].Both crystal structures were solved by direct method and refined by the full-matrixmethod based on F2bymeans of the SHELXLTL software package[24].Non-H atoms were refined anisotropicallyusingall reflectionswith I>2σ(I). All H atomswere generated geometrically and refined using a“riding”model with Uiso=1.2Ueq(C).The asymmetric units and the packing views were drawn with DIAMOND(Brandenburg and Putz,2005).Angles between some planeswere calculated using DIAMOND, and other calculations were carried out using SHELXLTL.Crystal data and structures refinement for1 and 2 are listed in Table1.

Scheme 1 Preparation of comp lexes 1 and 2

Table1 Crystal data and structure refinement for 1 and 2
CCDC:1451129,1;1451130,2.
1.3Physical techniques
PL emission spectra were measured at room temperature using a spectra fluorophotometer(JASCO, FP-6500)with a xenon lamp(150W)asa lightsource. Powder X-ray diffraction(PXRD)data were recorded on a Rigaku D/MAX 2000 PC X-ray diffraction instrumentwith Cu Kαradiation(λα1=0.015 405 98 nm,λα2=0.015 444 26 nm)under the generator voltage of 40 kV and tube current of 40 mA,by using continuous scan type from 5.0°to 80.0°at room temperature.
2 Results and discussion
2.1Synthesis and crystal structures of complexes 1 and 2
Compared by IR spectra of complex 1 andmidn,a serial of new strong peaks ranges from 1 652 to 1 439 cm-1appear while the absorption of cyano group at 2 355 cm-1νas(C≡N)andνs(C≡N)disappear in 1 and 2,indicating that the[2+3]Sharpless reaction between cyano groups and azide anions have finished[16-19]. Especially,a sharp peak at 2 095 cm-1existing in 1 without in 2 suggest that there are possible azide anions in complex 1.
Single crystal X-ray diffraction analyses reveal that1 belongs to triclinic crystal system with P1 space group.The fundamental building unit of 1 consists of two crystallgraphically Zn(Ⅱ)ions,one midt ligand, one protonated Hmidt ligands(one of the tetrazole groups is protonated),one N3-anions,and one H2O molecule.As depicted in Fig.1,Zn1 and Zn2 have totally different coordinated environment.Zn1 adopts a slightly distorted hexa-coordinated octahedral geometry in which twoβN atoms(N11 and N14ii) from different tetrazolyl groups occupy the axial vertexposition with an angle of 175.01(10)°(N(14)ii-Zn(1)-N(11)),while the basic planar is constituted by four N atoms(N1,N10,N9iand N20)from two chelating midt ligands via theαN atoms of tetrazolyl group and exposed N atoms of imidazole ring.Zn2 has a distorted tetrahedral geometry which connects four independent αN(N16,N17,N6iiiand N7iii)atoms from different tetrazolyl groups.Interestingly,we can clearly discover that all the Zn1-N distances(ranging from 0.211 1(2) to 0.222 4(2)nm)are obviously longer than those of Zn2-N distances(0.197 5(2)~0.199 0(2)nm).

