Pax6 PAI亚结构域在黑色素细胞中对MITF、TYR、TYRP1和TYRP2的影响
2016-12-01聂瑞强杨玉静谢建山范瑞文许冬梅于秀菊段志成董常生
聂瑞强,杨玉静,谢建山,2,范瑞文,许冬梅,于秀菊,段志成,董常生
(1山西农业大学动物科技学院,山西太谷030801;2山西医科大学基础医学院,太原030001)
Pax6 PAI亚结构域在黑色素细胞中对MITF、TYR、TYRP1和TYRP2的影响
聂瑞强1,杨玉静1,谢建山1,2,范瑞文1,许冬梅1,于秀菊1,段志成1,董常生1
(1山西农业大学动物科技学院,山西太谷030801;2山西医科大学基础医学院,太原030001)
【目的】高度保守的PAX转录因子家族在黑色素细胞的分化和黑色素的生成中起重要的作用,其家族共有的PD 结构域是其与下游基因结合的主要位点,而PD结构域氨基端的PAI亚结构域在其与下游基因的结合过程中发挥重要的作用。研究表明Pax6在视网膜上皮黑色素细胞的分化中发挥至关重要的作用,本试验借助研究Pax6 PAI亚结构域的功能来对PAX转录因子家族共有的PD结构域和PAI亚结构域进行研究。【方法】首先通过Psipred对Pax6 PD 结构域的结构进行分析,使用NCBI对Pax6 PD结构域与下游基因的结合位点进行分析,使用Jaspar对MITF、TYR、TYRP1和TYRP2启动子中Pax6 PD结构域可能的作用位点进行预测。使用普通PCR克隆Pax6 PAI亚结构域,将其连入T载体,酶切后连入慢病毒载体,并送公司测序确认。将构建好的PAI亚结构域过表达载体通过细胞转染导入到培养的小鼠黑色素细胞中,使其过量表达。收集细胞,分别通过观察绿色荧光蛋白检测转染效率,使用RT-PCR 和Western blot 来检测MITF 、TYR 、TYRP1和TYRP2在mRNA 和蛋白水平的变化,同时检测黑色素细胞中黑色素生成量的变化。【结果】通过NCBI分析可知,Pax6 PD结构域与下游基因的作用位点主要集中在氨基端的PAI 亚结构域。通过Jaspar预测分析,得知,MITF启动子-695处存在Pax6 PD 结构域的结合位点,TYR启动子-873和-1133处存在Pax6 PD 结构域的结合位点,TYRP1启动子-629处存在Pax6 PD 结构域的结合位点,TYRP2启动子-655处存在Pax6 PD 结构域的结合位点。在黑色素细胞中过表达Pax6 PAI亚结构域后,与空载组相比,试验组MITF mRNA升高2.05倍(P<0.01),蛋白质升高1.7倍(P<0.01);TYR mRNA升高2.09倍,蛋白质升高2倍(P<0.05);TYRP1 mRNA升高2.93倍(P<0.05),蛋白质升高1.9倍(P<0.01);TYRP2 mRNA升高3.62倍(P<0.01),蛋白质升高1.37倍。同时试验组的黑色素含量是空载组黑色素含量的1.33倍(P<0.001)。【结论】在小鼠黑色素细胞中,过表达Pax6 PAI亚结构域可以促进MITF、 TYR、TYRP1和TYRP2的表达,进而使黑色素细胞黑色素的生成量增加。
PAI 亚结构域;Pax6;黑色素
0 引言
