Anti-tumor activity of tanshinone IIA in combined with cyclophosphamide against Lewis mice with lung cancer
2016-11-24QiLiKeHuSiTangLiFangXuYuChuanLuo
Qi Li, Ke Hu, Si Tang, Li-Fang Xu, Yu-Chuan Luo
Department of Respiratory Medicine, Renmin Hospital of Wuhan University, 430060 China
Anti-tumor activity of tanshinone IIA in combined with cyclophosphamide against Lewis mice with lung cancer
Qi Li, Ke Hu✉, Si Tang, Li-Fang Xu, Yu-Chuan Luo
Department of Respiratory Medicine, Renmin Hospital of Wuhan University, 430060 China
ARTICLE INFO
Article history:
Accepted 17 September 2016
Available online 20 November 2016
Lung cancer
Bax
Bcl-2
VEGF
Angiostatin
Endostatin
Objective: To explore the anti-tumor activity of tanshinone IIA in combined with cyclophosphamide against Lewis mice with lung cancer and the effect on cellular immune function. Methods: Lewis tumor cells were inoculated subcutaneously into the right armpit of mice in each group (n=20) to establish Lewis lung cancer mice model. After model establishment, mice in the model group were given normal saline by lavage, qd. Mice in treatment I group were given intraperitoneal injection of TanIIA, 15 mg/kg, qd. Mice in treatment II group were given intraperitoneal injection of CTX, 25 mg/kg, qd. Mice in treatment III group were given intraperitoneal injections of TanIIA and CTX, in which the administration method of TanIIA was the same as in treatment I group, continuously for 2 weeks, and the dosage of CTX was the same as in treatment II group, 24h after model establishment, every other day. Mice were sacrificed 2 weeks after establishment. The tumor tissues were collected to calculate the anti-tumor rate. Immunohistochemistry was used to detect the expressions of Bcl-2, Bax, VEGF, Angiostatin, and Endostatin. FCM was used to detect T lymphocyte subsets in spleen and liver of mice. Results: The tumor weight in treatment I, II, and III groups was significantly lower than that in the model group (P<0.05). The tumor weight in treatment III group was significantly lower than that in treatment I and II groups (P<0.05). The anti-tumor rate in treatment II and III groups was significantly higher than that in treatment I group (P<0.05). Bcl-2 expression in the tumor tissues of treatment I, II, and III groups was significantly lower than that in the model group (P<0.05), while Bax expression was significantly higher than that in the model group (P<0.05). Bcl-2 expression in the tumor tissues of treatment I and II groups was significantly higher than that in treatment III group (P<0.05), while Bax expression was significantly lower than that in treatment III group (P<0.05). CD4+and CD4+/CD8+in treatment I, II, and III groups were significantly higher than those in the model group (P<0.05). CD4+in treatment III group was significantly higher than that in treatment I and II groups (P<0.05), while CD4+/CD8+was significantly higher than that in treatment II group (P<0.05). The comparison of CD8+among each group was not statistically significant (P>0.05). NK cell activity in treatment I, II, and III groups was significantly higher than that in the model group (P<0.05). NK cell activity in treatment III group was significantly higher than that in treatment I and II groups (P<0.05). Conclusions: TanIIA in combined with CTX can down regulate Bcl-2 expression in lung cancer tissues, up regulate Bax expression, inhibit the neovascularization of tumor tissues, and enhance the immunological function, with a significant anti-tumor activity.
