Protoscolicidal and immunomodulatory activity of Ziziphora tenuior extract and its fractions
2016-11-24MojtabaShahnaziAbbasAzadmehrAmmarAndalibianRezaHajiaghaeeMehrzadSaraeiMahmoodAlipour
Mojtaba Shahnazi, Abbas Azadmehr, Ammar Andalibian Reza Hajiaghaee, Mehrzad Saraei Mahmood Alipour
1Department of Parasitology, Qazvin University of Medical Sciences, Qazvin, Iran
2Cellular & Molecular Research Institute, Qazvin University of Medical Sciences, Qazvin, Iran
3Department of Immunology, Babol University of Medical Sciences, Babol, Iran
4Department of Pharmacognosy& Pharmaceutics Department of Medicinal Plants Research Center, Institute of Medicinal Plants, ACECR, Karaj, Iran
5Department of Social Medicine, Qazvin University of Medical Sciences, Qazvin, Iran
Protoscolicidal and immunomodulatory activity of Ziziphora tenuior extract and its fractions
Mojtaba Shahnazi1,2, Abbas Azadmehr3✉, Ammar Andalibian1, Reza Hajiaghaee4, Mehrzad Saraei1, Mahmood Alipour5
1Department of Parasitology, Qazvin University of Medical Sciences, Qazvin, Iran
2Cellular & Molecular Research Institute, Qazvin University of Medical Sciences, Qazvin, Iran
3Department of Immunology, Babol University of Medical Sciences, Babol, Iran
4Department of Pharmacognosy& Pharmaceutics Department of Medicinal Plants Research Center, Institute of Medicinal Plants, ACECR, Karaj, Iran
5Department of Social Medicine, Qazvin University of Medical Sciences, Qazvin, Iran
ARTICLE INFO
Article history:
Accepted 23 September 2016
Available online 20 November 2016
Hydatid cyst
Protoscolicidal
Ziziphora tenuior
Immunomodulatory
Macrophage
Nitric oxide
Fraction
Objective: To evaluate the scolicidal and immunomodulatory effect of the Ziziphora tenuior (Z. tenuior) extract and its fractions. Methods: Protoscolices were treated with six concentrations (3, 5, 10, 25, 50, and 100 mg/mL) of Z. tenuior extract and its fractions (ethanol, petroleum ether, ethyl acetate and chloroform) in periods of 10, 20, 30, 40, 50 and 60 minutes, and viability of protoscolices was evaluated using the 1.0% eosin. To examine the immunomodulatory effects of Ziziphora and its fractions on macrophage cells, the non-toxic concentration of extract and different fractions determined by MTT assay, and the Griess reaction was used to measure the level of nitrite as an indicator of nitric oxide by the macrophage cells in 10, 100 and 200 μg/mL in 24 hours at 37 ℃. Results: In this study, the Z. tenuior extract at 10 mg/mL concentration was able to kill all protoscolices during 20 minutes. By increasing the concentration to 25 mg/ mL, the scolicidal time reduced to 10 minutes. Regarding the effect of different fractions of Z. tenuior, the ethanolic fraction showed the highest scolicidal activity. The extract demonstrated an inhibitory effect on the activity of macrophages and reduced nitric oxide production. Although the petroleum ether and ethanolic fractions of the extract reduced nitric oxide production, nevertheless, this effect was only significant at 10 and 100 μg/mL concentrations (P<0.05). Conclusion: The Z. tenuior extract and its fractions were effective against protoscolices yet the effect of total extract was considerable. Our findings indicates that the extract and its ethanolic and petroleum ether fractions could have anti-inflammatory properties.
1. Introduction
Hydatidosis is one of the zoonotic and parasitic infectious diseases and is considered as an important health concern in terms of well-being and economic. It is caused by the larval stages of cestodes (tapeworms) of the genus Echinococcus such as Echinococcus granulosus (E. granulosus). The canines and herbivores are the final and intermediate hosts of this parasite, respectively. The disease in humans is caused by colonization of metacestod forms of the parasite in various organs including the liver, lung, and brain and is endemic in many countries including Australia, South America, South Africa, East Europe, Middle East, and also in Iran [1-6].