【研究意义】哺乳动物皮肤黑色素细胞是由神经嵴祖细胞定向分化而来[1],其正常分化依靠相关基因在时间和空间上的正常表达,而基因的正常表达离不开功能相互交错的转录因子网络的调控[2]。高度保守的Paired box(PAX)转录因子家族属于Ι型转录因子,在黑色素细胞的定向分化和黑色素的产生中发挥重要的作用[3-4]。PAX家族的共同特征是都在N端含有128个氨基酸组成的paired domain(PD),PD本身就是一个独立的结构,它包含氨基端的PAI 亚结构域和羧基端的RED 亚结构域[5-6],这两个亚结构域都包含有螺旋-转角-螺旋结构[7],而PAI亚结构域是PAX家族与其下游调控基因结合的主要部位[8]。【前人研究进展】BERY于2015年证明在小鼠的皮质祖细胞中富含PAX转录因子家族的结合位点[9]。FUJIMURA于2015年证明在视网膜色素上皮细胞的分化转移中,Pax6调控其色素积淀和细胞增殖[10]。CARBE于2013年证明Pax6在眼的发育中发挥独特的作用,但在大脑的发育中,Pax6可以在功能上被含有相似PD结构域结合特异性的PAX家族基因所代替[11]。HUETTL于2015年证明Pax6不仅依靠其包括paired domain 和 homeodomain(HD)的完整分子结构执行其功能,而且每一个亚结构域也有其独特的功能[8]。【本研究切入点】Pax6已被证明在黑色素细胞的分化和黑色素的生成中发挥重要作用[12-13]。Pax6不仅包含PAX家族共有的PD,还包含有homeodomain和C端富含脯氨酸、丝氨酸、苏氨酸的PST区域。PD和HD通过识别不同的DNA靶点,既合作又独立的来调控不同的分子机制。FAVOR发现在Pax6的编码序列中第309个碱基C突变为T,从而使转录终止,形成了只含有Pax6 PAI完整结构的氨基酸序列[14],破坏了RED的螺旋-转角-螺旋结构,保留了PAI完整的螺旋-转角-螺旋结构。这为笔者以研究Pax6 PAI 亚结构域的功能为途径探究PAX转录因子家族PAI亚结构域的功能提供了基础。【拟解决的关键问题】本试验在细胞水平,过表达Pax6 PAI 亚结构域,来探究其能否作为反式作用因子调控下游基因的表达,并通过生物信息学的分析方法,尝试解释其作用机理。
1 材料与方法
试验于2015年3月至2016年1月在山西农业大学羊驼生物工程实验室完成。
1.1 试验材料
黑色素细胞培养基(Sciencell,美国),Trizol(Invitrogen,美国),RIPA 蛋白裂解液(碧云天,北京),RT-PCR Kit(康为,北京),MITF 多克隆抗体,TYR 多克隆抗体,TYRP1多克隆抗体,TYRP2多克隆抗体。
1.2 试验方法
1.2.1 小鼠Pax6 PAI亚结构域二级结构和下游基因结合位点分析 二级结构通过Psipred(http://bioinf.cs. ucl.ac.uk/psipred/)分析获得[15],下游基因结合位点通过NCBI(http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb. cgi)分析获得[16]。
1.2.2 小鼠MITF、TYR、TYRP1和TYRP2启动子中Pax6作用位点预测 通过Jaspar(http://jaspar.genereg.net/)预测获得。
1.2.3 小鼠Pax6 PAI亚结构域基因片段的克隆和真核表达载体构建 通过普通PCR克隆PAI 亚结构域基因片段,并将其连入T载体,再将其酶切后连接在慢病毒载体中,送公司测序确认。
