Acetylcholinesterase, butyrylcholinesterase and paraoxonase 1 activities in rats treated with cannabis, tramadol or both
2016-11-24OmarAbdelSalamEmanYounessYasserKhadrawyAmanySleem
Omar M.E. Abdel-Salam, Eman R. Youness, Yasser A. Khadrawy, Amany A. Sleem
1Department of Toxicology and Narcotics, National Research Centre, Cairo
2Department of Medical Biochemistry, National Research Centre, Cairo
3Department of Physiology, National Research Centre, Cairo
4Department of Pharmacology, National Research Centre, Cairo
Acetylcholinesterase, butyrylcholinesterase and paraoxonase 1 activities in rats treated with cannabis, tramadol or both
Omar M.E. Abdel-Salam1✉, Eman R. Youness2, Yasser A. Khadrawy3, Amany A. Sleem4
1Department of Toxicology and Narcotics, National Research Centre, Cairo
2Department of Medical Biochemistry, National Research Centre, Cairo
3Department of Physiology, National Research Centre, Cairo
4Department of Pharmacology, National Research Centre, Cairo
ARTICLE INFO
Article history:
Accepted 25 August 2016
Available online 20 November 2016
Cannabis sativa
Tramadol
Cholinesterases
Memory
Cognitive decline
Objective: To investigate the effect of Cannabis sativa resin and/or tramadol, two commonly drugs of abuse on acetylcholinesterase and butyrylcholinesterase activities as a possible cholinergic biomarkers of neurotoxicity induced by these agents. Methods: rats were treated with cannabis resin (5, 10 or 20 mg/kg) (equivalent to the active constituent△9-tetrahydrocannabinol), tramadol (5, 10 and 20 mg/kg) or tramadol (10 mg/kg) combined with cannabis resin (5, 10 and 20 mg/kg) subcutaneously daily for 6 weeks. Acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) activities were measured in brain and serum. We also measured the activity of paraoxonase-1 (PON1) in serum of rats treated with these agents. Results: (i) AChE activity in brain increased after 10-20 mg/ kg cannabis resin (by 16.3%-36.5%). AChE activity in brain did not change after treatment with 5-20 mg/kg tramadol. The administration of both cannabis resin (5, 10 or 20 mg/kg) and tramadol (10 mg/kg) resulted in decreased brain AChE activity by 14.1%, 12.9% and 13.6%, respectively; (ii) BChE activity in serum was markedly and dose-dependently inhibited by cannabis resin (by 60.9%-76.9%). BChE activity also decreased by 17.6%-36.5% by 10-20 mg/kg tramadol and by 57.2%-63.9% by the cannabis resin/tramadol combined treatment; (iii) Cannabis resin at dose of 20 mg/kg increased serum PON1 activity by 25.7%. In contrast, tramadol given at 5, 10 and 20 mg/kg resulted in a dose-dependent decrease in serum PON1 activity by 19%, 36.7%, and 46.1%, respectively. Meanwhile, treatment with cannabis resin plus tramadol resulted in 40.2%, 35.8%, 30.7% inhibition of PON1 activity compared to the saline group. Conclusions: these data suggest that cannabis resin exerts different effects on AChE and BChE activities which could contribute to the memory problems and the decline in cognitive function in chronic users.
1. Introduction
Cannabis sativa L (family Cannabaceae) (C. sativa) has remained the most widely used and abused drug worldwide[1]. The two most common cannabis preparations are marijuana which is the dried flowing tops and leaves of the female plants and hashish which is the compressed resin. Cannabis has long been used through the history of mankind for its recreational properties. Cannabis consumersoften report the subjective feeling of “being high”, euphoria, altered time perception and increased sensual awareness. With long-term cannabis appears to impair several cognitive functions[2,3]. There is impairment of short-term and working memory that might persist for variable time after abstinence from cannabis[4,5]. There is also aggravation of pre-existing psychosis or even a likeness of developing psychosis in cannabis users[6]. Brain MRI scans indicate structural changes in humans with a history of long-term[7] and heavy cannabis abuse while animal models shows neuronal degeneration[8,9] despite neuroprotection against excitotoxic brain injury (glutamate-induced death) being reported [10].