1. Introduction
Lung cancer is a common malignant tumor of respiratory system in the clinic, with an increasing morbidity due to the alterations of modern environment and life style, and has been one of the diseases which can severely threaten the human life and health [1-3]. The pathogenesis of lung cancer is not yet clear. Some scholars argue that its pathogenesis is closely associated with the long-term heavy smoking[4]. According to the statistics [5], in recent 50 years, the morbidity and fatality rate of lung cancer in males rank the first place among all malignant tumors. With the continuous maturity of radiotherapy and chemotherapy technology, the efficacy by medicine of lung cancer is improved greatly, but the total 5-year survival rate is only 10%-15%, with a poor long-term efficacy[6]. A large amount of researches verify that[7] operation, radiotherapy, and chemotherapy can produce a certain effect on the immunological function; therefore, how to effectively treat lung cancer and reduce the effect on immunological function as much as possible has been the hot tissue of clinical research. CTX, a common anti-tumor drug in the clinic, can intervene DNA and RNA functions in tumor cells, with a wide application[8, 9]. TanIIA can significantly resist the atherosclerosis, inhibit the platelet aggregation, kill various tumor cells, and promote their apoptosis [10]. In order to observe the antitumor activity of Tan IIA in combined with CTX against lung cancer mice and the effect on cellular immune function, C57BL/6J mice were selected to establish Lewis lung cancer mice model. After model establishment, mice were given Tan IIA and CTX to observe its anti-tumor activity against Lewis mice with lung cancer and the effect on T lymphocyte subsets.
2. Materials and methods
2.1. Experimental animals
A total of 80 male C57BL / 6J mice (18-20g), SPF, were obtained from Beijing HFK Bioscience Co. Ltd. The animals were housed under standard conditions of temperature (20±2) ℃ and relative humidity (36%), and had free access to diet and water. All the experiments were performed in the Animal Experimental Center of Wuhan University. The animals were treated and cared for strictly in accordance with the guidelines recommended by the Laboratory Animal Administration Rules. The experimental protocol was approved by our departmental ethics committee.
2.2. Drugs and chemicals
Tan IIA sulfonate sodium injection was purchased from Shanghai No.1 Biochemical and Pharmaceutical Co. Ltd (Specification: 10 mg/2 mL, Approval No. H31022558). CTX injection was purchased from Jiangsu Hengrui Medicine Co. Ltd (Specification: 0.2g/ piece, Approval No. H32020857). Lewis lung cancer cell lines were provided by the Immunology Department of China Medical University. The anti-mice CD4 kits and anti-CD8 monoclonal antibodies (marked by PE) were purchased from BD Company, US. BBSDSC super clean bench was purchased from Shanghai Biboase. Olympus biological microscope (Japan), AnKe80 -ZC desk centrifuge, 301- 268. 001 histotome, and Leica-DM2500 B microscope (German) were also purchased. Image-pro plus 6. 0 image processing software was adopted.
2.3. Model establishment
Lewis lung cancer cell lines were melted at 37 ℃, and centrifuged at 14 000 r /min. The supernatant was abandoned, and the suspension was diluted with cell concentration of 1 107 /mL for preservation. The prepared cell suspension (0.2 mL) was inoculated subcutaneously into the right armpit of mice, with 2 generations. After inoculation, the mice were randomized into the model group, treatment I, II, and III groups with 20 mice in each group. After model establishment, mice in the model group were given normal saline by lavage, qd. Mice in treatment I group were given intraperitoneal injection of TanIIA, 15 mg/kg, qd. Mice in treatment II group were given intraperitoneal injection of CTX, 25 mg/kg, qd. Mice in treatment III group were given intraperitoneal injections of TanIIA and CTX, in which the administration method of TanIIA was the same as in treatment I group, continuously for 2 weeks, and the dosage of CTX was the same as in treatment II group, 24h after model establishment, every other day.
2.4. Observation indicators
Mice were sacrificed 2 weeks after establishment. The tumor tissues were collected to calculate the anti-tumor rate. Anti-tumor rate (%)=(average tumor weight in the model group-average tumor weight in the treatment group)/average tumor weight in the model group×100%. Immunohistochemistry was used to detect the expressions of Bcl-2, Bax, VEGF, Angiostatin, and Endostatin. The spleen of mice in each group was collected under a sterile condition. The spleen cell suspension was prepared with cell concentration of 1 ×107/mL. FCM was used to detect T lymphocyte subsets in spleen of mice.
