Expression and mechanism of action of miR-196a in epithelial ovarian cancer
2016-11-24BoYangShengZeLiLingMaHongLiLiuJianLiuJunJunShao
Bo Yang, Sheng-Ze Li, Ling Ma, Hong- Li Liu, Jian Liu, Jun-Jun Shao
Department of Female Tumor, First Affiliated Hospital of Bengbu Medical College, Bengbu, Anhui, 233004, China
Expression and mechanism of action of miR-196a in epithelial ovarian cancer
Bo Yang✉, Sheng-Ze Li, Ling Ma, Hong- Li Liu, Jian Liu, Jun-Jun Shao
Department of Female Tumor, First Affiliated Hospital of Bengbu Medical College, Bengbu, Anhui, 233004, China
ARTICLE INFO
Article history:
Accepted 16 September 2016
Available online 20 November 2016
MiR-196a
Epithelial ovarian cancer
Migration
Invasion
HOXA10
Objective: To explore the expression, biological function and possible mechanism of action of microRNA molecular-196a (miR-196a) in epithelial ovarian cancer. Methods: RT-PCR was used to detect the expression quantities of epithelial ovarian tissue, benign ovarian tissue, normal ovary epithelial tissue, ovarian cancer cell lines and miR-196a in normal ovarian epithelial cells to analyze the relationship between the expression of miR-196a and the clinical pathologic parameters of ovarian cancer. Among those cell lines, the cell line of which miR-196a expressed the most or least was selected and transfected the ovarian cancer cell line by using negative control plasma and miR-196a inhibitor. After transfection, RT-PCR was used to test the expression quantity of miR-196a, Transwell chamber method was applied to determine the migration and invasion abilities of ovarian carcinoma cells and Western blot was employed to detect the expression of HOXA10 protein. Results: The relative expression quantities of miR-196a in ovarian cancer tissue and benign ovarian tissue were significantly higher than that in normal ovarian epithelial tissue, and the expression quantity of miR-196a in ovarian cancer tissue was distinctively higher than that in benign ovarian tissue (P < 0.05). Among 78 cases of epithelial ovarian cancer, the expression quantities of miR-196a in patients with low differentiation were all significantly higher than those in patients with high differentiation (P< 0.05). The expression of miR-196a showed no significant relation with age, clinical stage and whether CA125 was positive or not in patients (P > 0.05). Compared with normal ovarian epithelial cell line IOSE80, the expression quantities of miR-196a of all ovarian cancer cell lines increased obviously and differences were statistically significant (P < 0.05). Among them, the expression of miR-196a of ovarian cancer cell line SKOV3 was the highest, while it decreased significantly (4.678 ± 0.785 vs. 2.131 ± 0.345, t = 2.938, P < 0.05) after the ovarian cancer cell line SKOV3 was transfected by miR-196a inhibitor. The results of Transwell chamber method showed that the migration and invasion abilities of ovarian cancer cells SKOV3 were declined significantly after the expression of miR-196a was down-regulated and the difference showed statistical significance (P < 0.05). The results of Western blot revealed that the relative expression of HOXA10 decreased distinctly after the expression of miR-196a was down-regulated and also the difference showed statistical significance (P < 0.05). Conclusions: The miR-196a might serve as a cancer-promoting gene to promote the migration and invasion of epithelial ovarian cancer by downstream target gene HOXA10.
1. Introduction
The fatality rate of epithelial ovarian cancer has topped the listof gynecologic malignant tumors. Epithelial ovarian cancer is a common malignant tumor with concealed onset, difficult for early diagnosis, high rate of recurrence and migration, intractable treatment and poor prognosis, which seriously threatens women's life and health[1]. At present, researchers on the pathogenesis of epithelial ovarian cancer are still in the stage of exploration. Current studies mostly concentrate on levels of gene regulation such as histone modification, DNA methylation and non-coding RNA[2-4]. Non-RNA is different from protein-coding genes. It regulates andcontrols network to regulate cell differentiation, disease occurrence and the nature of biological evolution and heredity by new RNA-mediated genetic massage expression, which provides new thoughts for the pathogeneses and treatments of multiple important human diseases with more and more attentions.