Only recently and with the identification of the cannabinoidreceptors and their endogenous ligands, the biological action of cannabis beings to be delineated. The C21 terpenophenolic cannabinoids are the unique constituents of the C. sativa plant of which the principal psychoactive ingredient is △9-tetrahydrocannabinol (△9-THC). Other cannabinoids such as cannabinol, cannabidiol, and cannabivarin, cannabichromene, cannabigerol are devoid of psychotropic action and might even antagonize some of the pharmacological effects of △9-THC. The latter and other cannabinoids exist as their carboxylic acids and are converted (decaboxylated) into their corresponding phenols upon heating[11, 12]. Cannabinoids or their endogenous ligands bind to cannabinoid receptors CB1 and CB2 with the former being predominantly expressed in the brain and spinal cord and thus mediates most of the effects of cannabis on the central nervous system. On the other hand, the CB2 receptor is mainly expressed on the surface of the immune cells in the periphery[13].
Tramadol is a frequently prescribed centrally acting analgesic with μ-opioid receptor agonist properties. It also inhibits the reuptake of serotonin, and noradrenaline in the brain[14]. It is used to treat acute pain and of moderate to moderately severe chronic pain resulting from musculoskeletal disorders or that due to cancer[15]. The drug is becoming increasingly popular in several countries as a drug of misuse[16-18]. Subjects taking 675 mg or more of tramadol for 5 years or more exhibited an increase in comorbid anxiety, depressive, and obsessive-compulsive symptoms[18]. There is also an evidence of memory impairing action for tramadol[19]. Cannabis users are more likely to report use of other illicit drugs[21] including tramadol[20].
In brain, central cholinergic neurotransmission is crucial for cognitive functions including learning and memory formation[22]. Inhibitors of brain acetylcholinesterase such as donepezil and rivastigmine are the drugs being used to treat the cognitive decline due to aging or Alzheimer's disease by increasing extracellular acetylcholine, the signaling neurotransmitter of the cholinergic system[23]. Changes in central cholinergic activity thus will have an important impact on cognitive functions[22]. The aim of this study was therefore to investigate the effect of cannabis and/or tramadol on the activities of brain acetylcholinesterase and plasma butyrylcholinesterase, the enzymes involved in the hydrolysis of the acetylcholine[24]. We in addition measured the activity of paraoxonase-1 (PON1) in serum of rats treated with cannabis and/ or tramadol. The PON1 enzyme is involved in the detoxification of several organophosphorus compounds and many other xenobiotics and changes in its activity have been associated with a number of neurologic disorders[25,26].
2. Materials and methods
2.1. Animals
Male Sprague-Dawley rats, obtained from Animal House of the National Research Centre, Cairo, weighing between 130-140 g were group-housed under temperature- and light-controlled conditions with standard laboratory rodent chow and water provided ad libitum. Animal procedures were performed in accordance with the Ethics Committee of the National Research Centre and followed the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985).
2.2. Drugs and chemicals
C. sativa resin (Hashish) and tramadol were kindly provided by the Laboratory of Forensic Sciences of Ministry of Justice (Cairo, Egypt). Other chemicals and reagents were obtained from Sigma Chemical Co.(St. Louis, MO, U.S.A).
2.3. Preparation of cannabis resin extract
Cannabis resin extract was prepared from the dried resin of C. sativa. The extraction was performed using chloroform according to the method of Turner and Mahlberg[27] with modification. In brief, 10 g of the resin was grounded in a mortar, subjected to oven heat (100 ℃) for 1h to decarboxylate all its cannabinolic acids content. The resin was extracted in chloroform overnight and filtered. The filtrate was evaporated under a gentle stream of nitrogen and stored at 4 ℃ and protected from light in an aluminium-covered container. 1g of the residue (dry extract) was suspended in in 2% ethanolsaline. △9-tetrahydrocannabinol (△9-THC) content was quantified using gas chromatography–mass spectrometry (GC-MS). The resin contained ~ 20% △9-THC and 3% cannabidiol.