1.2.4 细胞培养和转染 将小鼠黑色素细胞培养于6孔板中,设置正常组、空载组和试验组。细胞转染时,将转染试剂与表达载体形成的脂质体加入正常培养基中,37℃培养细胞60 h,进行转染结果检测。
1.2.5 黑色素含量测定 用胰酶将黑色素细胞从细胞培养板上消化下来,用PBS清洗后细胞计数。用0.2 mol·L-1NaOH溶解细胞,使用酶标仪在475 nm 波长进行测值。用乌贼墨标准品做标准曲线[17]。
1.2.6 Real-time PCR检测 Trizol法提取转染细胞RNA,反转录获得cDNA。根据荧光定量PCR结果的CT 值计算试验结果,目的基因的相对表达水平=2–△△CT,所有数据用GraphPad Prism5.0进行统计分析,实时荧光定量PCR 结果均用均值±标准误(Means ± SEM)表示,其中各基因的表达量均经β-actin校正,两组之间的数据比较全部采用GraphPad Prism5.0 统计软件进行t检验,三组之间的比较全部采用单因素方差分析。
1.2.7 蛋白免疫印迹试验 RIPA蛋白裂解液提取转染细胞蛋白,200 ng上样量进行SDS-PAGE 电泳,后转至NC 膜。抗体所用浓度为1 000倍稀释。孵育二抗后,用ECL显色后暗室曝光,获得有条带的胶片,分析。用Image-ProPlus 6.0 软件对行条带面积和灰度值半定量分析,数据均用Means ± SEM 表示,两组之间的数据比较全部采用GraphPad Prism5.0 统计软件进行t检验,三组之间的比较全部采用单因素方差分析。
2 结果
2.1 小鼠Pax6 PAI 亚结构域
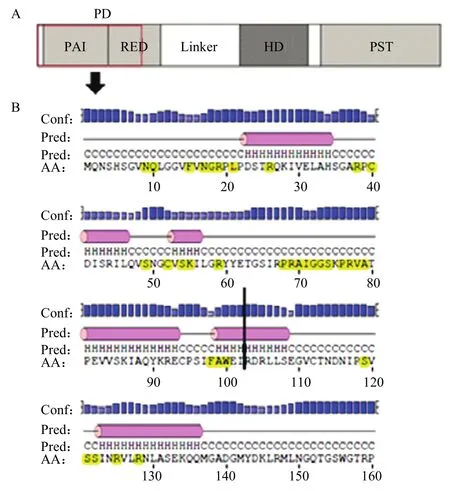
图1 Pax6 PD 结构域结构分析Fig. 1 The structure analysis of Pax6 PD domain
结构完整性分析发现,Pax6 PD 结构域 是由PAI和RED 亚结构域组成(图1-A),PAI和RED亚结构域都含有螺旋-转角-螺旋结构,当编码Pax6基因cds区的第307个碱基由C突变为T后,在307—309碱基处形成了一个终止密码子,从而使翻译提前终止,形成了一个由Pax6前309个碱基序列翻译出的102个氨基酸残基组成的氨基酸序列。完整的PD含有128个氨基酸残基,该突变位点将RED亚结构域的螺旋-转角-螺旋结构破坏,而在形成的氨基酸序列中保留了完整的PAI亚结构域。且通过对PAX家族保守性分析可知,PD与下游基因的结合位点主要集中在前102个氨基酸序列(图1-B)。
2.2 小鼠黑色素细胞培养及细胞转染
细胞接种12 h后,即可见细胞贴壁伸展,24 h后细胞呈典型的树突状。细胞密度达到70%时,添加脂质体和载体的混合物,60 h后通过荧光显微镜观察转染结果(图2)。
2.3 细胞转染后相关检测
2.3.1 Pax6 PAI亚结构域与MITF相互作用检测 提取转染后试验组和空载组RNA和蛋白后,使用RT-PCR和Western blot 检测(图3),并使用Jaspar预测PD 结构域在MITF启动子上的作用位点。通过分析结果显示:在MITF转录起始位点前695个碱基处预测出存在PD 结构域与MITF的结合位点(图4-A),同时通过RT-PCR和Western blot结果分析,与空载组相比,试验组MITF mRNA升高2.05倍(P<0.01,图4-B);蛋白质升高1.7倍(P<0.01,图4-C)。由此得出,PAI 亚结构域可以与MITF启动子作用促进MITF表达。