2.5. Statistical analysis
SPSS 16.0 software was used for the statistical analysis. The measurement data were expressed as mean±SD, and t test was used. P<0.05 was regarded as statistically significant.
3. Results
3.1. Comparison of the anti-tumor rate among four groups
The tumor weight in treatment I, II, and III groups was significantly lower than that in the model group (P<0.05). The tumor weight in treatment III group was significantly lower than that in treatment I and II groups (P<0.05). The anti-tumor rate in treatment II and III groups was significantly higher than that in treatment I group (P<0.05). The comparison of anti-tumor rate between treatment II and III groups was not statistically significant (P>0.05) (Table 1).

Table 1 Comparison of the tumor weight and anti-tumor rate among four groups (n=20).
3.2. Comparison of Bcl-2 and Bax expressions in the tumor tissues among four groups
Bcl-2 expression in the tumor tissues of treatment I, II, and III groups was significantly lower than that in the model group (P<0.05), while Bax expression was significantly higher than that in the model group (P<0.05). Bcl-2 expression in the tumor tissues of treatment I and II groups was significantly higher than that in treatment III group (P<0.05), while Bax expression was significantly lower than that in treatment III group (P<0.05) (Table 1).
3.3. Comparison of VEGF, Angiostatin, and Endostatin expressions in the tumor tissues among four groups
VEGF expression in treatment I, II, and III groups was significantly lower than that in the model group (P<0.05), while Angiostatin and Endostatin expressions were significantly higher than those in the model group (P<0.05). VEGF expression in treatment I and II groups was significantly higher than that in treatment III group (P<0.05), while Angiostatin and Endostatin expressions were significantly lower than those in treatment III group (P<0.05) (Table 2).
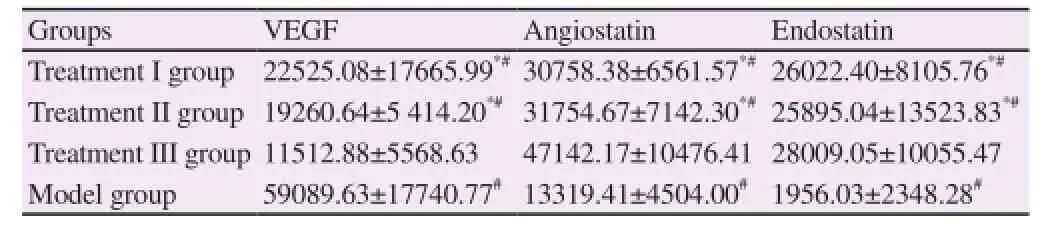
Table 2 Comparison of VEGF, Angiostatin, and Endostatin expressions in the tumor tissues among four groups (n=20).
3.4. Comparison of the immunological function among four groups
CD4+and CD4+/CD8+in treatment I, II, and III groups were significantly higher than those in the model group (P<0.05). CD4+in treatment III group was significantly higher than that in treatment I and II groups (P<0.05), while CD4+/CD8+was significantly higher than that in treatment II group (P<0.05). The comparison of CD8+among each group was not statistically significant (P>0.05). NK cell activity in treatment I, II, and III groups was significantly higher than that in the model group (P<0.05). NK cell activity in treatment III group was significantly higher than that in treatment I and II groups (P<0.05) (Table 3).
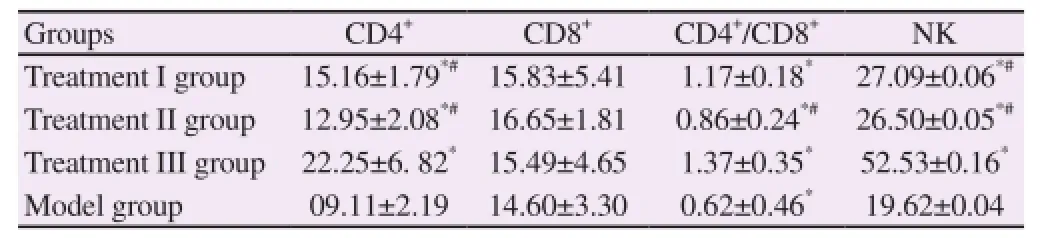
Table 3 Comparison of T lymphocyte subsets levels among four groups (%) (n=20).