MicroRNA molecular-196a (miR-196a) is a member of the noncode RNA family with a depth of 21-23 nucleotides, which combines with downstream target gene mRNA 3' untranslated regions (3'-UTR) adequately or deficiently to specifically explain or inhibit the translation of mRNA[5]. Many important life activities such as the growth and differentiation of cells on organisms are related to miRNA. It is supposed that miRNA regulates and controls about 1/3 genes of all human genomes, and it has been found that many diseases have relations with the abnormal expression of miRNA[6-8]. Since half of the miRNA are located in cancerregulated-related chromosomal domain fragile sites such as the regions of homozygous deletion, loss of heterozygosoty, oncogene and around tumor suppressor gene, breakpoint area and amplified region, the occurrence and development of tumors are closely related to miRNA. At the moment, it has been proved that the expression or loss of function of miRNA is closely associated with various tumors such as breast cancer[9], liver cancer[10], non-small cell lung cancer[11], gastric cancer[12], etc.
MiR-196a belongs to the family of homeotic genes (HOX) and plays different roles in different cells and tissues. Researches in recent years has found that the over-expression of miR-196a in cervical cancer[13], oral cancer[14] and gastric cancer[15] might play a role of cancer gene in regulating and controlling the growth, differentiation and metastasis of cancer cells. HOX is a kind of highly conserved homeobox DNA sequence consisting of 39 transcription factors which influence the growth and development of cells, embryos and tissues according to anterior-posterior acis (A-P) in the embryonic development period. It is found that the overexpression of HOXA10, a family number of HOX, in ovarian cancer is closely related to the occurrence of ovarian cancer[16].
At present, although the expression and biological function of miR-196a in ovarian cancer remain unknown, it is found through bioinformatics finding aid that HOXA10 is one of the downstream targets of miR-196a. Therefore, this study aimed to discuss the expression and biological function of miR-196a in epithelial ovarian cancer and to study whether miR-196a has a role to play by regulating and controlling the target gene HOXA10.
2. Materials and methods
2.1. Clinical tissue samples
A total of 78 epithelial ovarian cancer tissue samples acquired from December 2013 to December 2015 in our hospital were collected. Among them, 36 cases were benign ovarian tissues and the other 36 were normal pathological sections of ovary epithelial tissues. All samples were diagnosed definitely by pathological method. Patients with epithelial ovarian cancer received no radiotherapy, chemotherapy or immunotherapy before operation. The ages of the 78 patients ranged from (29-64) years with the average age of (47.75 ± 15.63) years. Out of those patients, 33 cases were diagnosed with mucinous cystadenocarcinoma and 45 suffered from serous papillary cystadenocarcinoma. As for pathological grades, 43 cases were of poorly differentiated stage and 35 were of highly to moderately differentiated stage. Clinical stages consisted of 8 cases of Ⅰ stage, 26 cases of Ⅱ stage, 32 cases of Ⅲ satge and 12 cases of Ⅳ stage. The age of the 36 patients with benign ovarian tumors ranged from 25-66 years with the average age of (46.28 ± 16.28) years, and 16 cases of them were diagnosed with mucinous cystadenoma and the other 20 cases were with papillary cystadenoma. The other 36 cases of normal ovarian epithelial tissues were all normal tissues collected from hysterectomies with ages of (27-63) years and the average age of (46.69 ± 16.10) years. The tissues were all quick-frozen by placing them in liquid nitrogen once they were excised for further application.
2.2. Cell culture
Ovarian cancer cell lines (SKOV3, OVCAR3, A2780, ES2) and normal ovarian epithelial cells (IOSE80) were all purchased from Shanghai Huiying Biological Technology co.ltd and cultured in 10% fetal bovine serum medium with saturated humidity and 5% CO2at 37 ℃. Cells growing in the logarithmic phase were selected and digested by 0.25% trypsin. The subsequent experiments were conducted after 2-3 stable passages.
2.3. RT-PCR
About 150 mg of the pathological tissue samples were selected, thawed, mixed, dissociated and centrifuged. RT-PCR was used to test the expression quantity of miR-196a of each pathological tissue sample and cell line. Firstly, TRIzol kits (Invitrogen, USA) was applied to extract the total thyroid RNA. The reverse transcription reaction system was set for RNA reverse transcription to compound the first-strand of modified miRNA cDNA. PCR amplification was conducted with the amplification system of RNU6B serving as the internal reference. The amplification procedure were 50 ℃, 15 min, 95 ℃, 10 min, 95 ℃, 5 s, 60 ℃, 30 s which circulated for 40 times. The recurring numbers reaching the cycle threshold value (Ct value) in the tube were recorded. RNU6B correction was used to acquire△Ct = CtmiR - 196a - CtRNU6B. 2-△△Ctmethod was used to calculate the relative expression quantity of miR-196a.