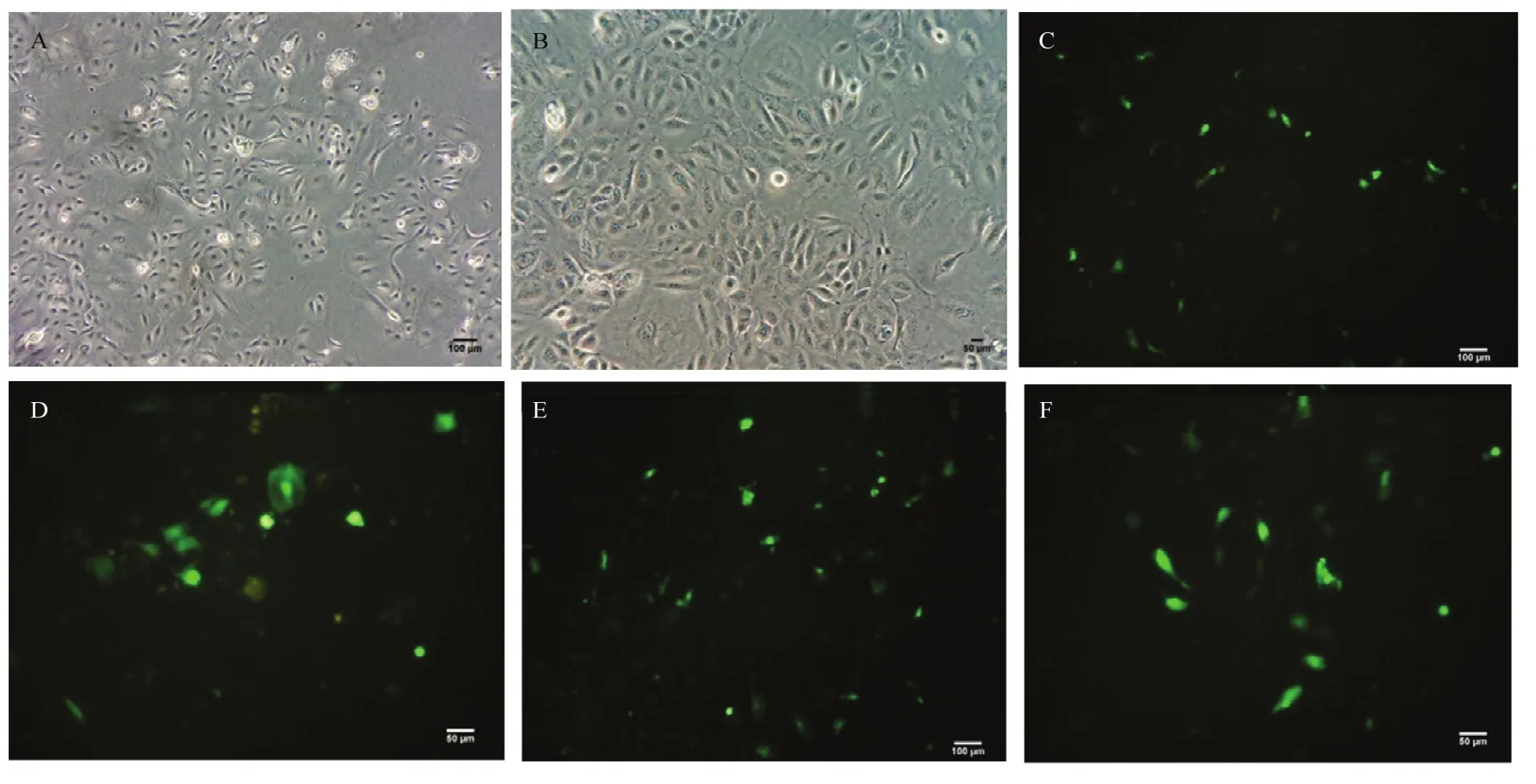
图2 黑色素细胞培养和转染Fig. 2 Melanocyte culture and transfection
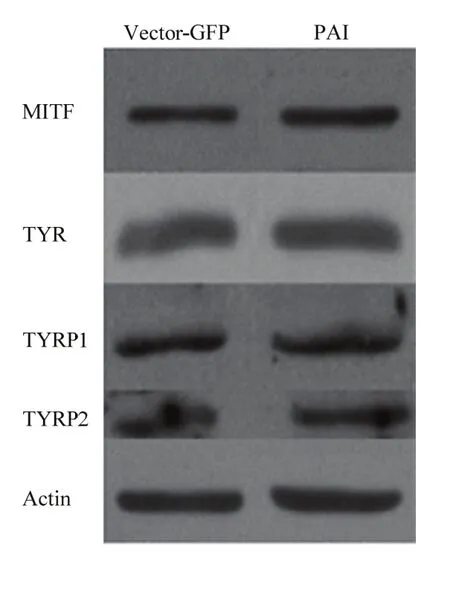
图3 Western blot 检测图Fig.3 The picture of Western blot
2.3.2 Pax6 PAI亚结构域与TYR相互作用检测 提取转染后试验组和空载组RNA和蛋白后,使用RT-PCR和Western blot 检测(图3),并使用Jaspar预测PD结构域在TYR启动子上的作用位点。通过统计分析获得结果显示:在TYR转录起始位点前873和1 133个碱基处预测出存在PD 结构域与TYR的结合位点(图5-A),同时通过RT-PCR和Western blot结果分析,与空载组相比,试验组TYR mRNA升高2.09倍(图5-B);蛋白质显著升高2倍(P<0.05)(图5-C)。说明PAI亚结构域仍然可以促进TYR的表达。
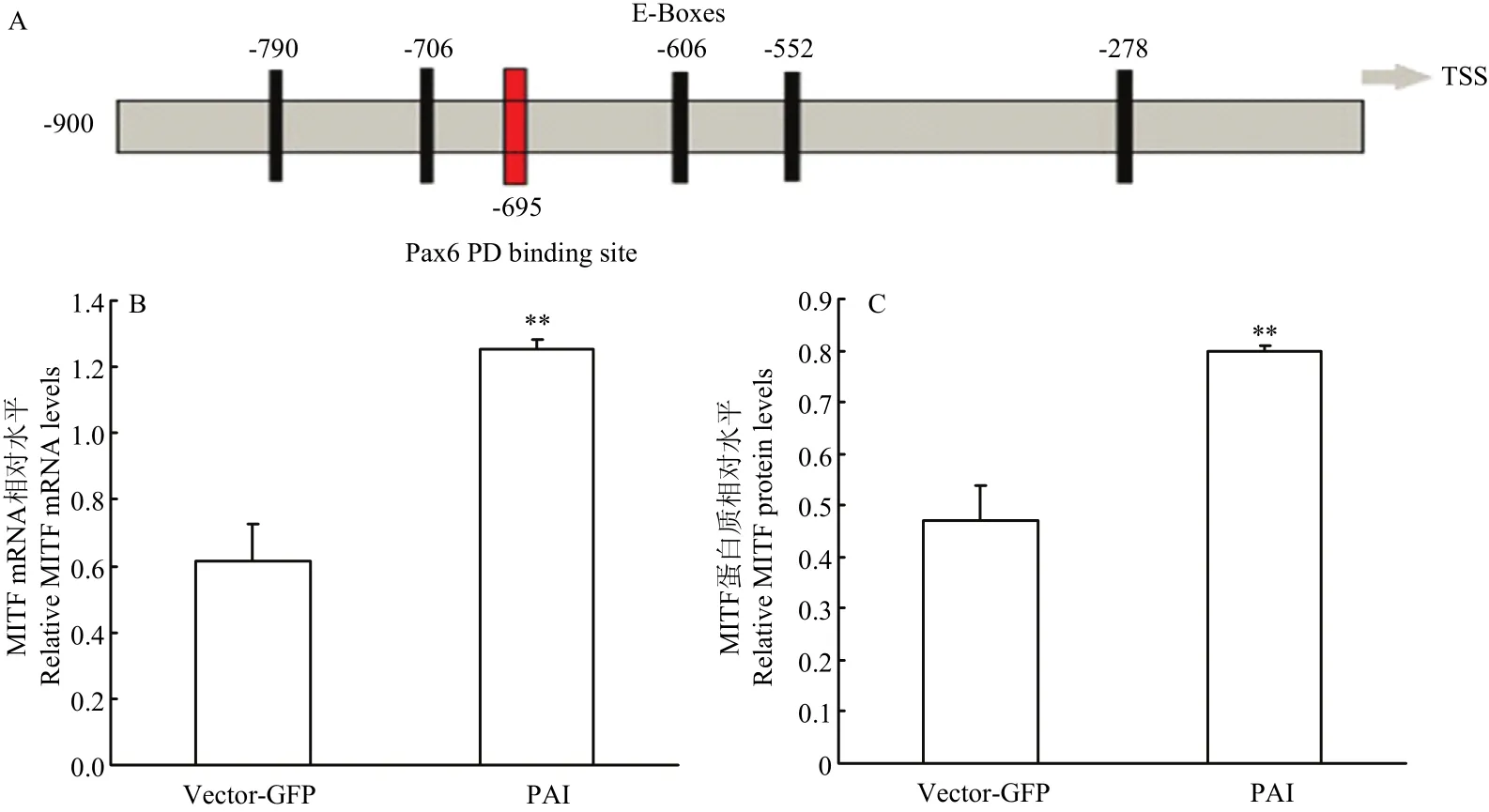
图4 MITF相关检测Fig. 4 The detection results of MITF
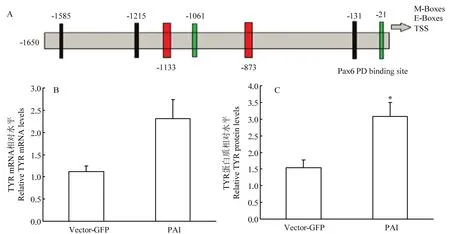
图5 TYR相关检测Fig.5 The detection results of TYR
2.3.3 Pax6 PAI亚结构域与TYRP1相互作用检测提取转染后试验组和空载组RNA和蛋白后,使用RT-PCR和Western blot 检测(图3),并使用Jaspar预测PD 结构域在TYRP1启动子上是否存在作用位点。通过分析结果显示:在TYRP1转录起始位点前629个碱基处预测出存在PD结构域与TYRP1的结合位点(图6-A),同时通过RT-PCR和Western blot结果分析,与空载组相比,试验组TYRP1 mRNA升高2.93倍(P<0.05)(图6-B);蛋白质升高1.9倍(P<0.01)(图6-C)。说明PAI 亚结构域仍然可以促进TYRP1的表达。
2.3.4 Pax6 PAI亚结构域与TYRP2相互作用检测提取转染后试验组和空载组RNA和蛋白后,使用RT-PCR和Western blot 检测(图3),并使用Jaspar预测PD 结构域在TYRP2启动子上是否存在作用位点。通过分析结果显示:在TYRP2转录起始位点前655个碱基处预测出存在PD 结构域与TYRP2的结合位点(图7-A),同时通过RT-PCR和Western blot结果分析,与空载组相比,试验组TYRP2 mRNA升高3.62倍(P<0.01)(图7-B);蛋白质升高1.37倍(图7-C)。说明PAI 亚结构域仍然可以促进TYRP2的表达。
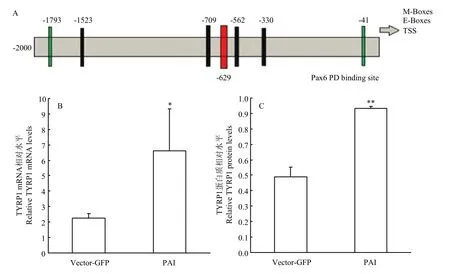
图6 TYRP1相关检测Fig. 6 The detection results of TYRP1
2.4 转染后黑色素含量测定
收集空载组和试验组黑色素细胞,通过酶标仪对黑色素含量进行测定。试验组的黑色素含量是空载组黑色素含量的1.33倍(P<0.001)(图8)。说明PAI 亚结构域仍然可以促进黑色素细胞中黑色素含量增多。
3 讨论