4. Discussion
Lung cancer is a common malignant tumor of respiratory system in the clinic, occurring more in males than in females, and ranks the first place of malignant tumor death among the urban population [11]. Non-small cell lung cancer is a common type in the clinic, including large cell carcinoma, adenocarcinoma, and squamous-cell carcinoma, whose cell division is slower than other cancer cells, with later spreading and metastasis [12-15]. Due to its concealment, most non-small cell lung cancer is discovered on physical examination, but already progresses into the middle and advanced stage, with a low 5-year survival rate [16-18]; therefore, how to conduct an effective treatment is of great significance in improving the patients' survival.
Multiple factors are involved in the lung cancer pathogenesis which is not yet completely clarified [19]. Some scholars argue that [20] the imbalance of cell apoptosis and proliferation may be a main factor for developing lung cancer. Cell apoptosis is an initiative and physiological death mechanism of cell itself, and is regulated by the apoptosis inhibiting factor and pro-apoptosis factor. Bcl-2 and Bax belong to Bcl-2 family genes, and are closely associated the occurrence of various humor tumors [21]. Bcl-2 a proto-oncogene cloned from the follicular B-cell non-Hodgkin lymphoma, and can inhibit the cell apoptosis, while Bax is a pro-apoptosis gene, and can resist Bcl-2 [22]. The results in the study showed that Bcl-2 expression in treatment I, II, and III groups was significantly lower than that in the model group (P<0.05), while Bax expression was significantly higher than that in the model group (P<0.05), suggesting that the 3 treatment protocols can down regulate Bcl-2 expression, and up regulate Bax expression. The results in the study also showed that Bcl-2 expression in the tumor tissues of treatment I and II groups was significantly higher than that in treatment III group (P<0.05), while Bax expression was significantly lower than that in treatment III group (P<0.05), indicating that Tan IIA in combined with CTX can promote the lung cancer cell apoptosis, with a more significant effect.
Some scholars argue that [22] when the tumor diameter is greater than 1mm, the surrounding tissues are unable to provide the required nutrition for its growth. The sequential growth of tumor must depend on new blood vessels to maintain the nutrition supply, otherwise, degeneration will occur in the tumor cells. The tumor angiogenesis is a multi-step complicated process, including vascular endothelial cell proliferation and migration, and extracellular matrix degradation. VEGF is an important factor to promote the angiogenesis, while Angiostatin and Endostatin are the main inhibiting factors for angiogenesis [23]. The results in the study showed that VEGF expression in treatment I, II, and III groups was significantly lower than that in the model group (P<0.05), while Angiostatin and Endostatin expressions were significantly higher than those in the model group (P<0.05), suggesting that the 3 treatment protocols can inhibit the angiogenesis of tumor tissues. The results in the study also showed that VEGF expression in treatment I and II groups was significantly higher than that in treatment III group (P<0.05), while Angiostatin and Endostatin expressions were significantly lower than those in treatment III group (P<0.05), indicating that Tan IIA in combined with CTX can better effectively inhibit the angiogenesisof tumor tissues in order to reach the goal of anti-tumor. In the study, the anti-tumor rate in treatment III group was the highest, which is associated with the effective inhibition on the tumor angiogenesis by Tan IIA and CTX.
Some experiments verify that [24, 25] the multiple immune escape mechanism formed in the growth process of cancer cells is an important factor for the continuous occurrence and development of tumor cells; therefore, the immunological function plays a vital role in the tumor treatment process. The results in the study showed that CD4+in treatment III group was significantly higher than that in treatment I and II groups (P<0.05), while CD4+/CD8+was significantly higher than that in treatment II group (P<0.05); moreover, NK cell activity in treatment III group was significantly higher than that in treatment I and II groups (P<0.05), indicating that this treatment protocol can effectively enhance the immunological function to strengthen the anti-tumor effect.