Currently, despite the risk of leakage of cyst contents and the spread of protoscolices in the body and even the possible risk of secondary hydatidosis in infected patients, the surgery is still themost effective treatment option [7,8]. Choosing effective and safe scolicidal agents and injection of such substances into hydatid cysts prior to operation is of great importance for many surgeons as it can reduce the risk of cyst leakage [7,9,10]. To date, various chemical substances have been used to inactivate protoscolices before surgery, however the application of these compounds, due to their side effect, are controversial and limited and still no candidate substance with desirable effect and minimum harm is reported [2,8,10]. Considering the aforementioned problems, the natural medicinal products (medicinal plants) has recently been considered as an alternative to synthetic materials [11]. Of these natural products and plants, the garlic (Allium sativum), Ajwain (Trachyspermum ammi L.) and Punica granatum have been reported as scolicidal materials, and to ensure their harmless, the researchers have suggested further investigations on these plants [12-14]. The Ziziphora plant is a member of the family Lamiaceae. Ziziphora genus composes of 40 species in the Mediterranean, Central Asia, and also in Iran. There are four species of this plant, namely Ziziphora clinopodioides (Z. clinopodioides), Ziziphora capitata (Z. capitata), Ziziphora persica (Z. persica), and Ziziphora tenuior (Z. tenuior). The Z. tenuior, as a medicinal plant, is used as an antiseptic to treat dysentery, uterine infections, and febrile illnesses. Ziziphora contains chemical ingredients such as pulegone (87%), thymol (3.4%), piperitenone (12.19%), mentha-2-enthol (31.5%), carvacrol (10.5%), menthone (46.4%), and neomenthon (78.4%) [15,16]. Pulegone has been shown to have anti-bacterial and anti-fungal properties and with significant effect on different strains of Salmonella and Candida albicans [17]. So far, several studies on the possible scolicidal effect of different fractions of Lamiaceae family including ethanol, ethyl acetate, chloroform, petroleum ether, water, water/ethanol, methanol, and acetone fractions, have been reported[16-18]. Due to the presence of different ingredients in each fractions, the effect these fractions produce, are different. For example, the polyphenolic compounds in ethyl acetate, chloroform, and n-butanol fractions was reported to be around 19.27%, 4.99%, and 3.94%, respectively, whereas the figures found for petroleum ether and ethanol fractions, were about 0.23% and 1.64%, respectively [18]. Various studies have been conducted on Z. tenuior. In a review study, Ziziphora was introduced as a valuable resource for the treatment of Plasmodium infections[19] with confirmed antibacterial [17] and antifungal properties [20]. Considering the antibacterial activity of Ziziphora, it has been shown that this medicinal plant produce a significant effect on Bacillus subtilis, Escherichia coli, and Staphylococcus epidermidis[21]. There are several controversial reports regarding the immunemodulatory properties of different fractions of Ziziphora as well as its diverse effects on the activity of macrophages and production of free radicals[22]. Researchers have shown that Z. clinopodioides can inhibit the production of TNF- and reduce oxidative stress [23]. Also, it was reported that this medicinal plan shows inhibitory effect on cellular myeloperoxidase activity [24]. Considering the fact that macrophage dysfunction and promoting an inappropriate Th2 type immune response is the main reason for the echinococcus evasion from the immune system, in the present study, we attempted to assess the scolicidal effects of the extract and different fractions of Z. tenuior and also to study the immunomodulatory properties of the study samples through the production of nitric oxide (NO) from RAW 264.7 cell lines.
2. Material and methods
2.1. Preparation of hydatid cysts and protoscolices
Liver containing hydatid cyst was transferred to the Department of Parasitology from Qazvin and Tehran slaughterhouses. Under sterile conditions, the contents of cysts were aspirated and placed in the sterile tubes, separately. Calcified and purulent cysts were excluded from the study. Briefly, following disinfection of cyst with iodine, the cysts contents were discharged by 5 and 10 mL syringes and poured into sterile test tubes. After washing with normal saline, the samples were examined for fertility status under light microscope and if fertile cysts were present, the viability of protoscolices was evaluated by 0.1% eosin staining and flame cells movement. Those cysts with more than 90% viability were used for further experiments [23,25].
2.2. Plant materials
Z. tenuior was purchased from the local herbal market and authenticated by a botanist. Voucher specimens were preserved in the central herbarium of medicinal plants (ACECR). The aerial parts of plant were air-dried at room temperature. Later, this was ground into powder.
2.2.1. Extraction
The aerial part of plant (50 g) was extracted using percolation method by ethanol (80%) at room temperature. The solvent was completely removed by drying under reduced pressure at 40 ℃ in a rotary evaporator. The samples were stored at 4 ℃ until use (3 g, 6% yield) [26].
