Clinical significance of dynamic detection for serum levels of MCP-1, TNF-α and IL-8 in patients with acute pancreatitis
2016-11-24YuanZhengYangYingXiangMianChenLiNaXianXiaoYanDeng
Yuan-Zheng Yang, Ying Xiang, Mian Chen, Li-Na Xian, Xiao-Yan Deng
1Department of Intensive Medicine, Affiliated Hospital of Hainan Medical College, Haikou, 570102, Hainan, China
2Department of Medical Oncology, Affiliated Hospital of Hainan Medical College, Haikou, 570102, Hainan, China
Clinical significance of dynamic detection for serum levels of MCP-1, TNF-α and IL-8 in patients with acute pancreatitis
Yuan-Zheng Yang1✉, Ying Xiang2, Mian Chen1, Li-Na Xian1, Xiao-Yan Deng1
1Department of Intensive Medicine, Affiliated Hospital of Hainan Medical College, Haikou, 570102, Hainan, China
2Department of Medical Oncology, Affiliated Hospital of Hainan Medical College, Haikou, 570102, Hainan, China
ARTICLE INFO
Article history:
Accepted 15 September 2016
Available online 15 November 2016
Pancreatitis
Monocyte chemotactic protein-1
Tumor necrosis factor-α
Interleukin-8
Objective: To observe dynamic changes of levels of monocyte chemotactic protein-1 (MCP-1), tumor necrosis factor-α (TNF-α) and interleukin-8 (IL-8) in patients with acute pancreatitis and to investigate its evaluation value on the severity of acute pancreatitis. Methods: A total of 109 patients with acute pancreatitis admitted were divided into mild acute pancreatitis group (MAP group, 42 cases), moderately severe acute pancreatitis (MSAP group, 35 cases) and severe acute pancreatitis (SAP group, 32 cases). ELISA was used to detect the serum levels of MCP-1, TNF-α and IL-8 of patients at day 1, day 4 and day 7 of admission to hospital. Results: The serum levels of MCP-1, TNF-α and IL-8 from MAP group, MSAP group and SAP group at day 1 of admission to hospital all significantly increased. There was a significant difference between MAP group and control group, MSAP group and MAP group, SAP group and MSAP group (P<0.05). The serum concentrations of IL-8 from MASP group and SAP group obviously increased at day 1, and there was significant difference between MASP group and MAP group, SAP group and MSAP group (P<0.05), while the difference between MAP group and control group was not obvious (P>0.05); The serum concentrations of MCP-1, TNF-α and IL-8 from MAP group all reached the highest level at day 4, which were significantly higher than the detection levels at day 1. In MSAP group and SAP group, the serum concentrations of MCP-1, TNF-α and IL-8 were the highest at day 1, which were significantly higher than the detection levels at day 4 and 7. At each detecting timing, the serum concentrations of MCP-1, TNF-α and IL-8 from MSAP group and SAP group were all higher than those of MAP group and MSAP group, respectively. Conclusions: The dynamic changes of serum levels of MCP-1, TNF-α and IL-8 in patients with acute pancreatitis have their rules, and the change rule of MAP group was different with that of MSAP and SAP group, which showed the reference value for the diagnosis and illness severity evaluation of acute pancreatitis.
1. Introduction
Acute pancreatitis is one of the common acute abdominaldiseases with the clinical characteristics of acute onset, quick development and difficulty of control. It can be classified in accordance with the illness severity as mild acute pancreatitis (MAP), moderately severe acute pancreatitis (MSAP) and severe acute pancreatitis (SAP). Some documents have indicated that about 20% of MAP patients will develop into SAP, and the multiple organ dysfunction syndromes will occur if the patients' systemic inflammatory response gets worse[1]. At present, it is believed that the inflammatory cell infiltration is the key factor for the occurrenceof acute pancreatitis. This study investigated the evaluation value on the severity of acute pancreatitis by detecting the dynamic changes of serum monocyte chemotactic protein-1 (MCP-1), tumor necrosis factor-α (TNF-α) and interleukin-8 (IL-8) in patients with acute pancreatitis.
2. Materials and methods
2.1. General materials
A total of 109 patients with acute pancreatitis treated in our hospital from June 2014 to March 2016 were selected as mild acute pancreatitis (MAP group), moderate severe acute pancreatitis group (MSAP group) and severe acute pancreatitis group (SAP group) according to the guide for diagnosis and treatment of acute pancreatitis. There were 42 cases in the MAP group. Among them, 28 cases were males and 14 were females aging from (24-66) years with the average age of (40.8±13.7) years. There were 35 cases in the MSAP group. Among them, 21 cases were males and 14 were females aging from (28-67) years with the average age of (42.2±17.8) years. There were 32 cases in the SAP group. Among them, 19 cases were males and 13 were females aging from (20-67) years with the average age of (43.7±15.8) years. The base materials of gender and age were comparable in each group.