2.4. Plasma transfection
Negative control plasma (NC) and miR-196a inhibitor were bought from Shanghai GenePharma Co.,Ltd. The ovarian cancer cell line of which miR-196a expressed the most or least was selected and inoculated in six-well plates of 2×106/well with DMEM medium to culture until the cell confluence exceeded 50%. According to the instructions of lipidosome LipofectamineTM200 (Promega, USA), ovarian cancer cell lines growing in the logarithmic phase were transfected by NC and miR-196a inhibitor plasmas respectively and cultured until the final volume reached 2 mL.
2.5. Transwell chamber method used to detect the migration and invasion of ovarian carcinoma cells
RT-PCR was repeated to detect the changes of the expression quantities of miR-196a in ovarian cancer cell lines after they were transfected by miR-196a inhibitor plasmas. Transwell chamber method was applied to determine the migration and invasion abilities of ovarian carcinoma cells. First of all, frozen Matrigel was added in serum-free DMEM culture solution for liquefaction and dilution with 100 μL in each Transwell chamber and then washed after peridium at 37 ℃. Two groups of Transwell chambers were set. One was used for culturing embedded peridium Matrigel (invasion experiemnt) and the other did not have peridium Matrigel (migration experiemnt). The upper culture embedded chamber of the Transwell chamber was added with 2.5×104cell suspension after transfected for 24 h and normal nutrient solution was added into in bottom at the same time for 24 h. Paraformaldehyde was used for fixing, staining and washing after the lipid in the embedded chamber was sucked out. After that, cell population was calculated with the light microscope.
2.6. Western blot method used to detect the expression of HOXA10 protein
The total protein was collected on ice and then denaturalized by boiling. BCA protein quantitative kits were used to determine the content of protein and ELIASA was applied to measure the absorbance at 562 nm. The protein concentration was calculated according to the standard curve. Gel concentrations, separation gel and spacer gel were prepared in accordance with the size of target protein. A proportion of 4:1 SDS-PAGE loading buffer was added for protein loading. Spacer gel was used for electrophoresis. The set was closed by defatted milk powder after transferring membrane with β-actin serving as the internal reference. Primary antibodies were added according to the dilution ratios of β-actin (1:5 000) and HOXA10 (1:1 000). Secondary antibodies (1:5 000) were added after incubation at room temperature for 1 h and washing. After 0.5 h of incubation at room temperature, they were rinsed, colored, exposed and developing films. Image J software was employed to analyze the relative expression quantity of the protein.
2.7. Statistical management
SPSS19.0 statistical software was used for statistical management. Measurement data were expressed by mean±SD and tested by t-test. Analysis of variance and repeated measurement data were compared between groups. P < 0.05 showed statistical significance.
3. Results
3.1. Comparison of the relative expression quantities of miR-196a in various tissues
Results of ANOVA showed that differences of the comparison of the relative expression quantities of miR-196a in ovarian cancer tissues, benign ovarian tissues and normal ovarian epithelial tissues were statistically significant (P < 0.05). Further pairwise comparison found that the relative expression quantities of miR-196a in ovarian cancer tissues and benign ovarian tissues were significantly higher than that in normal ovarian epithelial tissues, and the expression quantity of miR-196a in ovarian cancer tissues was distinctly higher than that in benign ovarian tissues (P < 0.05). The difference was statistically significant (P < 0.05) (Table 1).
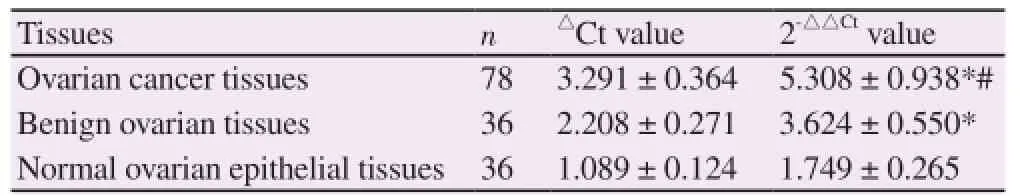
Table 1 Comparison of the relative expression quantities of miR-196a in various tissues.