2.3. Treatment of protoscolices with total extract and different fractions of Z. tenuior
In the current study, protoscolices were treated with five concentrations (3, 5, 10, 25, 50, and 100 mg/mL) of total extract and each fraction (ethanol, petroleum ether, ethyl acetate and chloroform) in periods of 10, 20, 30, 40, 50 and 60 minutes. Different concentrations were prepared by adding 3, 5, 10, 25, 50, and 100 mg of extract and each fraction into the tubes containing 9 mL of normal saline. Dissolving of the extract and different fractions was facilitated by adding 0.3 mL of DMSO to each tube followed by mixing the content of tube using an electric magnetic stirrer. Finally, by addition of normal saline, the total volume of each tube was adjusted to 10 mL. In each test tube, 2.5 mL of each concentration plus 100 mL of samples having about 1 000 protoscolex were addedand incubated at 37 ℃ for time intervals of 10, 20, 30, 40, 50, and 60 minutes. Following incubation, 10 mL normal saline was added to each tube and centrifuged for 1 minute at 300 rpm. The supernatant was removed by pipetting and eosin 0.1% was added to the sediment and mixed gently. After a 15-minute incubation time, the supernatant was removed; a smear was prepared from the precipitate and studied under light microscope. By analysis of almost 700 protoscolices and counting the stained (dead) and un-stained (live) ones, the percentage of dead protoscolices was calculated. To ensure the accuracy of the test and quality control, a control group (without plant extracts or fractions) was included in all tests and the experiments were repeated three times [9,27].
2.4. Macrophage cells culture, cell line RAW 264.7
The RAW 264.7 cells, a murine macrophage cell line, were obtained from the Pasteur Institute of Iran. The cells were cultured and incubated in RPMI 1640 medium with (FBS 10%, 100 U/mL of penicillin and 100 μg/mL of streptomycin) in 5% CO2atmosphere at 37 ℃. When 80% of flask was filled with cells, the cells were scraped from the flask surface following the addition of Try/EDTA (trypsin/ethylene diamante tetra acetic acid), removed from the bottom of the flask, placed into Falcon tubes, and centrifuged. The supernatants were removed and cells suspended in 2 mL of RPMI 1640 medium. Using a Neubauer slide and trypan blue staining, the cells were counted and cultured in individual well of a cell culture plate (5×104) [28].
2.5. Determination of non-toxic concentrations of extract and different fractions by MTT assay
The MTT [3- (4, 5-dimethyl-2-thiazolyl0-2, 5-diphenyle tetrazolium bromide)] assay is based on conversion of tetrazolium salt to formazan dye by mitochondria of living cells. The formazan dye is dissolvable in acidic solution of isopropanol. This test is a standard laboratory method for detecting the cytotoxicity effect of agents. To perform this test, the macrophages were cultured in the presence of different concentrations of extracts and after 48 hours, 10 mL of MTT solution was added to all wells and plates were incubated at 37℃ for 4 hours. Later, the acidic solution and DMSO were added and plates were re-incubated for half an hour and finally the plates were analyzed using an ELISA reader system at a wavelength of 570 nm. By performing MTT assay, the non-toxic concentration of herbs was defined for further experiments [29]. The percentage of cell viability in the negative control group was supposed 100 and for the cells affected by certain concentration of the extract, it was calculated by dividing the value obtained for the absorption of treated wells to the value observed for the absorption of negative controls multiplied by 100.
2.6. Measurement of nitric oxide
The macrophage cells (5×104) were added to the cell culture plates and later the LPS-activated macrophages (LPS 1 μg/mL) were treated with 10, 100, and 200 μg/mL concentrations of total extract and different fractions of Z. tenuior for 24 hours. Two control groups consisted of macrophages with culture medium as negative control and macrophages with culture medium plus LPS as positive control were used. Nitric oxide is very unstable and rapidly converts to nitrite and nitrate. Therefore, the amount of nitrite as an indicator of nitric oxide was measured by Griess method using commercially available kit (Nitric Oxide Colorimetric Assay Kit; BioVision, USA) for this purpose. After 24 hours, the supernatant of cell culture was collected and the optical density (OD) of the supernatant was measured at 540 nm according to the manufacturer's instructions. All experiments were repeated three times and each test was performed in triplicate [30].
2.7. Statistical analysis
Statistical analyses were conducted using Sigma Stat for Windows V13 (SPSS, Chicago, IL, USA). Data were analyzed using one-way ANOVA and Tukey Test. A P value less than 5% was considered as significant, statistically.
3. Results
3.1. Protoscolicidal activity of Z. tenuior extract and its fractions
In this study, Z. tenuior extract at 10 mg/mL concentration, was able to kill all protoscolices during 20 minutes. Our results also showed that increasing the concentration to 25 mg/mL reduced the scolicidal time to 10 minutes. Considering the effect of different fractions of Z. tenuior against protoscolices, the ethanolic fraction showed the highest effect followed by ethyl acetate, petroleum ether, and chloroform, respectively. The ethanolic and ethyl acetate fractions at 50 mg/mL concentration deactivated all protoscolices within 60 minutes (Figure 1). Increasing the concentration of ethanol and ethyl acetate fractions to 100 mg/mL reduced the scolicidal time to 30 and 50 minutes, respectively. Fractions of petroleum ether and chloroform at 100 mg/mL concentration killed 97% and 91% of protoscolices within 60 minutes, respectively (Table 1).