2.4. Study design
Rats were treated with C. sativa resin extract at 5, 10 or 20 mg/kg (expressed as △9-tetrahydrocannabinol), tramadol at 5, 10 or 20 mg/ kg or tramadol (10 mg/kg) in combination with C. sativa resin (5, 10 or 20 mg/kg) subcutaneously daily for 6 weeks. Rats were randomly divided into ten groups, six rats each. Group 1 received the vehicle (0.2 mL saline) daily. Group 2, 3, 4 received C. sativa resin at the doses of 5, 10 and 20 mg/kg, subcutaneously daily. Groups 5, 6, 7 received tramadol at doses of 5, 10 and 20 mg/kg subcutaneously daily. Groups 8, 9, 10 received tramadol at 10 mg/kg in combination with C. sativa resin (5, 10 or 20 mg/kg, subcutaneously daily). Rats were then euthanized by decapitation under ether anesthesia for tissue collection. The brain of each rat was rapidly dissected and snap-frozen in liquid nitrogen. Tissue samples were stored at -80 ℃ until further processing. Frozen samples were thawed and homogenized in a glass tube with a Teflon dounce pestle in ice-cold phosphate buffer solution (50 mM Tris-HCl, pH 7.4) and sonicated. Homogenized samples were then centrifuged at 9 000 g for 5 minutes at 4 ℃. The supernatant was stored at -80 ℃ until further analysis.
2.5. Determination of acetylcholinesterase activity
The procedure used was a modification of the method of Ellman et al.[28] as described by Gorun et al. [29]. The principle of the method is the measurement of the thiocholine produced as acetylthiocholine is hydrolyzed. The color was read immediately at 412 nm.
2.6. Determination of butyrylcholinesterase activity
Butyrylcholinesterase activity was measured spectrophotometrically using commercially available kit (Ben Biochemical Enterprise, Milan, Italy). In this assay, cholinesterase catalyzes the hydrolysis of butyrylthiocholine, forming butyrate and thiocholine. The thiocholine reacts with dithiobis-nitrobenzoic acid (DTNB) forming a colored compound. The increase in absorbance in the unit time at 405 nm is proportional at the activity of the cholinesterase in the sample.
2.7. Determination of paraoxonase activity
Arylesterase activity of paraoxonase was measured spectrophotometrically using phenylacetate as a substrate[30, 31] . In this assay, arylesterase/paraoxonase catalyzes the cleavage of phenyl acetate resulting in phenol formation. The rate of formation of phenol is measured by monitoring the increase in absorbance at 270 nm at 25 ℃. The working reagent consisted of 20 mM Tris/HCl buffer, pH 8.0, containing 1 mM CaCl2and 4 mM phenyl acetate as the substrate. Samples diluted 1:3 in buffer are added and the change in absorbance is recorded following a 20 s lag time. Absorbance at 270 nm was taken every 15 s for 120 s. One unit of arylesterase activity is equal to 1 μ M of phenol formed per minute. The activity is expressed in kU/L, based on the extinction coefficient of phenol of 1310 m/cm at 270 nm, pH 8.0 and 25 ℃. Blank samples containing water are used to correct for the spontaneous hydrolysis of phenylacetate.
2.8. Statistical analysis
Data are expressed as mean ± SE. Data were analyzed by one-way analysis of variance, followed by Duncan's multiple range test for post hoc comparison of group means. Effects with a probability of P<0.05 were considered to be significant.
3. Results
3.1. Acetylcholinesterase activity
Significant increase in brain AChE activity by 16.3% and 36.5% was observed in rats treated with 10-20 mg/kg cannabis resin alone compared to the saline control group [(9.84 ± 0.31) μmol SH/g/ min, (11.55±0.45) μmol SH/g/min vs. (8.46±0.22) μmol SH/ g/min]. No significant change in AChE activity was found after tramadol. The combined administration of cannabis resin/tramadol was, however, associated with significant decrease in brain AChE activity by14.1%, 12.9%, and 13.5%, respectively [ (7.27 ± 0.22) μ mol SH/g/min, (7.37±0.26) μmol SH/g/min, (7.32 ± 0.300) μmol SH/g/min vs. (8.46 ± 0.22) μmol SH/g/min) ] (Figure 1).
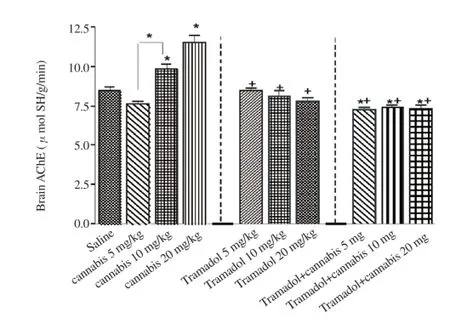
Figure 1. Brain acetylcholinesterase (AChE) activity in rats treated with cannabis resin, tramadol or both.