In conclusion, TanIIA in combined with CTX can down regulate Bcl-2 expression in lung cancer tissues, up regulate Bax expression, inhibit the neovascularization of tumor tissues, and enhance the immunological function, with a significant anti-tumor activity.
Conflict of interest statement
We declare that we have no conflict of interest.
References
[1] Yunchao Z, Yingjie J, Jinli Z. Impact of Xiaoyan decoction on muscle protein degradation in lung cancer cachexia mice. J Tradit Chin Med 2016;57(9):775-778.
[2] Shuyun L, Huidong Y, Mei Y. Animal experiment on anti-tumor activity of magnetic nanocarrier and doxorubicin with target simple external magnetic fields. Cancer Res Clin 2016;28(5):289-293,299.
[3] Nili R, Ping C. Role of IL-6 in progress of estrogen promoting lung adenocarcinoma in mice and its mechanism. Cancer Prev Res 2016;43(4):253-257.
[4] Lei P, Qinggan Z, Peifeng C. Effect of Qingre Xiaoji recipe on VEGF and bFGF expression in Lewis lung cancer mice with transplantation tumor. Zhejiang J Tradit Chin Med 2016;51(5):338-339.
[5] Hui Z, Yongjun Z, Aiqin Z. Experimental study on elemene in inhibiting the chemotherapy resistance in lung cancer with CDK8 gene expression reversion. Zhejiang J Tradit Chin Med 2016;51(1):59-61.
[6] Hu Y, Zhou J, Ye F, Xiong H, Peng L, Zheng Z, et al. BRD4 inhibitor inhibits colorectal cancer growth and metastasis. Int J Mol Sci 2015;16(1):1928-1948.
[7] Lei P, Fei Z, Peifeng C. Effect of konjac glucomannan on immune function in Lewis lung cancer cell transplanted mice. Zhejiang J Integr Tradit Chin Western Med 2016;26(4):324-327.
[8] Weidong Z, Mengzhao Y, Ye C. Beneficial effects of autophagy in tanshinone IIA reducing endotoxin-induced acute lung injury and its related mechanisms. Zhejiang J Integr Tradit Chin Western Med 2016;26(6):532-535.
[9] Wang DC, Wang LC, Wang LJ, Chen G, Zhao YX, Zhao ZF, et al. Inhibitory effect of imrecoxib combined with lobaplatin on tumor growth and lymph node metastasis of human lung cancer xenografts in nude mice. Chin J Oncol 2016;38(5):340-345.
[10] Xiao S, Ji Z, Junfeng Z. Effect of tanshinone IIA on TGF-β1/Smads signaling pathway in pulmonary fibrosis cells of rats. Zhejiang J Integr Tradit Chin Western Med 2016;26(5):414-417.
[11] Qiu Y, Hu Y, Zhang ZY, Ye L, Xu FH, Schneider ME, et al. Genetic association of osteopontin (OPN) and its receptor CD44 genes with susceptibility to Chinese gastric cancer patients. J Cancer Res Clin Oncol 2014;140(12):2143-2156.
[12] Xiong Z, Deng P, Hu C, Liu J, Yang H, Zhou J, et al. Quantitatively evaluating the evolution of the tumor perfusion in A549 lung adenocareinoma transplantation model induced by antiangiogenic treatment. Chin Med J 2016;96(4):306-310.
[13] Matthew Sherger, William Kisseberth, Cheryl London, Susan Olivo-Marston, Tracey L Papenfuss. Identification of the subpopulations of myeloid-derived suppressor cells and the function study in elderly tumorbearing mice. Chin J Geriatr 2016;35(6):651-655.
[14] Yonghong D, Jiong C, Xin L. 2-18F-flupropanate microPET imaging in Lewis lung cancer mice. Chin J Nucl Med Mol Imaging 2016;36(2):180-183.