3.2. The correlation between the expression of miR-196a in ovarian cancer tissues and clinical pathologic parameters
In 78 cases of epithelial ovarian cancer, the expression quantities of miR-196a in patients with low differentiation were all significantly higher than those in patients with high differentiation. The difference was statistically significant (P < 0.05). The expression of miR-196a showed no significant correlation with the age and clinical stage of patients and also had no correlation whether CA125 was positive or not in patients (P > 0.05) (Table 2).

Table 2 The correlation between the expression of miR-196a in ovarian cancer tissues and clinical pathologic parameters
3.3. The expression quantities of miR-196a in normal ovarian epithelial cell lines and ovarian cancer cell lines
Compared with normal ovarian epithelial cell line IOSE80, the expression quantity of miR-196a in each ovarian cancer cell line increased significantly and the differences were statistical significant (P < 0.05). Among them, the expression quantity of miR-196a in ovarian epithelial cell line SKOV3 was the highest (Table 3).
Expression quantity of miR-196a in each ovarian cancer cell line was as follows, IOSE80 (normal ovarian surface epithelium) was 1, SKOV3 was (4.678 ± 0.785), A2780 was (4.092 ± 0.803), OVCAR3 was (3.278 ± 0.660), ES2 was (2.863 ± 0.632).
3.4. Influence of down-regulation of miR-196a expression in migration and invasion of ovarian carcinoma cells SKOV3
The results of RT-PCR showed that the expression quantity of miR-196a of ovarian cancer cell line SKOV3 decreased significantly (4.678 ± 0.785 vs. 2.131 ± 0.345, t = 2.938, P < 0.05) after the ovarian cancer cell line SKOV3 was transfected by miR-196a inhibitor. The results of Transwell chamber method showed that the migration and invasion abilities of ovarian cancer cells SKOV3 were declined significantly after the expression of miR-196a was downregulated. Difference showed statistical significance (P < 0.05) (Table 3).

Table 3 Influence of down-regulation of miR-196a expression in migration and invasion of ovarian carcinoma cells SKOV3.
3.5. Influence of down-regulation of miR-196a expression in HOXA10 protein in ovarian carcinoma cells
After the ovarian cancer cell line SKOV3 was transfected by miR-196a inhibitor to down-regulate the expression of miR-196a, Western blot revealed was used to detect the expression of HOXA10 protein. The results showed that the relative expression of HOXA10 decreased distinctly. Difference showed statistical significance (P <0.05). Relative expression of HOXA10 of Group NC and miR-196a inhibitor were (0.938 ± 0.124) and (0.525 ± 0.083), respectively, with t = 2.920, P = 0.000.
4. Discussion
Epithelial ovarian cancer is a disease with concealed pathogenesis and unconspicuous symptoms. Due to lack of effective screening and early diagnosis method, most patients are at the mid-late stage when they go to see their doctors. The death rate of epithelial ovarian cancer maintains pretty high and is extremely apt to reoccur and migrate with poor prognosis. The survival rate is less than 30% in five years. At present, the pathogenesis of epithelial ovarian cancer is not clear yet. It is considered that understanding the molecular mechanism of the occurrence and development of epithelial ovarian cancer cells and unmasking the predictive molecular markers of differential expression to search for biological markers with high sensitivity for the early diagnosis of epithelial ovarian cancer will be conductive to improving the diagnosis and treatment level of epithelial ovarian cancer, which consequently promotes the prognosis of the disease.