本试验通过将Pax6 PAI 亚结构域在黑色素细胞中过量表达,结合生物信息学的分析方法,来探究PAX转录因子家族共有的PD结构域氨基端的PAI亚结构域在调控下游基因中的作用。前人的研究得出,Pax6在黑色素细胞的分化和黑色素的产生中发挥着至关重要的作用,且其与黑色素生成通路中的MITF[18-20]、TYR[21-22]、TYRP1[23-24]、TYRP2[25]和β-catenin[26-28]有着密切的联系。故本试验将MITF、TYR、TYRP1和TYRP2作为目标基因,使用RT-PCR和Western blot来检测这些基因表达的变化,同时对黑色素细胞的黑色素生成量进行测定。结果显示,通过预测发现,在MITF、TYR、TYRP1和TYRP2的启动子中都可以找到Pax6 PD 结构域的结合位点,过量表达PAI亚结构域后,黑色素细胞中MITF、TYR、TYRP1和TYRP2的表达量在mRNA和蛋白质水平都会升高,并且黑色素细胞的黑色素生成量也会增多。
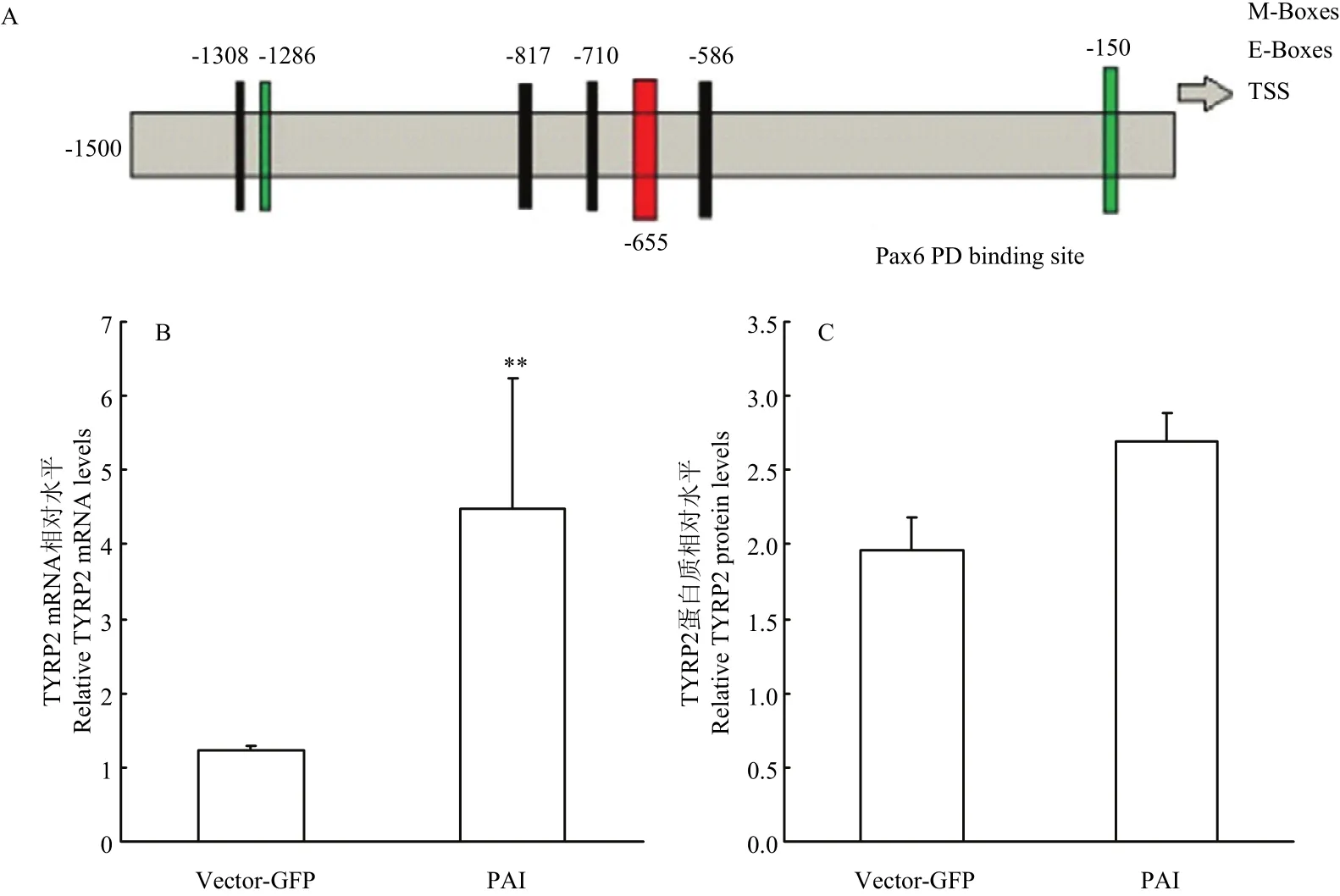
图7 TYRP2相关检测Fig.7 The detection results of TYRP2
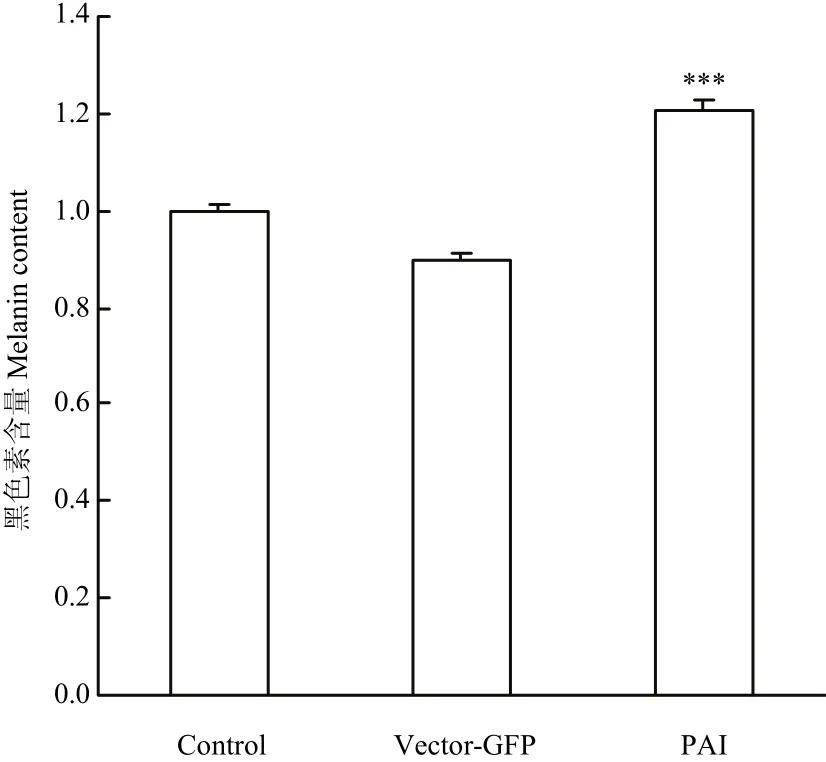
图8 过表达Pax6 PAI 亚结构域对黑色素细胞中黑色素生成的影响Fig. 8 Melanin production in melanocytes over-expressing Pax6 PAI subdomain structure
FUJIMURA在2015年通过试验得出,Pax6可以与MITF和β-catenin协同作用促进TYR和TYRP1的表达,但是Pax6只有在MITF存在的情况下才能激活TYR和TYRP1的启动子,Pax6在没有MITF存在的情况下是不会激活TYR和TYRP1的启动子[10]。PLANQUE证明Pax6与MITF协同作用调节下游基因的表达时,主要是与MITF的b-HLH-LZ 结构域相互作用[29]。YASUMOTO证明在Pax6表达存在缺陷的视网膜色素上皮细胞中,TYR的表达量降低1.58倍(P<0.05),TYRP1的表达量降低1.91倍(P<0.01)[23,30]。本试验结果得出,在过量表达PAI亚结构域的情况下,MITF mRNA升高2.05倍,蛋白质升高1.7倍,说明PAI亚结构域仍然可以通过调控MITF的启动子来促进MITF的表达。TYR mRNA升高2.09倍,蛋白质升高2倍;TYRP1 mRNA升高2.93倍,蛋白质升高1.9倍;TYRP2 mRNA升高3.62倍,蛋白质升高1.37倍,由此可以得出PAI 亚结构域仍然可以使TYR、TYRP1和TYRP2的表达量升高,但是本试验不能确定PAI亚结构域能否还可以与MITF的b-HLH-LZ结构域相互作用,其促进TYR和TYRP1的表达是与MITF协同作用的结果,还是通过促进MITF的表达间接促进TYR和TYRP1的表达,这需要进一步进行试验加以确认。同时尚未有研究报道Pax6作为转录因子是如何作用于TYRP2的,其促进TYRP2的表达是否也需要MITF的存在。与此同时,试验组的黑色素含量也与空载组相比升高1.33倍,说明PAI 亚结构域仍然可以通过促进调节黑色素生成相关基因的表达来促进黑色素细胞黑色素的生成(图9)。