3.2. Butyrylcholinesterase activity
Cannabis resin alone at a dose of 5, 10 and 20 mg/kg caused significant inhibition in serum BChE activity by 60.9%, 67.0% and 76.9% compared to the saline control group [(90.91 ± 2.80) U/L, (76.73±4.20) U/L, (53.76±3.10) U/L vs. (232.72 ± 5.90) U/L] (Figure 2). Serum BChE activity was also significantly decreased by 10-20 mg/kg tramadol (17.2% and 36.5% decrease: [(192.71± 4.90) U/L, (147.74±2.60) U/L vs. (232.72±5.90) U/L] and following treatment with both cannabis resin and tramadol (57.2%, 62.6%, and 63.9% decrease: [(99.70±6.00) U/L, (87.00 ± 4.10) U/L, (84.10±3.30) U/L vs. (232.72 ± 5.90)U/L].

Figure 2. Serum butyrylcholinesterase (BChE) activity in rats treated with cannabis resin, tramadol or both.*P<0.05 vs. saline group and between different groups as indicated in the figure.+P<0.05 vs. only cannabis resin.
3.3. Paraxonase 1 activity
In serum, a significant and marked increase in PON1 activitywas observed after treatment with 20 mg/kg of cannabis resin compared to the saline group by 25.7%, [(76.1±21.2) vs. (299.2±13.0) kU/L] (Figure 3). A significant decrease in serum PON1 activity by 19.0%, 36.7%, and 46.1% was observed after the administration of 5, 10 and 20 mg/kg of tramadol, respectively [(242.4±19.1) kU/L, (189.3±11.9) kU/L, (161.1±12.7) kU/L vs. (299.2±13.0) kU/L]. Meanwhile, treatment with cannabis resin plus tramadol resulted in decreased PON1 activity by 40.2%, 35.8%, 30.7% compared to the saline group [(179.0±15.4) kU/L, (182.2±8.8) kU/L, (207.4±16.9) kU/L vs. (299.2±13.0) kU/L].

Figure 3. Serum paraoxonase-1 (PON1) activity in rats treated with cannabis resin, tramadol or both.
4. Discussion
In the current study, we show that treating rats with a cannabis resin extract rich in delta △9-THC resulted in an increase in brain acetylcholinesterase (AChE) activity and at the same time caused marked inhibition of serum butyrylcholinesterase (BChE) activity. The serine esterases AChE (EC 3.1.1.7.) and BChE (EC 3.1.1.8.) can catalyze the hydrolysis of the neurotransmitter acetylcholine into choline and acetic acid. The enzyme AChE is present in brain, autonomic ganglia, skeletal muscle end-plate and in erythrocyte membrane. AChE hydrolyzes acetylcholine faster than the other choline esters. It is the key enzyme which terminates the action of ACh at cholinergic synapses and is highly efficient in modulating the levels of extracellular acetylcholine and in regulating cholinergic neurotransmission[32,33]. It is not surprising therefore that inhibitors of AChE e.g., donepezil are used for boosting residual cholinergic activity in Alzheimer's disease[34]. In this neurodegenerative disorder, loss of cholinergic innervations and deficits in cholinergic neurotransmission underlie the cognitive deterioration involving memory, language and higher executive functioning[22].
It follows that the increase in brain AChE activity by the cannabis resin observed in this study would result in decreased brain levels of ACh, which could explain at least in part, the cognitive and memory deficits in heavy users of cannabis. In this context, several studies have shown inhibition in extracellular acetylcholine concentration in several brain areas following i.p. injection of THC (3-6 mg/kg), the main active ingredient in cannabis[35-37]. In cortical, hypothalamic and striatal rat brain slices, both △8-THC and △9-THC were found to inhibit the synthesis of 3H-ACh with D8-THC being twice as effective as △9-THC. Meanwhile, the non-psychotropic cannabidiol did not alter ACh synthesis[38]. In rat hippocampus, inhibition of ACh release also followed i.p. injection of synthetic cannabinoids, WIN 55,212-2 (5.0 and 10 mg/kg i.p.) and CP 55,94 (0.5 and 1.0 mg/kg i.p.)with the effect being blocked by the CB1 antagonist SR 141716A[39]. On the other hand, very low doses of △9-THC (10-150 mg/kg) or the cannabinoid CB1 receptor agonists WIN 55,212-2 (10-150 mg/kg) and HU 210 (1 and 4 mg/kg) given intravenously to freely moving rats increased cortical and hippocampal acetylcholine release[40,41].