MiRNA is a class of small, single-stranded RNA molecules which could not encode proteins. On one hand, miRNA can combine with target gene 3'-UTR adequately to specifically degrade mRNA. On the other hand, miRNA influences the translation of mRNA by combining with target gene 3'-UTR inadequately. By far, it is known that miRNA in various human tumors expresses abnormally and loss functions[9-12]. However, multiple miRNA express over or low in epithelial ovarian cancer. For example, Li et al.[17] reported the low expression of miR-217 in epithelial ovarian cancer tissues inhibits human epithelial ovarian cancer cells by affecting target gene IGF1R, which indicated that miR-217 could serve as a potential target for treating epithelial ovarian cancer. Ge and his colleagues studied the possible effect of miR-215 in epithelial ovarian cancer and found that the expression quantity of miR-215 in epithelial ovarian cancer tissues and ovarian cancer cells decreased significantly, indicating that miR-215 might play an anticancer role in the epithelial ovarian cancer. It was found that the expression of XIAP was inhibited obviously after miR-215 was up-regulated. The decreased expressionof XIAP can inhibit the growth of ovarian cancer cells and promote apoptosis and chemosensitivity. This study explored the relationship between the expression of miR-196a in epithelial ovarian cancer and clinical pathological parameters. The results showed that the relative expression quantities of miR-196a in ovarian cancer tissue and benign ovarian tissue were significantly higher than that in normal ovarian epithelial tissue, and the expression quantity of miR-196a in ovarian cancer tissue was distinctly higher than that in benign ovarian tissue. Differences were statistically significant (P < 0.05). In 78 cases of epithelial ovarian cancer, the expression quantities of miR-196a in patients with low differentiation were all significantly higher than those in patients with high differentiation. The difference was statistically significant (P < 0.05). The results indicated that the expression of miR-196a was related to cell differentiation. The poorer the differentiation of cancer cells, the higher the expression quantity of miR-196a will be. That further proved the cancer gene function of miR-196a.
In the large among of biological characteristics, migration and invasion are two important ones in cancer cells and also the biological basis of distant migration of tumors[19,20]. Gene transfection technique was employed in this study. The cancer cell line SKOV3 with the highest expression quantity of miR-196a was selected and transfected by miR-196a inhibitor. The results showed that the expression quantity of miR-196a declined distinctly after transfected by miR-196a inhibitor. Transwell chamber method was further used to test the migration and invasion abilities of ovarian cancer cells and found that the migration and invasion abilities of ovarian cancer cells decrease after down regulating the expression quantity of miR-196a.
Researches has found that miR-196a plays a role of cancer gene in various cancers, but its relative signal mechanism has differences[21-28]. In head and neck tumors, miR-196a exert its biological functions by targeting HOXB9[21]. Huang and his colleagues[22] investigated the effect of miR-196a in pancreas. They discovered the high expression of miR-196a in pancreas and found that the expression of NFKBIA protein could be increased by down regulating the expression of miR-196a, while silencing NFKBIA protein expression could improve the migration and proliferation of pancreatic cancer cells. Zhang et al.[23] discovered that miR-196a adjusts the migration and proliferation of cervical cancer cells by targeting affecting netrin-4. Previous studies revealed that the over expression of HOXA10, a family member of HOX, was closely related to the occurrence of ovarian cancer[29-31]. It is found through bioinformatics finding aid that 3'-UTR HOXA10 mRNA possessed a specific binding sequence of miR-196a and it is one of the downstream targets of miR-196a. For that reason, Western blot method was further used to detect the expression of HOXA10 protein in this study and the results showed that the relative expression quantity of HOXA10 decreased significantly after down regulating the expression of miR-196a. Differences were statistically significant. The results illustrated that miR-196a may serve as a cancer-promoting gene that promotes the migration and invasion of epithelial ovarian cancer by downstream target gene HOXA10.
In conclusion, the increased expression quantity of miR-196a in epithelial ovarian cancer tissues was observed. The expression quantity of miR-196a was concerned with the cell differentiation degree. After the ovarian cancer cells were transfected by miR-196a inhibitor, the expression quantity of ovarian cancer cells declined evidently, the migration and invasion abilities decreased significantly and also the expression of HOXA10 protein was down-regulated. The effect of miR-196a in the occurrence and development of epithelial ovarian cancer and its possible regulatory pathway are preliminarily clear, which provides new clues for further researches on molecular mechanism. However, due to the small sample size of this study, the relationships between miR-196a and target gene HOXA10 and between the miR-196a and its expression regulation and the occurrence and development of epithelial ovarian cancer still require further deep-going researches.
Conflict of interest statement
We declare that we have no conflict of interest.
References
[1] Kohan-Ivani K, Gabler F, Selman A, Vega M, Romero C. Role of dihydrotestosterone (DHT) on TGF-β1 signaling pathway in epithelial ovarian cancer cells. J Cancer Res Clin Oncol 2016; 142(1): 47-58.
[2] Zhou X, Teng L, Wang M. Distinct prognostic values of four-Notchreceptor mRNA expression in ovarian cancer. Tumour Biol 2016; 37(5): 6979-6985.