Fig.1 Asymmetric unit view of 1 showing octahedral coordination geometry of Zn1 and tetrahedral coordination geometry of Zn2
As can be seen in Fig.2a,there also exist two crystallgraphically different midt ligands in 1.One midt ligand(named as midt1)acts as a 3-connected bridging spacer through N17,N16,N14,N11 and N20 linking three different zinc atoms such as Zn1,Zn2 and Zn1viii,while the other one(correspondingly called as midt2)acts as a 3-connected bridging spacer via N1,N10,N9,N7 and N6 connecting the other three zinc atoms such as Zn1,Zn1viand Zn2vii.Remarkably, we can clearly observe that the plane of midt1 is nearly perpendicular to that of midt2,the dihedral angle between them is 89.14°calculated by diamond software.As shown in Fig.2b,firstly all the midt1 ligands link different zinc atoms with“end to end”modes to produce an infinite linear structure along the a axis;meanwhile,all the midt2 ligands connect the other zinc atoms to form another linear structure along the c axis,then they cross-link to extend to a 2D sheet which parallel to the ac plane;lastly the neighboring 2D sheets are further packed by the strong supramolecular interactions such as intermolecular hydrogen bonds,π-πstacking of tetrazolyl rings and O-H…πinteractions,which extend the structure into a 3D network.It should be emphasized that there exist four totally different coordination modes of tetrazolyl groups inmidt1 and midt2 ligands.In midt1 (Fig.2a,left),one tetrazolyl group adoptsμ3coordination mode and the other usesμ1coordination mode by one ofαN atoms.In midt2(Fig.2a,right),both tetrazolyl groups adoptμ2coordination mode,but one of them uses twoαN atoms of tetrazolyl ring and the other employs oneαN atom and oneβN atom of tetrazolyl ring.According to the summary oncoordinationmodesof tetrazolylgroupsby Xiong etal[1], the above coordination modes belong to modeⅦ, modeⅠ,modeⅢand modeⅤrespectively.To our knowledge,few literatures reported that so many coordination modes of tetrazolyl groups are coexistent in the same complex before.

Table2 Selected bond lengths(nm)and angles(°)for complex 1

Table3 Selected hydrogen bonds for com p lex 1

Fig.2(a)A representation showing the different coordination modes ofmidt1 andmidt2 in 1;(b)Packing view of the 2D sheets along the b-axis and supramolecular interactions in 1
Although 2 also crystallizes in P1 space group,it has completely different structure from 1.The asymmetric unit of 2 is composed of half Mn(Ⅱ)cation and halfmidt ligand,one coordinated watermolecule and one lattice water molecule.As shown in Fig.3a, the coordination geometry around Mn(Ⅱ)center can be viewed as a slightly distorted octahedron which is surrounded by two coordinated water molecules and four N atoms of two chelating midt ligands.To our surprise,only half of the tetrazolyl groups in midt ligands of 2 participate in coordination.Because of the steric hindrance effect of the uncoordinated tetrazolyl groups,it prevent forming an infinite high dimensionalmetal organic framework.Nevertheless,it become an important factor to assembly themolecules into a three dimensional supramolecular structure, since there exist a lot of strong intermolecular hydrogen bonds between the uncoordinated tetrazolyl groups and watermolecules such as O(2W)-H(2WA)…N(5)iv(0.282 1(3)nm)and O(1W)-H(1WB)…N(4)v(0.280 8(3)nm).In additional,other interesting supramolecular interactions includingπ-πstacking interac-tionsbetween the neighboring tetrazolyl planes(0.345 4 nm)and imidazolyl planes(0.345 4 nm)also contribute to stabling the whole structure(Fig.3b).

Table4 Selected bond lengths(nm)and angles(°)for com p lex 2
One of themost striking features is that there lies a one dimensionalwater cluster in 2.As shown in Fig. 4,the basic building block can be regarded as a tetrameric water cluster which is constituted by two equivalents coordinated watermolecules(O1Wvi,O1W) and two equivalents lattice water molecules(O2Wvi, O2W).The short contacts and the reasonable angles between them indicate the existence of strong hydrogen bonds(Table2),which favors the construction of a tetrameric water cluster.Then,these building blocks are further extended by strong intermolecular hydrogen bonds between lattice water molecules(O(2W)-H(2WB)…O(2W)v0.287 0(5)nm),resulting in the formation of an infinite one dimensionalwater chain.
2.2Fluorescence properties and powder X-ray diffraction analysis

