PMMA系聚合物在锂离子电池凝胶电解质领域中的研究进展
2016-11-24焦晓宁周锦涛陈洪立
焦晓宁,周锦涛,陈洪立
(1.天津工业大学纺织学院,天津 300387;2.天津工业大学 先进纺织复合材料教育部重点实验室,天津300387)
PMMA系聚合物在锂离子电池凝胶电解质领域中的研究进展
焦晓宁1,2,周锦涛1,陈洪立1
(1.天津工业大学纺织学院,天津 300387;2.天津工业大学 先进纺织复合材料教育部重点实验室,天津300387)
综述了聚甲基丙烯酸甲酯(PMMA)作为锂离子电池凝胶电解质的特性及应用情况.PMMA基凝胶电解质与锂金属电极的界面稳定性好,室温离子电导率高达10-3S/cm数量级,循环充放电性能好;但较差的机械强度限制了其应用,因此常采取共混、涂覆以及共聚等不同方式与其他高聚物、无机纳米粒子、聚烯烃膜乃至非织造膜等结合,制备出复合凝胶电解质使用.指出未来研究趋势是采用静电纺丝技术制备出分层复合聚合物电解质.
PMMA;锂离子电池;凝胶电解质;分层复合
锂离子电池拥有能量大、无记忆效应等优点,在便携式电子产品、电动汽车以及储能系统等领域中应用广泛[1].正负极材料、电解液和多孔隔膜组成了锂离子电池的工作系统,其中隔膜既要保证Li+正常通过,又要确保正负极不直接接触,起至关重要的作用[2].锂离子电池最初采用液体电解质,因暴露出易生长枝晶、漏液等问题,逐渐被聚合物锂离子电池代替,后者使用凝胶聚合物电解质(GPE)来代替电解液[3].GPE由高聚物、锂盐和增塑剂形成,同时拥有固体粘聚性和液体分散传导性,Li+可借助微孔中的液态电解质分子在两极间实现自由往返,电池安全性大为提高[4].GPE受热稳定性好,且拥有不错的离子电导率(>10-3S/ cm)和电化学稳定窗口(>4.5 V),其制备方法主要有溶液浇筑法[5]、相分离法[6-7]以及静电纺丝法[8-10].相对于传统方法,静电纺丝法是最简单、最有应用前景的方法,可用于制备厚度均匀、成分均一的纤维膜,且具有无数贯通的微孔[11],方便Li+传输,电化学性能因此有很大提高[12].
1973年,Fenton等最先开始研究 GPE,随后Wright等发现聚环氧乙烯(PEO)与碱金属盐形成的络合物(PEO-MX)具有电导性,但其离子电导率达不到应用水平[13].1975年Feullade和Perche发现PVDF-MX等亦具备离子电导性,1979年Armand等使用其制造电池.1985年,Iijima等[14]首次将聚甲基丙烯酸甲酯(PMMA)用于GPE,当PMMA质量分数为15%时,室温离子电导率可达10-3S/cm数量级[15].
PMMA基GPE最大特点是与金属锂电极的电化学稳定性好、界面阻抗较低,而且甲基丙烯酸甲酯(MMA)单元中的羰基(-CO-)侧基与碳酸酯类增塑剂中氧原子的相互作用较强,因此能包含大量液体电解质,是已知亲电解液能力最高的聚合物[4,7],Li+迁移数也要优于PEO、PVDF基凝胶电解质.优异的电化学性能和成本优势,使PMMA获得研究者的青睐[15],然而由于机械强力不足、吸液后表面易受破坏[4],限制了其在GPE中单独使用,而多与其他成分组合使用.
本文以PMMA系聚合物在锂离子电池凝胶电解质中的应用为背景,介绍PMMA与其他组分不同结合方式的研究进展.
1 与其他聚合物结合
1.1 物理共混
不同聚合物组分共混可以减少结晶、促进链段运动,因此能提高电导率和机械性能.PMMA机械强度较弱,研究者常使用PVDF、P(VDF-HFP)、PAN等断裂强度高的聚合物与其混合制备多孔膜,然后浸入电解液中得到GPE.