[3] Chen Y, Chen Q, Liu Q, Gao F. Human epididymis protein 4 expression positively correlated with miR-21 and served as a prognostic indicator in ovarian cancer. Tumour Biol 2016; 37(6): 8359-8365.
[4] Liu Z, Xiao YU, Ning S, Li ZY, Zhu Y, Hu G. Effect of taxol on the expression of FoxM1 ovarian cancer-associated gene. Oncol Lett 2016; 11(6): 4035-4039.
[5] Nagy ZB, Wichmann B, Kalmár A, Barták BK, Tulassay Z, Molnár B. miRNA isolation from FFPET specimen: A technical comparison of miRNA and total RNA isolation methods. Pathol Oncol Res 2016; 22(3): 505-513.
[6] Shalaby SM, El-Shal AS, Shoukry A, Khedr MH, Abdelraheim N. Serum miRNA-499 and miRNA-210: A potential role in early diagnosis of acute coronary syndrome. IUBMB Life 2016; 68(8): 673-682.
[7] Zhou Y, Chen Q, Lew KS, Richards AM, Wang P. discovery of potential therapeutic miRNA targets in cardiac ischemia-reperfusion injury. J Cardiovasc Pharmacol Ther 2016; 21(3): 296-309.
[8] Chen S, Qi X, Chen H, Li M, Gu J, Liu C, et al. Expression of miRNA-26a in platelets is associated with clopidogrel resistance following coronary stenting. Exp Ther Med 2016; 12(1): 518-524.
[9] Liang AL, Zhang TT, Zhou N, Wu CY, Lin MH, Liu YJ. miRNA-10b sponge: An anti-breast cancer study in vitro. Oncol Rep 2016; 35(4): 1950-1958.
[10] Huang FY, Wong DK, Seto WK, Lai CL, Yuen MF. Estradiol induces apoptosis via activation of miRNA-23a and p53: implication for gender difference in liver cancer development. Oncotarget 2015; 6(33): 34941-3452.
[11] Sun D, Li X, Ma M, Liu J, Xu Y, Ye L, et al. The predictive value and potential mechanisms of miRNA-328 and miRNA-378 for brain metastases in operable and advanced non-small-cell lung cancer. Jpn J Clin Oncol 2015; 45(5): 464-473.
[12] Hu J, Shan Z, Hu K, Ren F, Zhang W, Han M, et al. miRNA-223 inhibits epithelial-mesenchymal transition in gastric carcinoma cells via Sp1. Int J Oncol 2016; 49(1): 325-335.
[13] Liu P, Xin F, Ma CF. Clinical significance of serum miR-196a in cervical intraepithelial neoplasia and cervical cancer. Genet Mol Res 2015; 14(4): 17995-18002.
[14] Lu YC, Chang JT, Huang YC, Huang CC, Chen WH, Lee LY, et al. Combined determination of circulating miR-196a and miR-196b levels produces high sensitivity and specificity for early detection of oral cancer. Clin Biochem 2015; 48(3): 115-121.
[15] Li HL, Xie SP, Yang YL, Cheng YX, Zhang Y, Wang J, et al. Clinical significance of upregulation of mir-196a-5p in gastric cancer and enriched KEGG pathway analysis of target genes. Asian Pac J Cancer Prev 2015; 16(5): 1781-1787.
[16] Jiang Y, Chu Y, Tang W, Wan Y, Zhang L, Cheng W. Transcription factor WT1 and promoter CpG hypomethylation coactivate HOXA10 expression in ovarian cancer. Curr Pharm Des 2014; 20(11): 1647-1654.
[17] Li J, Li D, Zhang W. Tumor suppressor role of miR-217 in human epithelial ovarian cancer by targeting IGF1R. Oncol Rep 2016; 35(3): 1671-1679.
[18] Ge G, Zhang W, Niu L, Yan Y, Ren Y, Zou Y. miR-215 functions as a tumor suppressor in epithelial ovarian cancer through regulation of the X-chromosome-linked inhibitor of apoptosis. Oncol Rep 2016; 35(3): 1816-1822.
[19] Miao C, Ren Y, Chen M, Wang Z, Wang T. Microcystin-LR promotes migration and invasion of colorectal cancer through matrix metalloproteinase-13 up-regulation. Mol Carcinog 2016; 55(5): 514-524.