Figure 1. Live protoscolices after staining with 0.1% eosin (A), Dead protoscolices after exposure to Z. tenuior L. extract and fractions and staining with 0.1% eosin (B).

Table 1 Protoscolicidal effects of total extract of Z. tenuior and their fractions at different concentrations and exposure times.
3.2. Immunomodulatory activity of Z. tenuior extract and its fractions
Z. tenuior extract showed immunomodulatory effect on nitric oxide. To determine the appropriate non-toxic concentrations of extract and fractions using MTT assay, the results demonstrated that the extract at concentrations up to 400 μg/mL had no significant toxicity on the cell line used in the study (Figure 2). The results also revealed that the extract and fractions of Ziziphora had an immunomodulatory effect on the nitric oxide production from RAW 264.7 macrophage cells. Although the total extract had an inhibitory effect on macrophage activity and NO production, however, it was statistically insignificant at 3 concentrations (10, 100, and 200 μg/ mL) used during the study. The chloroform fraction at 10 to 200 μg/mL concentrations, compared with the positive control, failed to show any significant increase in nitric oxide production. Ethyl acetate fraction at 200 μg/mL concentration produced more nitric oxide compared to other fractions; nevertheless, this effect was not significant compared to the positive control. Petroleum ether and ethanol fractions reduced the nitric oxide production which was significant at 10 and 100 μg/mL concentrations (Figure 3).
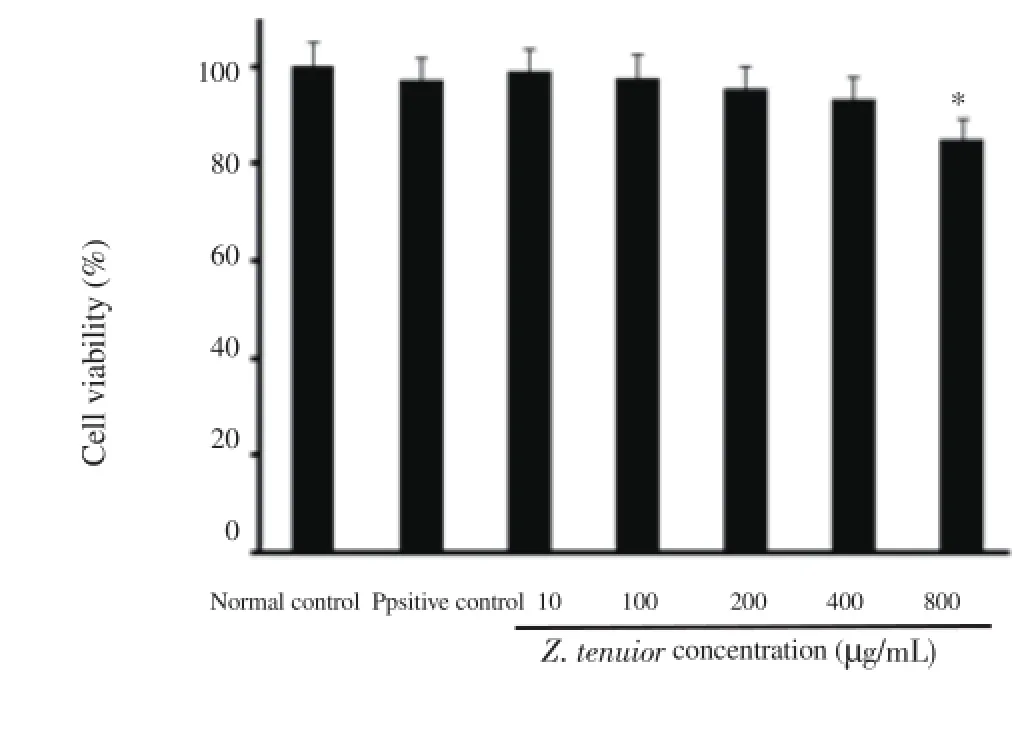
Figure 2. Effects of Z. tenuior L. extract on the cell viability of RAW 264.7 cells.

Figure 3. Effects of Z. tenuior L. extract on the Nitrite leveles of RAW 264.7 cells.