2.2. Primary reagent and instrument
All indices were analyzed by using ELISA, in which MCP-1 kit was purchased from American IBI Group. TNF-α and IL-8 kits were purchased from Sunlong Biotech Co. Ltd (Hangzhou, China), and 680 automatic enzyme-linked immunosorbent assay systems were adopted (Bio-Rad, US). All procedures were strictly operated according to the specification.
2.3. Sample collection and index observation
Five milliliter peripheral venous blood was collected from fasting patients in each group on the 1st, 4th, and 7th day after admission. Serum was taken after high speed centrifugation, and then stored in low-temperature and constant-temperature refrigerator (-80 ℃). Using the same method to stored the serum of control group. The concentration of serum MCP-1, TNF-α and IL-8 in patients were detected using ELISA, in which the serum of control group was only taken on the day of physical examination, and no continuous dynamic testing was made.
2.4. Statistical analysis
SPSS version 17.0 was used for statistical analysis. Measurement data were expressed by mean±SD and t-test was used for comparison among groups. Enumeration data were detected by using Χ2. P<0.05 was considered to have statistical difference.
3. Results
3.1. Comparison of MAP, MSAP, SAP on the day of admission and serum MCP-1, TNF-α and IL-8 in control group
The concentration of serum MCP-1and TNF-α in MAP, MSAP and SAP groups were all evaluated. There was significantly difference among MAP group and control group, MSAP group and MAP group, SAP group and MSAP group (P<0.05). The concentration of serum IL-8 in MSAP and SAP groups was obviously increased. There was significantly difference among MSAP group and MAP group, SAP group and MSAP group (P>0.05), as shown in Table 1.
3.2. Dynamic change of serum MCP-1, TNF-α, IL-8 concentrations in MAP, MSAP and SAP groups
In MSAP group and SAP group, serum MCP-1 concentration reached the peak on Day 1 and decreased gradually on Day 4 and Day 7, while in MAP group, it reached the peak on Day 4 and decreased into the level of the first day on Day 7 (P>0.05). In all monitoring time, serum MCP-1 concentration of MSAP group washigher than that of MAP group and in SAP group, it was higher than MSAP group (all P<0.05).
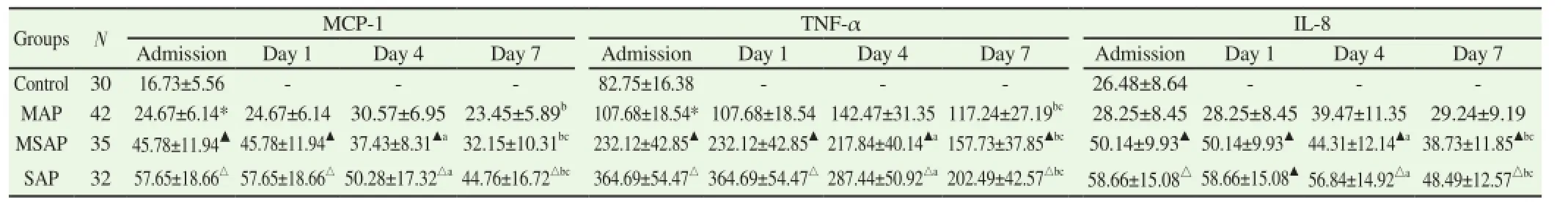
Table 1 Dynamic change of serum MCP-1, TNF-α, IL-8 concentrations in each group.
In MSAP group and SAP group, serum TNF-α concentration reached the peak on Day 1 and decreased gradually on Day 4 and Day 7, while in MAP group, it reached the peak on Day 4. In all monitoring time, serum TNF-α concentration of MSAP group was higher than that of MAP group and in SAP group, it was higher than MSAP group (all P<0.05).
In MSAP group and SAP group, serum IL-8 concentration reached the peak on Day 1 and decreased gradually on Day 4 and Day 7, while in MAP group, it reached the peak on Day 4 and decreased into the level of the admission day (P>0.05). In all monitoring time, serum IL-8 concentration of MSAP group was higher than that of MAP group and in SAP group, it was higher than MSAP group (all P<0.05).