The chemistry of herbal cannabis, however, is complex, as there are over 70 different cannabinoids identified so far[11]. Some cannabinoids potentiate whilst others eg., cannabidiol antagonize some of the pharmacological actions of the principal and psychotropic one i.e., △9-THC[42]. Cannabis is not merely cannabinoids and contains several terpenoids and among these -Pinene, a bicyclic monoterpene is an AChE inhibitor[43]. Clearly, the net effect will therefore depends on the relative contribution of different cannabis components and it is possible that in high potency cannabis with high content of △9-THC, the action of the latter will prevail/predominate.
Butyrylcholinesterase (EC 3.1.1.8; BChE) also known as pseudocholinesterase or plasma cholinesterase is expressed in many tissues but is found primarily in the liver and plasma. The enzyme differs from AChE in substrate specificity. BChE, preferentially hydrolyses butyrylcholine and also acetylcholine[24, 44]. The exact physiological function of BChE is not understood. It can hydrolyze heroin and might function as a detoxifying enzyme for natural compounds. The genetic deficiency of this enzyme in humans results in no apparent physiological consequences[24]. The role of BChE in cholinergic neurotransmission is also much less clear compared with that of AChE. Recent evidence from rodent experiments, however, suggests that, brain-targeted BChE inhibitors elevates extracellular ACh levels and improve the cognitive performance of aged rats[45]. The findings in the present study indicates that the cannabis resin extract exerted marked inhibitory effect on serum BChE activity. It is not clear whether the potent inhibition of BChE activity by the cannabis resin will be reflected in decreased degradation of brainACh. This is because the hydrolysis of ACh is carried out mainly by AChE, with a minor role for BChE if any[24,44]. Moreover and as shown in this study, brain AChE activity increased by the cannabis resin which will lead to decreased brain ACh availability.
In the present study, we also demonstrated that the repeated administration of cannabis resin extract increased paraoxonase-1 (PON1) activity in serum. This enzyme belongs to the paraoxonase family which also comprises PON2 and PON3 isoforms. Paraoxonase 1 is a calcium-dependent esterase that hydrolyzes the active metabolites (oxons) of several organophosphorus insecticides including parathion, chlorpyrifos and diazinon and an individual's PON1 status determines the sensitivity to these chemicals. Paraoxonase 1 also hydrolyzes aromatic esters such as phenyl acetate (arylesterase activity) and a variety of aromatic and aliphatic lactones (lactonase activity). It is synthesized in the liver and released into blood where it binds to high density lipoproteins and prevents their oxidation[25, 31]. The activity of PON1 decreases in patients with Alzheimer's disease and other dementias[46], and autism[47]. PON1 possesses an antioxidant role [25, 31] and is inactivated by increased oxidative stress[48, 49] which might explain the decrease in enzyme activity in patients suffering from these neurologic disorders.
The present study also investigated the ability of tramadol, an opiate like analgesic with abuse liability and addictive properties[17,20,50] on the activity of AChE, BChE and PON1. In contrast to the effect of the cannabis resin extract, we found that tramadol did not alter AChE activity in the rat brain. When combined with tramadol, cannabis, however, did not increase brain AChE activity, thereby, suggesting a modulatory action for tramadol on the cannabis-induced increase in AChE activity. In contrast, BChE activity was inhibited by tramadol doses of 10 and 20 mg/kg although to much less extent compared with the cannabis resin. Serum BChE activity might thus serve as a marker in those who abuse tramadol. Meanwhile, there was no additive effect for the combination of cannabis-tramadol in inhibiting BChE activity. PON1 activity, however, was markedly inhibited by tramadol and also by cannabis-tramadol. This finding is important in view of the evidence that the catalytic efficiency of PON1 determines the severity of toxicity following exposure to some of the organophosphorus compounds[51,52]. Thus patients on tramadol might be susceptible to some of the consequences of pesticide exposure and among these lies the risk for developing neurodegenerative disorders like Parkinson's disease or Alzheimer's disease[26].