PD结构域是PAX转录因子家族共有的结构域,而位于PD结构域氨基端的PAI亚结构域在PD结合下游基因启动子中发挥重要的作用,而PD结构域羧基端的RED亚结构域是否也有其独特的功能?尚待进一步深入的研究。
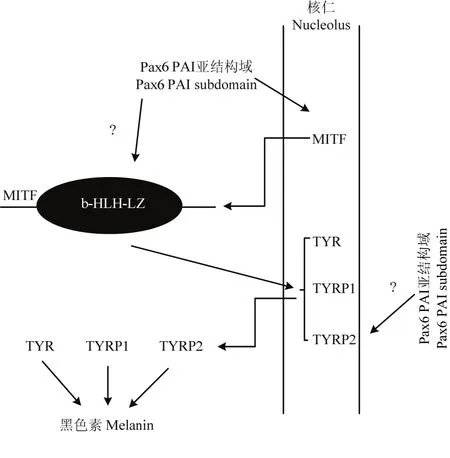
图9 Pax6 PAI亚结构域调控黑色素生成通路Fig. 9 The pathway of Pax6 PAI subdomain in regulating melanogenesis
4 结论
在小鼠黑色素细胞中,过表达Pax6 PAI 亚结构域可以促进MITF、TYR、TYRP1和TYRP2的表达,进而使黑色素细胞黑色素的生成量增加。
[1] COHEN M A, WERT K J, GOLDMANN J, MARKOULAKI S, BUGANIM Y, FU D, JAENISCH R. Human neural crest cells contribute to coat pigmentation in interspecies chimeras after in utero injection into mouse embryos. Proceedings of the National Academy of Sciences of the United States of America 2016, 113: 1570-1575.
[2] CHEN Y, PAN L, SU Z, WANG J, LI H, MA X, LIU Y, LU F, QU J, HOU L. The transcription factor TBX2 regulates melanogenesis in melanocytes by repressing Oca2. Molecular and Cellular Biochemistry 2016, 415(1/2):103-109.
[3] HEVER A M, WILLIAMSON K A, VAN HEYNINGEN V. Developmental malformations of the eye: The role of PAX6, SOX2 and OTX2. Clinical Genetics, 2006, 69(6):459-470.
[4] MONSORO-BURQ A H. PAX transcription factors in neural crest development. Seminars in Cell & Developmental Biology, 2015, 44: 87-96.
[5] WEI F, LI M, CHENG S Y, WEN L, LIU M H, SHUAI J. Cloning, expression, and functional characterization of the rat Pax6 5a orthologous splicing variant. Gene, 2014, 547(1):169-174.
[6] PAIXAO-CORTES V R, SALZANO F M, BORTOLINI M C. Origins and evolvability of the PAX family. Seminars in Cell & Developmental Biology, 2015, 44:64-74.