4. Discussion
Having appropriate scolicidal agents is one of the major concerns of surgeons for hydatid cyst surgery. Several important factors must be considered by relevant clinicians and researchers when searching for appropriate scolicidal agents. This indicates that an appropriate scolicidal compound should be used at the lowest dose possible while having the potential to destroy all protoscolices within the cyst at the shortest time with no toxic effect on patients and the ability to remain stable once diluted by the fluid inside the cyst. Also, scolicidal agents must deactivate daughter cysts as well [31]. Z. clinopodioides, a member of the Lamiaceae family, has been used to treat many diseases and various investigations have been carried out to assess the effects of this plant on different organisms including bacteria, fungi, and parasites [18]. The results of the current study confirmed the anti-parasitic effect of Z. tenuior, reported previously [19]. The results of our study showed the extract and different fractions of Z. tenuior were effective against protoscolices although the effect of the total extract was more significant. Consistent with our finding, in a study conducted on the antibacterial and antifungal effects of the Carpolobia lutea plant extracts and fractions, the authors also showed the extract was more effective compared to the fractions [32]. Our results also revealed that increasing the concentration of extract and each of the fractions, caused increased protoscolicidal effect. In some studies, similar finding concerning the presence of an association between the increased concentration of Z. tenuior and an increase in anti-bacterial and anti-fungal properties was reported [33,34]. In our experiments with different fractions, the highest scolicidal effect was observed for ethanolic fraction while the chloroform fraction showed the lowest effect on protoscolices. Nwidu et al. demonstrated that the ethanolic fraction of Carpolobia lutea plant, compared to its chloroform and ethyl acetate fractions, was with better antimicrobial results against Candida albicans [34]. Therefore, there may be some compounds in the ethanolic fraction that, in addition to the antifungal effects, could also demonstrate an anti-protoscolices effect. Unlike the present study, in a research on anticancer effects of seven species of Silver soreader on cancerous and normal cells, the ethanolic and ethyl acetate fractions showed the lowest effect [35]. In our study, except to the ethanolic fraction that showed the highest effect, the ethyl acetate fraction revealed the greatest scolicidal effect followed by petroleum ether and chloroform fractions which destroyed smaller percentage of protoscolices. Similar to our study, in an investigation on the effects of different fractions of the Scrophularia plant on 50 resistant strains of Pseudomonas aeruginosa, it was shown that chloroform fraction was with no significant effect on Pseudomonas aeruginosa[36]. In contrast to our finding, examining the antibacterial effect of different fractions of hazelnut leaves led to this conclusion that the chloroform fraction produces stronger effect compared to petroleum ether and ethyl acetate fractions [37]. On the contrary, in another study the researchers found the petroleum ether fraction of walnut green skin more lethal than those observed for chloroform fraction, a finding similar to that shown in our present study [38]. Therefore, the diverse effects of extracts and fractions on microorganisms could be associated with the type and amount of ingredients present in each extract and fractions as well as the difference in the type of organisms under study. Previously, the scolicidal effect of Thyme, a member of the Lamiaceae family; the same family with ziziphora, was reported [39]. Also, in a study on the lavender plant from the Lamiaceae family, anti-fungal and antibacterial effects were observed and the reason given for such effect was attributed to the presence of specified compounds such as pulegone, cineol, thymol, and carvacrol [40,41]. Therefore, the antiparasitic and scolicidal effect of Z. tenuior and its fractions can be attributed to the presence of similar compounds in Lamiaceae family. Macrophages are considered as professional antigen presenting cells (APCs) in the body. They phagocytose microbial agents and destroy them in phagolysosomes through the production of free toxic radicals such as nitric oxide (NO). Nitric oxide is a key factor in promoting an inflammatory process, thus a reduction in the production of NO leads to inhibition of inflammation [28,42]. Our finding showed that Z. tenuior reduced the activity of macrophages and the level of nitric oxide. In agreement with our study, a previous investigation showed that Ziziphora clinopoides inhibited inflammation and help heal the sick by inhibiting the production of tumor necrosis factor alpha (TNF- α) and reducing the oxidative stress [23]. In contrast to our investigation, in a study by Naeini, it was reported that the aqueous extract of Z. clinopodioides increased the activity of macrophages with higher level of toxic oxygen free radicals [22]. Differences in the results of various studies may be due to the differences in the type of extracts and the concentration of active ingredients present in the extracts. Studies have shown that phenolic compounds can inhibit the production of free radicals and the antioxidant properties