In summary, the findings of the present study suggest a modulatory effect for cannabis resin extract on the activities of AChE and BChE. This action of cannabis is likely to contribute to the memory deficits and the decline in cognitive function observed in chronic users. The study also demonstrates an inhibitory effect for tramadol on BChE and PON1 activities. It is suggested that the changes in the activities of both enzymes could be a marker for the drug-induced neurotoxicity.
Conflicts of interest statement
The authors declare that there are no competing conflicts of interest.
Acknowledgement
This works is supported by NRC grant (Grant No.10001004).
References
[1] Volkow ND, Baler RD, Compton WM, Weiss SRB. Adverse health effects of marijuana use. N Engl J Med 2014; 370: 2219-2227.
[2] Desrosiers NA, Ramaekers JG, Chauchard E, Gorelick DA, Huestis MA. Smoked cannabis' psychomotor and neurocognitive effects in occasional and frequent smokers. J Anal Toxicol 2015; 39(4):251-261.
[3] Maldonado R, Berrendero F, Ozaita A, Robledo P. Neurochemical basis of cannabis addiction. Neuroscience 2011; 181:1-17.
[4] McClure EA, Lydiard JB, Goddard SD, Gray KM. Objective and subjective memory ratings in cannabis-dependent adolescents. Am J Addict 2015; 24(1):47-52.
[5] Riba J, Valle M, Sampedro F, Rodríguez-Pujadas A, Martínez-Horta S, Kulisevsky J, et al. Telling true from false: cannabis users show increased susceptibility to false memories. Mol Psychiatry 2015; 20(6):772-777.
[6] McClure EA, Lydiard JB, Goddard SD, Gray KM. Objective and subjective memory ratings in cannabis-dependent adolescents. Am J Addict 2015; 24(1):47-52.
[7] Battistella G, Fornari E, Annoni JM, Chtioui H, Dao K, Fabritius M, et al. Long-term effects of cannabis on brain structure. Neuropsychopharmacology 2014; 39(9):2041-2048.
[8] Chan GC, Hinds TR, Impey S, Storm DR. Hippocampal neurotoxicity of Delta9-tetrahydrocannabinol. J Neurosci 1998; 18(14):5322-5332.
[9] Abdel-Salam OME, Youness ER, Shaffee N. Biochemical, immunological, DNA and histopathological changes caused by Cannabis sativa in the rat. J Neurol Epidemiol 2014; 2: 6-16.
[10] El-Remessy AB, Khalil IE, Matragoon S, Abou-Mohamed G, Tsai NJ, Roon P, et al. Neuroprotective effect of (-) Delta9-tetrahydrocannabinol and cannabidiol in N-methyl-D-aspartate-induced retinal neurotoxicity: involvement of peroxynitrite. Am J Pathol 2003; 163(5):1997-2008.
[11] Elsohly MA, Slade D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci 2005; 78(5):539-548.
[12] Pertwee RG. Pharmacological actions of cannabinoids. Handb Exp Pharmacol 2005;168:1-51.
[13] Di Marzo V, Stella N, Zimmer A. Endocannabinoid signalling and the deteriorating brain. Nat Rev Neurosci 2015; 16(1):30-42.
[14] Bloms-Funke P, Dremencov E, Cremers TI, Tzschentke TM. Tramadol increases extracellular levels of serotonin and noradrenaline as measured by in vivo microdialysis in the ventral hippocampus of freely-moving rats. Neurosci Lett 2011; 490:191–195.
[15] Grond S, Sablotzki A. Clinical pharmacology of tramadol. Clin Pharmacokinet 2004; 43(13):879-923.
[16] Tjäderborn M, Jönsson AK, Sandström TZ, Ahlner J, Hägg S. Nonprescribed use of psychoactive prescription drugs among drug-impaired drivers in Sweden. Drug Alcohol Depend 2016; 161:77-85.
[17] Randall C, Crane J. Tramadol deaths in Northern Ireland: a review ofcases from 1996 to 2012. J Forensic Leg Med 2014; 23:32-36.
[18] El-Hadidy MA, Helaly AM. Medical and psychiatric effects of long-term dependence on high dose of tramadol. Subst Use Misuse 2015; 50(5):582-589.