Fig.4 Views of the structure of one dimensionalwater chain in 2
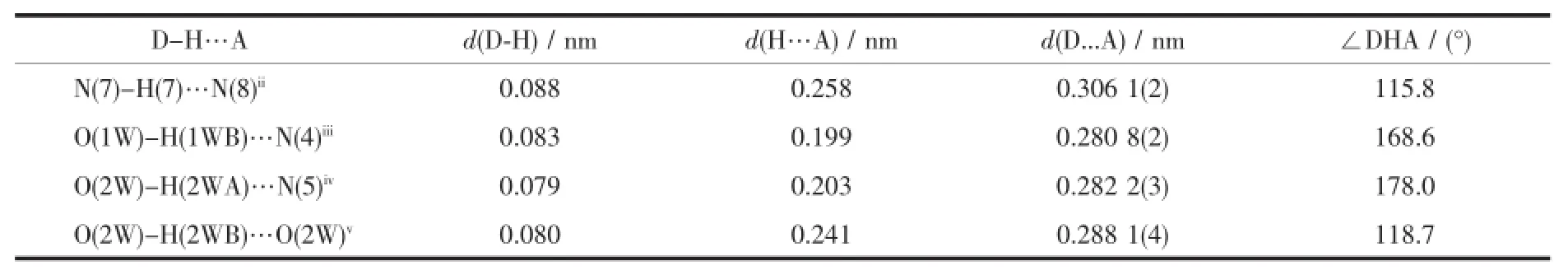
Table5 Selected Hyd rogen bonds for 2
We studied the solid-state luminescent emissionspectra of 1 and 2 at room temperature(Fig.5). Complex 1 display a very strong emission at353.4 nm. In comparison with 1,complex 2 appears a distinct“Einstein”shift,the maximum emissive peak occurs at382.0 nm,meanwhile the relative intensity decreases. According to previous research and our investigation on tetrazolate complexes,the emission peak ranging from 350 to 380 nm is attributed to the ligand to ligand change transition[11-19],since the luminescent emission from d-d charge transition of Zn(Ⅱ)and Mn(Ⅱ)mainly occurs in 450 and 650 nm respectively.Besides, in comparison with 2 the significant improvement of 1 at intensity is tentatively attributed to the weak Zn…Zn(3d-4s)with d10cluster-centered(CC)excited states. There may exist CC excited states because of the vibronic progression in the spectra and the short Zn…Zn distances in the structures which favor the formation of metal-metal bond in light of Cotton′s work through DFT calculations.

Fig.5 Solid state fluorescentemission spectra of 1 and 2
To confirm the phase purity of 1 and 2,the powder X-ray diffraction weremeasured[25-27].As shown in Fig.6,the powder XRD of complexes 1 and 2 show that their products are very highly crystalline.The results also revealed that the reaction was quantitative because no starting materials were detected.Their stimulated powder XRD patterns based on crystal structure analysis allowed unambiguous identification via comparison of the experimental and computed powder XRD patterns.