[7] EPSTEIN J A, GLASER T, CAI J, JEPEAL L, WALTON D S, MAAS R L. Two independent and interactive DNA-binding subdomains of the Pax6 paired domain are regulated by alternative splicing. Genes & Development, 1994, 8(17):2022-2034.
[8] HUETTL R E, ECKSTEIN S, STAHL T, PETRICCA S, NINKOVIC J, GOTZ M, HUBER A B. Functional dissection of the Pax6 paired domain: Roles in neural tube patterning and peripheral nervous system development. Developmental Biology, 2015,413:86-103.
[9] BERY A, MEROT Y, RETAUX S. Genes expressed in mouse cortical progenitors are enriched in Pax, Lhx, and Sox transcription factor putative binding sites. Brain Research, 2015,1633:37-51.
[10] FUJIMURA N, KLIMOVA L, ANTOSOVA B, SMOLIKOVA J, MACHON O, KOZMIK Z. Genetic interaction between Pax6 and β-catenin in the developing retinal pigment epithelium. DevelopmentGenes and Evolution, 2015, 225(2):121-128.
[11] CARBE C, GARG A, CAI Z, LI H, POWERS A, ZHANG X. An allelic series at the paired box gene 6 (Pax6) locus reveals the functional specificity of Pax genes. The Journal of Biological Chemistry, 2013, 288(17):12130-12141.
[12] ZHANG S J, LI Y F, TAN R R, TSOI B, HUANG W S, HUANG Y H, TANG X L, HU D, YAO N, YANG X. A new gestational diabetes mellitus model, hyperglycemia-induced eye malformation via inhibiting Pax6 in chick embryo. Disease Models & Mechanisms, 2016, 9:177-186.
[13] 聂瑞强, 杨玉静, 谢建山, 范瑞文, 高文俊, 董常生. 黑色素细胞中过量表达Pax610Neu基因对MITF和TYR的影响. 中国农业科学, 2016, 49(11):2214-2221. NIE R Q, YANG Y J, XIE J S, FAN R W, GAO W J, DONG C S. The influences of over-expressing Pax610Neuon MITF and TYR in melanocytes. Scientia Agricultura Sinica, 2016, 49(11):2214-2221.(in Chinese)
[14] FAVOR J, PETERS H, HERMANN T, SCHMAHL W, CHATTERJEE B, NEUHAUSER-KLAUS A, SANDULACHE R. Molecular characterization of Pax6(2Neu) through Pax6(10Neu): an extension of the Pax6 allelic series and the identification of two possible hypomorph alleles in the mouse Mus musculus. Genetics, 2001, 159(4): 1689-1700.
[15] SHUKLA S, MISHRA R. Predictions on impact of missense mutations on structure function relationship of PAX6 and its alternatively spliced isoform PAX6(5a). Interdisciplinary Sciences, Computational Life Sciences, 2012, 4(1):54-73.
[16] MARCHLER-BAUER A, DERBYSHIRE M K, GONZALES N R, LU S, CHITSAZ F, GEER L Y, GEER R C, HE J, GWADZ M, HURWITZ D I. CDD: NCBI's conserved domain database. Nucleic Acids Research, 2015, 43(Database issue):D222-226.
[17] DONG Y, WANG H, CAO J, REN J, FAN R, HE X, SMITH G W, DONG C. Nitric oxide enhances melanogenesis of alpaca skin melanocytes in vitro by activating the MITF phosphorylation. Molecular and Cellular Biochemistry, 2011, 352(1/2):255-260.
[18] SINGH R K, MALLELA R K, CORNUET P K, REIFLER A N, CHERVENAK A P, WEST M D, WONG K Y, NASONKIN I O. Characterization of three-dimensional retinal tissue derived from human embryonic stem cells in adherent monolayer cultures. Stem Cells and Development, 2015, 24(23):2778-2795.
[19] PARVINI M, PARIVAR K, SAFARI F, TONDAR M. Generation of eye field/optic vesicle-like structures from human embryonic stem cells under two-dimensional and chemically defined conditions. In vitro Cellular & Developmental Biology Animal, 2015, 51(3): 310-318.
[20] 朱芷葳, 贺俊平, 于秀菊, 程志学, 董常生. Mitf-M在羊驼皮肤组织的表达与序列分析及免疫组织化学定位. 中国农业科学, 2012, 45(4):794-800. ZHU Z W, HE J P, YU X J, CHENG Z X, DONG C S. Expression, sequence analysis and immunohistochemical localization of Mitf-M transcription factor in alpaca skin. Scientia Agricultura Sinica, 2012, 45(4):794-800. (in Chinese)
[21] SUZUKI K T, ISOYAMA Y, KASHIWAGI K, SAKUMA T, OCHIAI H, SAKAMOTO N, FURUNO N, KASHIWAGI A, YAMAMOTO T. High efficiency TALENs enable F0 functional analysis by targeted gene disruption in Xenopus laevis embryos. Biology Open, 2013, 2(5): 448-452.
[22] YAHALOM C, SHARON D, DALIA E, SIMHON S B, SHEMESH E, BLUMENFELD A. Combined occurrence of autosomal dominant aniridia and autosomal recessive albinism in several members of a family. Ophthalmic Genetics, 2015, 36(2):175-179.
[23] RAVIV S, BHARTI K, RENCUS-LAZAR S, COHEN-TAYAR Y, SCHYR R, EVANTAL N, MESHORER E, ZILBERBERG A, IDELSON M, REUBINOFF B. PAX6 regulates melanogenesis in the retinal pigmented epithelium through feed-forward regulatory interactions with MITF. PLoS Genetics, 2014, 10(5):e1004360.