[19] Hosseini-Sharifabad A, Rabbani M, Sharifzadeh M, Bagheri N. Acute and chronic tramadol administration impair spatial memory in rat. Res Pharm Sci 2016; 11(1):49-57.
[20] Loffredo CA, Boulos DN, Saleh DA, Jillson IA, Garas M, Loza N, et al. Substance use by Egyptian youth: current patterns and potential avenues for prevention. Subst Use Misuse 2015; 50(5):609-618.
[21] Berge J, Håkansson A, Berglund M. Alcohol and drug use in groups of cannabis users: results from a survey on drug use in the Swedish general population. Am J Addict 2014; 23(3):272-279.
[22] Schliebs R, Arendt T. The cholinergic system in aging and neuronal degeneration. Behav Brain Res 2011; 221(2):555-563.
[23] Campos C, Rocha NB, Vieira RT, Rocha SA, Telles-Correia D, Paes F, et al. Treatment of Cognitive Deficits in Alzheimer's disease: A psychopharmacological review. Psychiatr Danub 2016; 28(1):2-12.
[24] Massoulié J, Sussman J, Bon S, Silman I. Structure and functions of acetylcholinesterase and butyrylcholinesterase. Prog Brain Res 1993; 98:139-146.
[25] Furlong CE. Paraoxonases: an historical perspective. In: Mackness B, Mackness M, Aviram M, Paragh G. (eds.) The paraoxonases: their role in disease development and xenobiotic metabolism. Dordrecht: Springer; 2008, p.3-31.
[26] Menini T, Gugliucci A. Paraoxonase 1 in neurological disorders. Redox Rep 2014; 19(2):49-58.
[27] Turner JC, Mahlberg PG. Separation of acid and neutral cannabinoids in Cannabis sativa L. using HPLC. In: Agurell S, Dewey WL, Willete RE (eds.). Chemical pharmacol ther agents. USA: Academic Press;1984, p.79–88.
[28] Ellman GL, Courtney KD, Andreas V Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharm 1961;7:88 – 90.
[29] Gorun V, Proinov I, Baltescu V, Balaban G, Barzu O. Modified Ellman procedure for assay of cholinesterases in crude enzymatic preparation. Anal Biochem 1978; 86:324 –326.
[30] Higashino K, Takahashi Y, Yamamura Y. Release of phenyl acetate esterase from liver microsomes by carbon tetrachloride. Clin Chim Acta 1972; 41: 313-320.
[31] Watson AD, Berliner JA, Hama SY, La Du BN, Faull KF, Fogelman AM, et al. Protective effect of high density lipoprotein associated paraoxonase. Inhibition of the biological activity of minimally oxidized low density lipoprotein. J Clin Invest 1995; 96(6):2882-2891.
[32] Silman I, Sussman JL. Acetylcholinesterase: 'classical' and 'non-classical' functions and pharmacology. Curr Opin Pharmacol 2005; 5(3):293-302.
[33] Pohanka M. Inhibitors of acetylcholinesterase and butyrylcholinesterase meet immunity. Int J Mol Sci 2014; 15(6):9809-98025.
[34] Tan CC, Yu JT, Wang HF, Tan MS, Meng XF, Wang C, et al. Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer's disease: a systematic review and meta-analysis. J Alzheimers Di 2014; 41(2):615-631.
[35] Nava F, Carta G, Battasi AM, Gessa G. D(2) dopamine receptors enable delta(9)-tetrahydrocannabinol induced memory impairment and reduction of hippocampal extracellular acetylcholine concentration. Br J Pharmacol 2000; 130(6):1201-1210.
[36] Solinas M, Scherma M, Fattore L, Stroik J, Wertheim C, Tanda G, et al. Nicotinic alpha 7 receptors as a new target for treatment of cannabis abuse. J Neurosci 2007; 27(21):5615-5620.
[37] Egashira N, Ishigami N, Mishima K, Iwasaki K, Oishi R, Fujiwara M. Delta9-Tetrahydrocannabinol-induced cognitive deficits are reversed by olanzapine but not haloperidol in rats. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32(2):499-506.
[38] Friedman E, Hanin I, Gershon S. Effect of tetrahydrocannabinols on 3H-acetylcholine biosynthesis in various rat brain slices. J Pharmacol Exp Ther 1976; 196(2):339-345.