[15] Chen Yuan, Ai Xiao-Jia, Wang Zhi-Qi, Tian Sha, Zhou Qing, Pei Gang, et al. Study on anti-lung cancer efficiency of centipede extracts in vitro and vivo experiments. Chin J Inform Tradit Chin Med 2016;23(5):61-63.
[16] Zhu Ya-fang, Jiang Feng, Wu Chao-hua, Zhou Xue, Shen Xiang-chun, TAO Ling. Preparation and characterization of pharmaceutical properties of tanshinone IIA microspheres. Chin Herb Med 2016;39(1):138-142.
[17] Tong Yeling, Dai Guanhai, Ren Zeming, Chen Xuan, Yang Feng. Inhibition effect of chloroform extracts from Yangmei (Myrica rubra) bark on Lewis lung cancer of mice. Chin J Tradit Med Sci Technol 2016;23(1):50-52.
[18] Shiman X, Yongai L, Jinwei Q. Pathological study on early lung adenocarcinoma model in mice. Acta Lab Anim Sci Sin 2016;24(1):1-6.
[19] Jin W, Sen L, Youpeng Z. Effect of Tan IIA on the microvessel density, BFGF, and VEGF in bladder cancer mice with transplantation tumor. Chin J Exp Surg 2016;33(5):1275-1277.
[20] Bu W, Zhihua Z, Xiulong Z, Xin Gu, Jianghua T. Effect of tetramethylpyrazine in combination with cisplatin on the growth and angiogenesis of transplanted Lewis lung carcinoma in mice. Chin J Clin Pharmacol 2016;32(1):51-54.
[21] Haile H, Jing L, Xinglin G. Inhibition effect of Tan IIA sulfonate on the expression of STIM1/Orai1 in pulmonary artery smooth muscle cells induced by hypoxia. Chin J Tuberc Respir Dis 2016;39(1):62-65.
[22] Wei W, Bixia D, Li Z. Effect and mechanism of radiosensitization of poly (ADP-Ribose) polymerase inhibitor on Lewis cells and xenografts. Chin J Lung Cancer 2016;19(1):16-23.
[23] Dekun W, Hanwei W, Jian P. Intervention effect of sodium tanshinone IIA sulfonate on ANG-TGF-PN signal pathways in infarction tissue of rats with heart failure. J Emerg Tradit Chin Med 2016;25(6):949-952.
[24] Mo Z, Zhongmin J. Effects of phillyrin on VEGF and endostatin expression in Lewis lung carcinoma. Chin J Pathophysiol 2016;32(1):167-171,178.
[25] Zihang X, Jiemiao H, Xiao C. Research progress of mouse lung cancer models. China Med Herald 2016;13(9):63-67.
Document heading 10.1016/j.apjtm.2016.09.003
10 July 2016
in revised form 10 September 2016
Qi Li, Department of Respiratory Medicine, Renmin Hospital of Wuhan University, 430060 China.
Tel: 13429868677
E-mail: liqi13429868677@163.com
✉ Ke Hu, Department of Respiratory Medicine, Renmin Hospital of Wuhan University, 430060 China.
Tel: 18971035988
E-mail: hukejx@163.com
Foundation Project: The study was supported by the Scientific and Research Planning Project of Education Department in Hubei Province with the grant number of Q20131106.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Modifiable determinants of attitude towards dengue vaccination among healthy inhabitants of Aceh, Indonesia: Findings from a communitybased survey
- Clinical significance of dynamic detection for serum levels of MCP-1, TNF-α and IL-8 in patients with acute pancreatitis
- Expression and mechanism of action of miR-196a in epithelial ovarian cancer
- Protective effect of antioxidant on renal damage caused by Doxorubicin chemotherapy in mice with hepatic cancer
- Mechanism of action of Zhuyu Annao pill in mice with cerebral intrahemorrhage based on TLR4
- Acetylcholinesterase, butyrylcholinesterase and paraoxonase 1 activities in rats treated with cannabis, tramadol or both