of these compounds have been proved [43]. In this respect, a study showed that thyme essential oil (the same family with Z. tenuior) produces strong immunomodulatory effectdue to the presence of phenolic thymol and carvacrol compounds, thereby, the authors suggested that the immunomodulatory properties of Z. clinopodioides can be attributed to the presence of these compounds [43]. In the current study, among different fractions tested, petroleum ether and ethanol fractions reduced the production of nitric oxide whereas chloroform and ethyl acetate fractions, at their highest concentrations (200 μg/mL), increased the level of nitric oxide production, indicating a higher antioxidant capacity for ethyl acetate and chloroform fractions, compared to other fractions. Consistent with our finding, a research showed that the polar aqueous and ethyl acetate fractions of Rosemary (within the same family as Z. tenuior) have high antioxidant effect [44]. Also, in another study, strong antioxidant effect was reported for ethyl acetate fraction of thyme, possibly due to the presence of polar compounds such as rosmarinic acid and other phenolic acids [45]. Therefore, in our study, the effect of ethyl acetate and chloroform fractions may be attributed to a higher content of phenolic compounds in these fractions, compared to others; however, further studies to identify the active ingredients of Z. tenuior extract are needed. Shuge and colleagues showed that the ethyl acetate fraction of Z. clinopodioides has the highest antioxidant capacity, compared to other fractions, due to the presence of higher levels of polyphenolic compounds (19.27%) and flavonoids (65.61%). They also demonstrated that the chloroform fraction produced the second highest antioxidant activity after ethyl acetate fraction whereas the petroleum ether fraction, compared to other fractions, showed the lowest antioxidant activity possibly due to lower content of polyphenolic compounds present in the fraction (0.23%) [18]. Hence, in this study, the effect of petroleum ether and ethanol fractions of Ziziphora in reducing the production of nitric oxide may be related to lower content of polyphenolic compounds in these fractions. According to the results obtained in the present study, it can be concluded that the Z. tenuior extract with significant scolicidal effect and anti-inflammatory activity could be a good alternative option for the current compounds already used in hydatid cyst surgery. Furthermore, it should be noted that the ethanolic fraction was the only fraction with both scolicidal and antiinflammatory effect. Except for the total extract of Ziziphora plant, the ethanolic fraction showed the highest scolicidal action and compared to the total extract, it demonstrated greater inhibitory effect on macrophages and nitric oxide production.
Conflict of interest statement
The authors declare no conflict of interests regarding the publication of this paper.
Acknowledgments
We would like to thank the Research Division of Qazvin University of Medical Sciences for supporting the project financially and also the Medicinal Plants Research Center, Institute of Medicinal Plants, (ACECR), Karaj, Iran.
References
[1] Cucher MA, Macchiaroli N, Baldi G, Camicia F, Prada L, Maldonado L, et al. Cystic echinococcosis in South America: systematic review of species and genotypes of Echinococcus granulosus sensu lato in humans and natural domestic hosts. Trop Med Int Health 2016; 21(2):166-175.
[2] Jenkins DJ, Lievaart JJ, Boufana B, Lett WS, Bradshaw H, Armua-Fernandez MT. Echinococcus granulosus and other intestinal helminths: current status of prevalence and management in rural dogs of eastern Australia. Aust Vet J 2014; 92(8): 292-298.
[3] Pakala T, Molina M, Wu GY. Hepatic echinococcal cysts: A review. J Clin Transl Hepatol 2016; 4(1): 39-46.
[4] Sarkari B, Mansouri M, Khabisi SA, Mowlavi G. Molecular characterization and seroprevalence of Echinococcus granulosus in wild boars (Sus scrofa) in south-western Iran. Ann Parasitol 2015; 61(4): 269-273.
[5] Iraqi W. Canine echinococcosis: the predominance of immature eggs in adult tapeworms of Echinococcus granulosus in stray dogs from Tunisia. J Helminthol 2016; 6:1-4.
[6] Mandal S, Mandal MD. Human cystic echinococcosis: epidemiologic, zoonotic, clinical, diagnostic and therapeutic aspects. Asian Pac J Trop Med 2012; 5(4): 253-260.
[7] Lötsch F, Naderer J, Skuhala T, Groger M, Auer H, Kaczirek K, et al. Intra-cystic concentrations of albendazole-sulphoxide in human cystic echinococcosis: a systematic review and analysis of individual patient data. Parasitol Res 2016; 115(8): 2995-3001.
[8] Gomez I Gavara C, López-Andújar R, Belda Ibáñez T, Ramia Ánge JM1, Moya Herraiz Á, Orbis Castellanos F, et al. Review of the treatment of liver hydatid cysts. World J Gastroenterol 2015; 21(1): 124-131.
[9] Tripathy S, Sasmal P, Rao PB, Mishra TS, Nayak S. Cetrimidechlorhexidine-induced multiorgan failure in surgery of pulmonary hydatid cyst. Ann Card Anaesth 2016; 19(3):557-560.
[10] Shi H, Lei Y, Wang B, Wang Z, Xing G, Lv H, et al. Protoscolicidal effects of chenodeoxycholic acid on protoscoleces of Echinococcus granulosus. Exp Parasitol 2016; 67:76-82.
[11] Gangwar M, Verma VC, Singh TD, Singh SK, Goel RK, Nath G. Invitro scolicidal activity of Mallotus philippinensis (Lam.) Muell Arg. fruit glandular hair extract against hydatid cyst Echinococcus granulosus. Asian Pac J Trop Med 2013; 6(8): 595-601.