Liang等[16]将不同质量比的PVDF/PMMA混合,采用静电纺丝制备出多孔纤维膜,结果表明当质量比为90∶10时所得GPE室温离子电导率最高(2.54×10-3S/cm).Ding等[17]采用静电纺丝方法制备P(VDF-HFP)/ PMMA(2∶1,w/w)GPE用于锂离子电池,测试表明P(VDF-HFP)结晶性受到削弱,复合膜吸液率高达377%,电池漏液情况得到改善;离子电导率2.0×10-3S/cm,首次放电比容量接近145 mAh/g,150次充放电循环以后放比容量仍然达到133.5 mAh/g.Mahant等[8]将PVDF与PMMA(8∶2,w/w)进行共混然后静电纺丝制得多孔膜,具有85%的孔隙率和285%的吸液率,电化学稳定窗口和室温离子电导率分别达到5.0 V和2.95× 10-3S/cm.高虹等[18]在PVDF/PMMA(7∶3,w/w)共混体系中添加了聚乙二醇PEG作增塑剂,采用相转化法制备出PVDF/PMMA/PEG聚合物隔膜,测得最佳工艺条件下制备的GPE离子电导率为2.848×10-3S/cm.赵剑蒙[19]利用同轴静电纺丝制备出PVDF/PMMA(芯层/皮层)复合隔膜,特殊的纤维结构使得该复合膜具有不错的机械性能和电化学性能,室温下的离子电导率和首次放电比容量分别达到3.07×10-3S/cm和157.1 mAh/g.
Florat等[20]制备出PAN/PMMA混合静电纺丝锂离子电池隔膜,研究发现PAN/PMMA质量比为75∶25时所得GPE性能最好.Rao等[10]研究了静电纺丝制备聚合物多孔膜的可行性,配制了10%的PAN/PMMA(摩尔比4∶1)混合纺丝液,所得纤维的直径为450 nm,室温离子电导率高达3.6×10-3S/cm,这与隔膜高孔隙率(86%)密不可分,电化学稳定窗口在5 V以上,0.1 C下首次放电容量为139 mAh/g,达到了正极材料(LiFePO4)理论容量的82.4%.Prasanth等[21]制备了PAN/PMMA/PS三组分混合静电纺丝多孔膜,发现质量比为80∶10∶10的混合膜制成GPE的界面稳定性最好,室温离子电导率为3.9×10-3S/cm,在Li/LiMn2O4电池充放电循环测试中,0.1 C下首次放电比容量约为120 mAh/g.
Zhong等[22]制备出PVC/PMMA静电纺丝复合纤维膜,实验表明PMMA的加入促进了PVC基体对电解液的吸收和保持,Li+运动效率得以提升,离子电导率高达3.36×10-3S/cm,电化学稳定窗口达5.0 V.Jung等[23]配制PMMA/PVC(不同质量比)溶液,制备出静电纺多孔膜,并进行DSC、XRD、SEM及电化学测试分析.结果显示PMMA质量分数为10%的GPE性能表现最优异,所组装电池在0.5 C倍率下充放电循环100次后放电比容量仅仅缩减了2 mAh/g(从142 mAh/g降至140 mAh/g).
1.2 化学反应
除了简单共混的方式,研究者亦通过共聚、交联、接枝等化学改性方法引入PMMA,以增加聚合物体系的无定型相.共聚是将不同种类单体通过氧化还原反应聚合在一起,以其中一种强力高的聚合物做基体,多种物质优势互补故整体性能更优秀.Ryoo等[5]在N2氛围下,通过引发剂作用将2种单体(丙烯腈和甲基丙烯酸甲酯)共聚合成P(AN-MMA),并采用溶液浇筑法制备出GPE,实验表明该电解质与金属锂片界面间的稳定性良好,不同温度下的离子电导率在0.86~1.6×10-3S/cm范围内.Lee等[24]在此基础上研究了SiO2纳米粒子对GPE电化学性能的影响,结果表明,加入SiO2后离子电导率大大增加,-15℃下为0.17×10-3S/ cm,而25℃下达1.3×10-3S/cm.