[39] Gessa GL, Mascia MS, Casu MA, Carta G. Inhibition of hippocampal acetylcholine release by cannabinoids: reversal by SR 141716A. Eur J Pharmacol 1997; 327(1):R1-2.
[40] Acquas E, Pisanu A, Marrocu P, Di Chiara G. Cannabinoid CB(1) receptor agonists increase rat cortical and hippocampal acetylcholine release in vivo. Eur J Pharmacol 2000; 401(2):179-185.
[41] Acquas E, Pisanu A, Marrocu P, Goldberg SR, Di Chiara G. Delta9-tetrahydrocannabinol enhances cortical and hippocampal acetylcholine release in vivo: a microdialysis study. Eur J Pharmacol 2001; 419(2-3):155-161.
[42] McPartland JM, Duncan M, Di Marzo V, Pertwee RG. Are cannabidiol and (9) -tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br J Pharmacol 2015; 172(3):737-753.
[43] Perry NS, Houghton PJ, Theobald A, Jenner P, Perry EK. In-vitro inhibition of human erythrocyte acetylcholinesterase by salvia lavandulaefolia essential oil and constituent terpenes. J Pharm Pharmacol 2000; 52(7):895-902.
[44] Darvesh S, Hopkins DA, Geula C. Neurobiology of butyrylcholinesterase. Nat Rev Neurosci 2003; 4(2):131-138.
[45] Greig NH, Utsuki T, Ingram DK, Wang Y, Pepeu G, Scali C, et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer beta-amyloid peptide in rodent. Proc Natl Acad Sci USA 2005; 102(47):17213-17218.
[46] Cervellati C, Trentini A, Romani A, Bellini T, Bosi C, Ortolani B, et al. Serum paraoxonase and arylesterase activities of paraoxonase-1 (PON-1), mild cognitive impairment, and 2-year conversion to dementia: A pilot study. J Neurochem 2015; 135(2):395-401.
[47] Abdel-Salam OME, Youness ER, Mohammed NA, Abu Elhamed WA. Nuclear Factor-Kappa B and Other Oxidative Stress Biomarkers in Serum of Autistic Children. Open J Mol Integr Physiol 2015; 5:18-27.
[48] Aviram M, Rosenblat M, Billecke S, Erogul J, Sorenson R, Bisgaier CL, et al. Human serum paraoxonase (PON 1) is inactivated by oxidized low density lipoprotein and preserved by antioxidants. Free Radic Biol Med 1999; 26(7-8):892-904.
[49] Nguyen SD, Sok DE. Oxidative inactivation of paraoxonase1, an antioxidant protein and its effect on antioxidant action. Free Radic Res 2003; 37(12):1319-1330.
[50] Ferrari A, Tiraferri I, Palazzoli F, Licata M. Tramadol abuse in a binge pattern in a young depressed woman. Eur Addict Res 2014; 20(2):82-86.
[51] Cole TB, Jansen K, Park S, Li WF, Furlong CE, Costa LG. The toxicity of mixtures of specific organophosphate compounds is modulated by paraoxonase 1 status. Adv Exp Med Biol 2010; 660:47-60.
[52] Jansen KL, Cole TB, Park SS, Furlong CE, Costa LG. Paraoxonase 1 (PON1) modulates the toxicity of mixed organophosphorus compounds. Toxicol Appl Pharmacol 2009; 236(2):142-153.
Document heading 10.1016/j.apjtm.2016.09.009
20 May 2016
in revised form 20 August 2016
✉First and Omar M.E. Abdel Salam, Department of Toxicology and Narcotics, National Research Centre, Tahrir St., Dokki, Cairo, Egypt. E-mail: omasalam@hotmail.com
Fax: 202-33370931
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Modifiable determinants of attitude towards dengue vaccination among healthy inhabitants of Aceh, Indonesia: Findings from a communitybased survey
- Clinical significance of dynamic detection for serum levels of MCP-1, TNF-α and IL-8 in patients with acute pancreatitis
- Expression and mechanism of action of miR-196a in epithelial ovarian cancer
- Protective effect of antioxidant on renal damage caused by Doxorubicin chemotherapy in mice with hepatic cancer
- Mechanism of action of Zhuyu Annao pill in mice with cerebral intrahemorrhage based on TLR4
- Anti-tumor activity of tanshinone IIA in combined with cyclophosphamide against Lewis mice with lung cancer