[24] 马淑慧, 薛霖莉, 徐刚, 侯亚琴, 耿建军, 曹靖, 赫晓燕, 王海东,董常生. 黑色素细胞中过量表达miR-137对TYRP-1和TYRP-2的影响. 中国农业科学, 2013, 46(16):3452-3459. MA S H, XUE L L, XU G, HOU Y Q, GENG J J, CAO J, HE X Y, WANG H D, DONG C S. The Influences of over-expressing miR-137 on TYRP-1 and TYRP-2 in melanocytes. Scientia Agricultura Sinica, 2013, 46(16): 3452-3459. (in Chinese)
[25] YANG S, ZHANG J, JI K, JIAO D, FAN R, DONG C. Characterization and expression of soluble guanylate cyclase in skins and melanocytes of sheep. Acta Histochemica, 2016,118:219-224.
[26] ZEMKE M, DRAGANOVA K, KLUG A, SCHOLER A, ZURKIRCHEN L, GAY M H, CHENG P, KOSEKI H, VALENTA T, SCHUBELER D. Loss of Ezh2 promotes a midbrain-to-forebrain identity switch by direct gene derepression and Wnt-dependent regulation. BMC Biology, 2015, 13:103.
[27] CANTU C, ZIMMERLI D, HAUSMANN G, VALENTA T, MOOR A, AGUET M, BASLER K. Pax6-dependent, but β-catenin-independent, function of Bcl9 proteins in mouse lens development. Genes & Development, 2014, 28(17):1879-1884.
[28] 贾小云, 金雷皓, 苗潋涓, 丁娜, 范瑞文, 董常生. miR-663通过靶向TGF-β1调控羊驼黑色素细胞的黑色素生成. 中国农业科学, 2015,48(1):165-173. JIA X Y, JIN L H, MIAO L J, DING N, FAN R W, DONG C S. Melanin synthesis of alpaca melanocytes regulated by miR-663 through targeting TGF-β1. Scientia Agricultura Sinica, 2015, 48(1): 165-173. (in Chinese)
[29] PLANQUE N, LECONTE L, COQUELLE FM, MARTIN P, SAULE S. Specific Pax-6/microphthalmia transcription factor interactions involve their DNA-binding domains and inhibit transcriptional properties of both proteins. The Journal of Biological Chemistry, 2001, 276(31):29330-29337.
[30] YASUMOTO K, YOKOYAMA K, TAKAHASHI K, TOMITA Y, SHIBAHARA S. Functional analysis of microphthalmia-associated transcription factor in pigment cell-specific transcription of the human tyrosinase family genes. The Journal of Biological Chemistry 1997, 272(1):503-509.
(责任编辑 林鉴非)
Influences of Pax6 PAI Subdomain on MITF, TYR, TYRP1 and TYRP2 in Melanocytes
NIE Rui-qiang1, YANG Yu-jing1, XIE Jian-shan1,2, FAN Rui-wen1, XU Dong-mei1, YU Xiu-ju1, DUAN Zhi-cheng1, DONG Chang-sheng1
(1College of Animal Science and Veterinary Medicine, Shanxi Agricultural University, Taigu 030801, Shanxi;2School of Basic Medical Sciences, Shanxi Medical University, Taiyuan 030001)
【Objective】 Highly conserved PAX family has important effects on the differentiation of melanocytes and production of melanin. It has mainly binding sites with target gene in PD domain that is included by all of PAX family, on the otherhand, the PAI subdomain, which is located in amino terminal of PD domain, has most important effects on PD domain which bound with target gene. Many reports show that Pax6 has the most important effects on the differentiation of retinal pigment epithelial cells. This experiment studies the function of PD domain and PAI subdomain of PAX family by analysing the function of Pax6 PAI subdomain.【Method】The structure of Pax6 PD domain was analyzed by Psipred. The target gene binding sites of Pax6 PD domain was analyzed by NCBI. The binding sites of Pax6 PD domain to the promoter of MITF, TYR, TYRP1, and TYRP2 were analyzed by Jasper. The coding sequences of Pax6 PAI subdomain was amplified by PCR and the Pax6 PAI subdomain was cloned into the T-Vector, meanwhile, confirmed by sequencing. The fragment was then subcloned into a mammalian expression vector, resulting in a construction that contained a promoter driving the expression of green fluorescent protein (GFP). The plasmid vector was confirmed by sequencing. Then, the mouse melanocytes were transfected with the vector using Liposome 2000. Three methods were used in the result test, they were quantitative real-time PCR, western blot and melanin content measurement. 【Result】The target gene binding sites of Pax6 PD domain was mainly distributed in PAI subdomain which is located in amino terminal of PD domain. There was a binding site of Pax6 PD domain at -695 base of MITF promoter; Two binding sites of Pax6 PD domain at -873 base and -1133 base of TYR promoter; One binding site of Pax6 PD domain at -629 base of TYRP1 promoter; And one binding site of Pax6 PD domain at -655 base of TYRP2 promoter. The RT-PCR and western blot results showed that the four target genes and melanin content were significantly increased. MITF mRNA was significantly increased by 2.05 times (P<0.01), TYR mRNA was increased by 2.09 times, TYRP1 mRNA was increased by 2.93 times(P<0.05), TYRP2 mRNA was increased by 3.62 times (P<0.01). Compared with the control group, MITF protein was significantly increased by 1.7 times (P<0.01), TYR protein was increased to 2 times (P<0.05), TYRP1 protein was increased by 1.9 times(P<0.01), TYRP2 protein was increased to 1.37 times. Meanwhile, the melanin content was significantly increased by 1.33 times (P<0.001).【Conclusion】Results of the study demonstrated that the Pax6 PAI subdomain still promoted the expression of MITF, TYR, TYRP1, and TYRP2, while increased the production of melanin of melanocytes.
PAI subdomain; Pax6; melanin
2016-01-26;接受日期:2016-07-29
国家高技术研究发展计划(863计划, 2013AA102506)、国家公益性行业(农业)科研专项(201303119)、山西农业大学创新团队建设计划项目(CXTD201201)
联系方式:聂瑞强,E-mail:libernie@126.com。通信作者董常生,E-mail:cs_dong@sxau.edu.cn