[12] Rahimi-Esboei B, Ebrahimzadeh MA, Fathi H, Rezaei Anzahaei F. Scolicidal effect of Allium sativum flowers on hydatid cyst protoscolices. Eur Rev Med Pharmacol Sci 2016; 20(1):129-132.
[13] Moazeni M, Saharkhiz MJ, Hosseini AA. In vitro lethal effect of ajowan (Trachyspermum ammi L.) essential oil on hydatid cyst protoscoleces. Vet Parasitol 2012; 187(1-2): 203-208.
[14] Labsi M, Khelifi L, Mezioug D, Soufli I, Touil-Boukoffa C. Antihydatic and immunomodulatory effects of Punica granatum peel aqueous extract in a murine model of echinococcosis. Asian Pac J Trop Med 2016; 9(3): 211-220.
[15] Batooli H, Akhbari M, Mohammad S, Hosseinizadeh J. Effect of different distillation methods on quantity and quality of essential oil oftwo Ziziphora L. species. J Herbal Drug 2012; 3 (3): 135-146.
[16] Verdian-rizi M. Essential oil composition and biological activity of Ziziphora clinopodioides Lam. from Iran. Am Eurasian J Agric Sci 2008; 2(1): 69-671.
[17] Najafi F, Tavakkoli Z. Comparing essential oil composition and antibacterial effects of Ziziphora tenuior L. in two regions of Iran. Iran J Med Arom Plants 2011; 2: 239-248.
[18] Shuge T, Yang S, Xiaoying Z, Liang G. Total polyphenolic (flavonoids) content and antioxidant capacity of different Ziziphora clinopodioides Lam. extracts. Pharmacogn Mag 2011; 7(25): 65-68.
[19] Adams M, Alther W, Kessler M, Kluge M, Hamburger M. Malaria in the Renaissance: remedies from European herbals from the 16th and 17th century. J Ethnopharmacol 2011; 133(2): 278-288.
[20] Shokri H, Sharifzadeh A, Ashrafi Tamai I. Anti-Candida zeylanoides activity of some Iranian plants used in traditional medicine. J Mycol Med 2012; 22(3): 211-216.
[21] Salehi P, Sonboli A, Eftekhar F, Nejad Ebrahimi S, Yousefzadi M. Essential oil composition, antibacterial and antioxidant activity of the oil and various extracts of Ziziphora clinopodioides subsp. rigida (BOISS.) RECH. f. from Iran. Bio Pharm Bull 2005; 8(10):1892-1896.
[22] Naeini A, Khosravi A, Tdjbakhsh H, Ghazanfari T, Yaraee R, Shokri H. Evaluation of the immunomodulatory activity of Ziziphora tenuior extracts. Comp Clin Pathol 2010; 19: 459-463.
[23] Amini-Shirazi N, Hoseini A, Ranjbar A, Mohammadirad A, Khoshakhlagh P, Yasa N, et al. Inhibition of tumor necrosis factor and nitrosative/oxidative stresses by Ziziphora clinopoides (Kahlioti); a molecular mechanism of protection against dextran sodium sulfateinduced colitis in mice. Toxicol Mech Methods 2009; 19(2):183-189.
[24] Ghafari H, Yasa N, Mohammadirad A, Dehghan G, Zamani MJ, Nikfar S, et al. Protection by Ziziphora clinopoides of acetic acid-induced toxic bowel inflammation through reduction of cellular lipid peroxidation and myeloperoxidase activity. Hum Exp Toxicol 2006; 25(6): 325-332.
[25] Smyth JD, Barrett NJ. Procedure for testing the viability of human hydatid cysts following surgical removal, especially after chemotherapy. Trans R Soc Trop Med Hyg 1980; 74: 649–652.
[26] Hajiaghaee R, Monsef-Esfahani HR, Khorramizadeh MR, Saadat F, Shahverdi AR, Attar F. Inhibitory effect of aerial parts of Scrophularia striata on matrix metalloproteinases expression. Phytother Res 2007; 21(12):1127-1129.
[27] Dalimi A, Motamedi Gh, Hosseini M, Mohammadian B. Echinococcosis/ hydatidosis in western Iran. Vet Parasitol 2002; 105: 161-171.
[28] Amirghofran Z, Azadmehr A, Javidnia K. Haussknechtia elymatica: a plant with immunomodulatory effects. Iran J Immunol 2007;4(1):26-31.
[29] Azadmehr A, Hajiaghaee R, Mazandarani M. Induction of apoptosis and G2 /M cell cycle arrest by Scrophularia striata in a human leukaemia cell line. Cell Prolif 2013; 46(6): 637-643.
[30] Azadmehr A, Afshari A, Baradaran B, Hajiaghaee R, Rezazadeh S, Monsef-Esfahani H. Suppression of nitric oxide production in activated murine peritoneal macrophages in vitro and ex vivo by Scrophularia striata ethanolic extract. J Ethnopharmacol 2009; 124(1): 166-169.