4. Discussion
The aggregation and excessive activation of leukocyte in pancreas are the critical factors to cause pancreatitis and make the patient's condition worsened as well as cause multiple organ failure and even death[3]. The aggregation of leukocyte in the area of inflammation is a complicated multi-step process. Many cytokines assist the biological function of leukocyte like recognition and aggregation of cells and reparative process of tissues in later period of inflammation. The related cytokines in serum like TNF-α, procalcitonin and a variety of interleukins and so on have been confirmed that they can reflect the degree of severity of pancreatitis[4-7].
Cytokines can coordinate and induce the interaction between cells and in general, the concentration is very low in the human body. Stimulated by external factors, its concentration will increase rapidly in a short time and will decrease gradually after disappearance of external stimulating factors[8]. In the process of the occurrence and development of pancreatitis, the affected tissues and endothelial reticular system stimulated by endotoxin and inflammation can rapidly increase cytokine levels in the short term. However, the biological characteristics and functional structures of each kind of cytokines are all different and could produce various biological effects after affecting on specific target cells. The network system constituted by various cytokines plays a different role in MAP, MSAP and SAP groups which is embodied by the dynamic variation rule of different serum concentrations of cytokines. In this study, we monitor the serum MCP-1, TNF-α and IL-8 levels in patients with pancreatitis in several time-points and discuss its change rule and the results showed that all serum MCP-1, TNF-α and IL-8 levels in patients with MAP reached the maximum value on Day 4, while MSAP and SAP patients reached the maximum value on Day 1, which indicated that these factors are closely correlated with the process of diseases development. The longer pathogenic course of MAP is, the slower development is. Therefore, the peak values of cytokine changes like MCP-1, TNF-α and IL-8 occur later, while the progression of diseases of MSAP and SAP is generally fast and proceeds rapidly. Hence, cytokine levels reach the peak values rapidly.
Kamath et al[9] thought that MCP-1 is the key cytokine in the disease course of acute pancreatitis which mediated leucocyte infiltration causing local damage for the pancreas and released inflammatory factors to the whole body to affect other organs. There were reports pointed out that the expression of MCP-1 and mRNA in the pancreatic tissue in the caerulein-induced edematous and necrotizing pancreatitis rat models increased after induced successfully for 6 hours and it was observed by the immunocytochemistry method that pancreatic cells and neutrophil MCP-1 protein infiltrating to the pancreatic tissue expressed positively[10]. TNF-α is produced by activated macrophages. Aside from its tumor killing effect, TNF-α is an important inflammatory factor as well. It can not only mediate primary inflammatory directly, but also serve as an important chemokine in the occurrence of secondary inflammation[11]. When TNF-α was in disorder or over-produced, it can destroy microcirculation and inhibit effects such as fibrinolysis to damage the pancreas and tissues around by activating inflammatory cells and cytotoxic effect. One hour after acute pancreatitis occurred, the level of serum TNF-α elevated and reached a peak in 6 hours later[12]. Therefore, TNF-α plays a key role of all the processes in the development of acute pancreatitis. IL-8 is a chemokine and inducer of neutrophils. Acute pancreatitis was stimulated by active substances released by pancreatic tissues. The monocyte-macrophages system was activated and a large amount of cytokines including IL-8 and inflammation mediators were released, which, as a result, aggravated pancreatic damage and leaded to systemic complications. Reported showed that the level of IL-8 in serum was related to the severity of acute pancreatitis[13]. There are also researches revealed that IL-8 has predictive value in fatal pancreatitis[14]. In this study, the concentrations of serum MCP-1, TNF-α and IL-8 in the MAP group peaked at the 4th day and increased significantly as compared to those determined on the admission day. Compared with IL-8, the concentrations of MCP-1 and TNF-α in patients with MAP rose earlier, while IL-8 showed no significant change on the admission day, which was correspondent with the disease progression of MAP and the biological functions of MCP-1, TNF-α and IL-8. In groups of MSAP and SAP, the highest concentrations of serum MCP-1, TNF-α and IL-8 appeared on the admission day and those concentrations were all distinctly higher than those determined at the 4th and 7th days. At all monitoring time points, the concentrations of serum MCP-1, TNF-α and IL-8 in theMSAP group were all higher than those in the MAP group but lower than those of the SAP group. Although the concentrations were on a declining trend after the peak concentrations appeared, they still maintained on high levels at the 4th and 7th days. That dynamic change was consistent with the occurrence time of MSAP and SAP inflammations. It is suggested that the dynamic monitoring of MCP-1, TNF-α and IL-8 is conducive for the severity evaluation of patients with acute pancueatitis.
In conclusion, serum MCP-1, TNF-α and IL-8 levels in patients with acute pancreatitis have a dynamic variation rule, which is different with the change rule of MAP, MSAP and SAP. That is of great reference value in the diagnosis and severity evaluation for patients with acute pancreatitis and can be used as the early auxiliary reference for the diagnosis and treatment of acute pancreatitis.