[20] Cho JH, Hong WG, Jung YJ, Lee J3, Lee E4, Hwang SG, et al. -Ionizing radiation-induced activation of the EGFR-p38/ERK-STAT3/CREB-1-EMT pathway promotes the migration/invasion of non-small cell lung cancer cells and is inhibited by podophyllotoxin acetate. Tumour Biol 2016; 37(6): 7315-7325.
[21] Darda L, Hakami F, Morgan R, Murdoch C, Lambert DW, Hunter KD. The role of HOXB9 and miR-196a in head and neck squamous cell carcinoma. PLoS One 2015; 10(4): e0122285.
[22] Huang F, Tang J, Zhuang X, et al. MiR-196a promotes pancreatic cancer progression by targeting nuclear factor kappa-B-inhibitor alpha. PLoS One 2014; 9(2): e87897.
[23] Zhang J, Zheng F, Yu G, Zhuang Y, Cheng W, Chen W, et al. miR-196a targets netrin 4 and regulates cell proliferation and migration of cervical cancer cells. Biochem Biophys Res Commun 2013; 440(4): 582-588.
[24] Lee SJ, Seo JW, Chae YS, Kim JG, Kang BW, Kim WW, et al. Genetic polymorphism of miR-196a as a prognostic biomarker for early breast cancer. Anticancer Res 2014; 34(6): 2943-2949.
[25] Villegas-Ruiz V, Juárez-Méndez S, Pérez-González OA, Arreola H, Paniagua-García L, Parra-Melquiadez M, et al. Heterogeneity of microRNAs expression in cervical cancer cells: over-expression of miR-196a. Int J Clin Exp Pathol 2014; 7(4): 1389-1401.
[26] Liu M, Du Y, Gao J, Liu J, Kong X, Gong Y, et al. Aberrant expression miR-196a is associated with abnormal apoptosis, invasion, and proliferation of pancreatic cancer cells. Pancreas 2013; 42(7): 1169-1181. [27] Mu YP, Tang S, Sun WJ, Gao WM, Wang M, Su XL. Association of miR-193b down-regulation and miR-196a up-regulation with clinicopathological features and prognosis in gastric cancer. Asian Pac J Cancer Prev 2014; 15(20): 8893-8900.
[28] Wang PY, Gao ZH, Jiang ZH, Li XX, Jiang BF, Xie SY. The associations of single nucleotide polymorphisms in miR-146a, miR-196a and miR-499 with breast cancer susceptibility. PLoS One 2013; 8(9): e70656.
[29] Tanwar PS, Kaneko-Tarui T, Lee HJ, Zhang L, Teixeira JM. PTEN loss and HOXA10 expression are associated with ovarian endometrioid adenocarcinoma differentiation and progression. Carcinogenesis 2013; 34(4): 893-901.
[30] Ko SY, Lengyel E, Naora H. The Müllerian HOXA10 gene promotes growth of ovarian surface epithelial cells by stimulating epithelialstromal interactions. Mol Cell Endocrinol 2010; 317(1-2): 112-119.
[31] Cheng W, Jiang Y, Liu C, Shen O, Tang W, Wang X. Identification of aberrant promoter hypomethylation of HOXA10 in ovarian cancer. J Cancer Res Clin Oncol 2010; 136(8): 1221-1227.
Document heading 10.1016/j.apjtm.2016.09.002
16 July 2016
in revised form 17 August 2016
✉First and Bo Yang, Department of Female Tumor, First Affiliated Hospital of Bengbu Medical College, Bengbu, Anhui, 233004, China.
Tel: 13865006005
E-mail: yangbo4836@163.com
Foundation project: It was supported by the General Project of Provincial Natural Science Research of the Anhui Higher Education Institutions (Grant No. KJ2015B096by).
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Modifiable determinants of attitude towards dengue vaccination among healthy inhabitants of Aceh, Indonesia: Findings from a communitybased survey
- Clinical significance of dynamic detection for serum levels of MCP-1, TNF-α and IL-8 in patients with acute pancreatitis
- Protective effect of antioxidant on renal damage caused by Doxorubicin chemotherapy in mice with hepatic cancer
- Mechanism of action of Zhuyu Annao pill in mice with cerebral intrahemorrhage based on TLR4
- Acetylcholinesterase, butyrylcholinesterase and paraoxonase 1 activities in rats treated with cannabis, tramadol or both
- Anti-tumor activity of tanshinone IIA in combined with cyclophosphamide against Lewis mice with lung cancer