[31] Amirghofran Z, Azadmehr A, Bahmani M, Javidnia K. Stimulatory effects of Euphorbia cheiradenia on cell mediated immunity and humoral antibody synthesis. Iran J Immunol 2008; 5(2):115-123.
[32] Nwidu L, Nwafor P, Vilegas W. Antimicrobial activity of Carpolobia lutea extracts and fractions. Afr J Tradit Complement Altern Med 2012; 9(3): 323-328.
[33] Tabatabaei Yazdi F, Mortazavi A, Koocheki A, Afsharian SH, Alizadeh Behbahani B. Antimicrobial properties of plant extracts of Thymus vulgaris L., Ziziphora tenuior L. and Mentha spicata L. against important foodborne pathogens in vitro. Sci J Microbiol 2013; 2(2): 23-30.
[34] Verdian-rizi M. Essential oil composition and biological activity of Ziziphora clinopodioides Lam. from Iran. Am Eurasian J Agric Sci 2008; 2(1): 69-71.
[35] Emami A, Zamani Taghizadeh Rabe S, Ahi A, Mahmoudi M. Study on toxic effects of Artemisisa Spp. fractions from Iran on human cancer cell lines. ZUMS J 2010; 18 (70): 58-67.
[36] Ayobi H, Jamalifar H, Pour Mohammadi F, Goodarzi S, Fazeli M, Attar F, et al. Antibacterial effects of Scrophularia striata extract on Pseudomonas aeruginosa. JMP 2014; 4 (52): 73-80.
[37] Billah M, Islam R, Khatun H. Antibacterial, antidiarrhoeal, and cytotoxic activities of methanol extract and its fractions of Caesalpinia bonducella (L.) Roxb leaves. BMC Complement Altern Med 2013; 13:101.
[38] Wang CY, Chiao MT, Yen PJ, Huang WC, Hou CC, Chien SC, et al. Modulatory effects of Echinacea purpurea extracts on human dendritic cells: A cell- and gene-based study. Genomics 2006; 88 (6): 801–808.
[39] Yones DA, Taher GA, Ibraheim ZZ. In vitro effects of some herbs used in Egyptian traditional medicine on viability of protoscolices of hydatid cysts. Korean J Parasitol 2011; 49(3): 255-263.
[40] Zuzarte M, Gonçalves MJ, Cavaleiro C, Canhoto J, Vale-Silva L, Silva MJ, et al. Chemical composition and antifungal activity of the essential oils of Lavandula viridis L'Hér. J Med Microbiol 2011; 60 (5): 612- 618.
[41] Sokovi M, Glamo lija J, Marin PD, Brki D, van Griensven LJ. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules 2010; 15(11): 7532-7546.
[42] Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science 2010; 327(5966): 656-661.
[43] Yanishlieva NV, Marinova EM, Gordon MH, Raneva VG. Antioxidant activity and mechanism of action of thymol and carvacrol in two lipid systems. Food Chem 1999; 64(1): 59-66.
[44] Erkan N, Ayranci G, Ayranci E. Antioxidant activities of rosemary (Rosmarinus officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem 2008; 110(1): 76-82.
[45] Sharififar F, Moshafi MH, Mansouri SH, Khodashenas M, Khoshnoodi M. In vitro evaluation of antibacterial and antioxidant activities of the essential oil and methaol extract of endemic Zataria multiflora Boiss. Food Control 2007; 18: 800-805.
Document heading 10.1016/j.apjtm.2016.09.008
20 July 2016
in revised form 2 August 2016
Dr. Mojtaba Shahnazi, Department of Parasitology, Qazvin University of Medical Sciences, bahonar St, Qazvin, Iran.
Tel/Fax: 982833360904
E-mail: shahnazi58@yahoo.com
✉ Dr. Abbas Azadmehr, Ph.D, Department of Immunology, Babol University of Medical Sciences, Babol, Iran.
Tel: +981132194667
E-mail: azadmehr2010@gmail.com
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Protective effect of antioxidant on renal damage caused by Doxorubicin chemotherapy in mice with hepatic cancer
- Anti-tumor activity of tanshinone IIA in combined with cyclophosphamide against Lewis mice with lung cancer
- Acetylcholinesterase, butyrylcholinesterase and paraoxonase 1 activities in rats treated with cannabis, tramadol or both
- Mechanism of action of Zhuyu Annao pill in mice with cerebral intrahemorrhage based on TLR4
- Clinical significance of dynamic detection for serum levels of MCP-1, TNF-α and IL-8 in patients with acute pancreatitis
- Modifiable determinants of attitude towards dengue vaccination among healthy inhabitants of Aceh, Indonesia: Findings from a communitybased survey