Conflict of interest statement
We declare that we have no conflict of interest.
References
[1] Yue W, Liu Y, Ding W, Jiang W, Huang J, Zhang J, et al. The predictive value of the prealbumin-to-fibrinogen ratio in patients with acute pancreatitis. Int J Clin Pract 2015; 69(10): 1121-1128.
[2] Division of Pancreatic Surgery, Branch of Sugery, Chinese Medical Association. Guide for diagnosis and treatment of acute pancreatitis (2014). Chin J Prac Surg 2015; 35(1): 4-7.
[3] Zhang DY, Gao C, Xie SM, Chen WC. Dynamic changes of peripheral blood IL-6, HGF and Ang-2 in patients with acute pancreatitis. Chin J Gastroenterol 2015; 20(7): 398-402.
[4] Dias BH, Rozario AP, Olakkengil SA, V A. Procalcitonin strip test as an independent predictor in acute pancreatitis. Indian J Surg 2015; 77(Suppl 3): 1012-1017.
[5] Song R, Yu D, Park J. Changes in gene expression of tumor necrosis factor alpha and interleukin 6 in a canine model of caerulein-induced pancreatitis. Can J Vet Res 2016; 80(3): 236-241.
[6] Jiao SH, Liu PL, Wen YH. Application value of joint detection of serum CRP, LPS, IL-1β and ICAM1 for diagnosis of acute pancreatitis. J Clin Hepatol 2016; 1: 131-134.
[7] Sun YP. Severity evaluation of TNF- and IL-6 for acute pancreatitis. Chin J Crit Care Med 2015; 35(z2): 85-86.
[8] Fietta P, Costa E, Delsante G. Interleukins (ILs), a fascinating family of cytokines. Part Ⅱ: ILs from IL-20 to IL-38. Theor Biol Forum 2015; 108(1-2): 19-40.
[9] Kamath MG, Pai CG, Kamath A, Kurien A. Monocyte chemoattractant protein-1, transforming growth factor-beta1, nerve growth factor, resistin and hyaluronic acid as serum markers: comparison between recurrent acute and chronic pancreatitis. Hepatobiliary Pancreat Dis Int 2016; 15(2): 209-215.
[10] Yu MM, Liu JJ, Wang YN, Lian G, Wang YH, Liu GQ, et al. Protective effects of grape polyphenol on pancreatic tissues of mice with caeruleininduced acute pancreatitis. Chin J Pathophys 2014; 30(10): 1820-1826.
[11] Wendling D, Prati C. Paradoxical effects of anti-TNF-α agents in inflammatory diseases. Expert Rev Clin Immunol 2014; 10(1): 159-169.
[12] Jiang Y, An Y, Jiang D, Wu B, Yang Y, Sun D. TNF-α Regulating Interleukin-33 induces acute pancreatic inflammation in rats. Ann Clin Lab Sci 2016; 46(1): 54-59.
[13] Zheng Q, Yang C. Differences between the expression of inflammatory markers in patients with acute pancreatitis and severe segree of correlation analysis. Sichuan Med J 2015; 36(11): 1552-1554.
[14] Liang CH, Tan Q, Xiong W. Diagnosis value of detection of HBP joint IL-8 PCT in secondary infection of severe acute pancreatitis. Zhejiang Clin Med J 2016; 18(7): 1327-1328.
Document heading 10.1016/j.apjtm.2016.09.001
15 July 2016
in revised form 16 August 2016
✉First and Yuan-Zheng Yang, Department of Intensive Medicine, Affiliated Hospital of Hainan Medical College, Haikou, 570102, Hainan, China.
Tel: 15103048576
E-mail: hhyangyuanzheng@163.com
Foundation project: It was supported by Health and Family Planning Commission of Hainan Province, China, Scientific Research Project (Grant No. 14A210207).
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Modifiable determinants of attitude towards dengue vaccination among healthy inhabitants of Aceh, Indonesia: Findings from a communitybased survey
- Expression and mechanism of action of miR-196a in epithelial ovarian cancer
- Protective effect of antioxidant on renal damage caused by Doxorubicin chemotherapy in mice with hepatic cancer
- Mechanism of action of Zhuyu Annao pill in mice with cerebral intrahemorrhage based on TLR4
- Acetylcholinesterase, butyrylcholinesterase and paraoxonase 1 activities in rats treated with cannabis, tramadol or both
- Anti-tumor activity of tanshinone IIA in combined with cyclophosphamide against Lewis mice with lung cancer