Fig.6 Powder XRD patterns of complexes 1(a)and 2(b)
3 Conclusions
We have synthesized two tetrazole complexes by using bisnitriles compoundmidtas[2+3]cycloaddition reaction substrate.The investigation on 1 and 2 suggest that they have totally different structures and 1 display a strong blue emission.
References:
[1]Zhao H,Qu Z R,Ye H Y,et al.Chem.Soc.Rev.,2008,37: 84-100
[2]Li Y W,Ma H,Chen Y Q,et al.Cryst.Growth Des.,2012, 12:189-196
[3]Tian D,Chen Q,Li Y,etal.Angew.Chem.Int.Ed.,2014,53: 837-841
[4]He K H,SongW C,LiY W,et al.Cryst.Growth Des.,2012, 12:1064-1068
[5]He K H,Li YW,Chen Y Q,etal.Cryst.Growth Des.,2012, 12:2730-2735
[6]Li L,Zhang S,Han L,et al.Cryst.Growth Des.,2013,13: 106-110
[7]Wang H,Yang W,Sun ZM,et al.Chem.Asian J.,2013,8:982-989
[8]Kusaka S,Sakamoto R,Kitagawa Y,et al.Chem.Asian J., 2012,7:907-910
[9]Tang Y Z,Xiong JB,Gao JX,etal.Inorg.Chem.,2015,54: 5462-5466
[10]Gao JX,Xiong JB,Xu Q,et al.Cryst.Growth Des.,2016, 16:1559-1564
[11]Li L,Zhang S,Han L,et al.Cryst.Growth Des.,2013,13: 106-110
[12]Wang C,Zhang T,Lin W.Chem.Rev.,2012,112:1084-1104
[13]Zhao S,Zhang J,Zhang S,et al.Inorg.Chem.,2014,53: 2521-2527
[14]Zhao S,Gong P,Luo S,et al.J.Am.Chem.Soc.,2014,136: 8560-8563
[15]Li L,Ma J,Song C,et al.Inorg.Chem.,2012,51:2438-2442
[16]Tang Y Z,Zhou M,Huang J,et al.Inorg.Chem.,2013,52: 1679-1681
[17]Tang Y Z,Wang G X,Ye Q,etal.Cryst.Growth Des.,2007, 7:2382-2386
[18]TAN Yu-Hui(谭育慧),XIONG Jian-Bo(熊剑波),HUANG Jun(黄珺),et al.Chinese J.Inorg.Chem.(无机化学学报), 2014,30(7):1621-1628
[19]Zhong D C,Wen Y Q,Deng JH,et al.Angew.Chem.Int. Ed.,2015,54:11795-11799
[20]Tong X L,Wang D Z,Hu T L,et al.Cryst.Growth Des., 2009,9:2280-2286
[21]Wang D Z.Polyhedron,2012,35:142-148
[22]Sheldrick G M.SHELXS-97,Program for X-ray Crystal Structure Determination,University of Göttingen,Germany, 1997.
[23]Sheldrick G M.SHELXS-97,Program for Crystal Structure Solution,University of Göttingen,Germany,1997.
[24]Flack H D.Acta Crystallogr.Sect.A,1983,A 39:876-881
[25]Zhang Y,Liao W Q,Fu D W,et al.J.Am.Chem.Soc., 2015,137:4928-4931
[26]Ye H Y,Zhou Q H,Niu X H,et al.J.Am.Chem.Soc., 2015,137:13148-13154
[27]Tang Y Z,Yu Y M,Xiong J B,et al.J.Am.Chem.Soc., 2015,137:13345-13351
In Situ Syntheses,Diversified Coordination M odes and Strong Photo-Lum inescent Properties of Two Tetrazole Comp lexes
GAO Ji-Xing XU Qing TAN Yu-Hui LIU Yi WEN He-Rui TANG Yun-Zhi*
(Institute of Engineering and Research,JiangxiUniversity of Science and Technology,Ganzhou,Jiangxi 341000,China)
Two tetrazole complexes,{[Zn2(midt)(Hmidt)](N3)·H2O}n(1)and[Mn(midt)2·(H2O)2]·H2O(2)(midt=1-methyl-1H-imidazole-4,5-ditetrazole),were synthesized via hydrothermal reaction of 1-methyl-1H-imidazole-4,5-dicarbonitrile(midn)and NaN3in the presence of ZnCl2or MnSO4·7H2O as Lewis acid.X-ray single crystal diffraction analyses indicate that both 1 and 2 have different crystal structures although they crystallize in the same P1 space group.Complex 1 demonstrates an interesting two dimensional polymeric structure which contains two crystalgraphically different Zn(Ⅱ)ions and midt ligandswith diversified coordination modes,while 2 shows a 3D supramolecular structure involving a water clusters chain.Moreover,1 and 2 display strong blue emission at 353 and 382 nm at room temperature.CCDC:1451129,1;1451130,2.
Sharpless reaction;photoluminescent;tetrazole complexes;crystal structure
O614.24+1;O614.7+11
A
1001-4861(2016)07-1267-08
10.11862/CJIC.2016.165
2016-02-03。收修改稿日期:2016-06-03。
国家自然科学基金(No.21261009,21471070,21461010)和江西省主要学术学科带头人资助项目。
*通信联系人。E-mail:tangyunzhi75@163.com,Tel(Fax):+86-797-8312708;会员登记号:S06N2898M1306。