交联主要是将共混物或共聚单体进行网络化,以增强其力学性能.Xu等[25]通过交联将聚乙烯基乙二醇二甲基醚(PEGDME)与PMMA制成了高机械强度和空间稳定性的均相凝胶电解质膜,实验数据表明该膜在-50~200℃范围内未发生相迁移,热稳定性良好.此外,接枝在提供稳定化学键的同时也改善了不同聚合物间的相容性,使电解质膜性能更好并且更加稳定.例如,将PMMA接枝到P(VDF-HFP)上可以得到P(VDF-HFP)-g-PMMA梳状共聚物,利用其制备的GPE具有较高的保液能力和不错的电化学性能[26].
刘建生[27]使用乙酸乙烯酯(VAc)与丙烯腈(AN)单体进行乳液聚合得到交联共聚物P(AN-co-VAc),并采用相转化法制备P(AN-VAc)自支撑膜,最后用PMMA对其进行表面复合改性,得到P(AN-co-VAc)/ PMMA复合膜.结果显示复合PMMA后的GPE电导率由1.4×10-3S/cm提到1.88×10-3S/cm,电化学稳定窗口则由4.8 V提升至5.2 V.
2 与纳米颗粒相互作用
2.1 直接添加
无机纳米填料具有强力高、耐高温、绝缘和化学稳定性好等优点,利用共混、溶胶凝胶和原位聚合的方法将其添加到聚合物中,能够改善聚合物的成膜性能,起到增强增韧的作用.而且纳米颗粒比表面积大,便于Li+嵌入和脱出,既保证了较小的充放电极化程度,又兼具较高的可逆容量,因此在改善GPE热稳定性和电化学性能方面表现出重大作用.
Zhou等[28]采用原位聚合法在PVDF/PMMA(8∶2,w/w)纺丝液中添加3%(相对于聚合物)的TiO2纳米颗粒,制备出静电纺丝纤维膜.结果表明:添加纳米颗粒后,混纺膜纤维直径由850 nm降至570 nm,吸液率、孔隙率及电化学性能(离子电导率:3.9×10-3S/cm,电化学稳定窗口∶5.1 V)均获得提升.
Song等[29]将不同含量TiO2纳米颗粒直接加入PVDF-HFP/PMMA混合液中,采用溶液浇筑法制备出GPE.结果发现加入5%TiO2后的GPE各方面性能最好,其130℃的热收缩率由23.4%(不添加TiO2)降至14.4%,室温下测试的离子电导率和电化学稳定窗口分别达到2.49×10-3S/cm和5.0 V,0.2 C倍率下经过50次循环后放电比容量仍能达到首次放电比容量(188.1 mAh/g)的92.1%.
2.2 核-壳结构
考虑到无机纳米粒子在有机溶剂中较弱的分散能力(例如SiO2粒子表面呈亲水疏油性,不利于在有机溶剂中润湿、分散),以及与聚合物存在的性质差异,上述直接添加的方式容易出现团聚现象.而表面化学改性的方法可以利用改变化学键的方式来减少活性基团(-OH)数目,从而改善无机颗粒的分散性.一种典型的方法是通过制备核-壳结构使无机粒子具有聚合物的性质[30-32].
Cui等[33]采用原子转移自由基聚合法(ATRP)得到PMMA-g-TiO2,然后将6%(相对于PVDF)的PMMA-g-TiO2溶于PVDF溶液中,利用静电纺丝制备出复合多孔膜.测试结果表明:加入PMMA-g-TiO2后的复合膜相对于纯PVDF膜性能获得提升,浸入电解液5 h后吸液率从310%增至360%,20℃离子电导率由2.51×10-3S/cm提升至2.95×10-3S/cm,电化学稳定窗口由5.1 V提升至5.3 V.
Yang等[31]首先采用改进的Stöber法[34]在实验室制备出SiO2纳米颗粒,然后利用无皂乳液聚合得到具有核-壳结构的SiO2-PMMA亚微球,如图1所示.再将其涂覆在PE隔膜一侧,制备出功能陶瓷涂层膜(FCC).其中SiO2耐热性能好,能够提高FCC耐热收缩性能,进而提升电池安全性;而PMMA形成凝胶电解质后,持液能力增强,电池性能得到提升.测试结果表明:FCC膜的热收率由纯PE膜的31.4%提高到12.9%,吸液率由57.7%提升到89.5%,离子电导率由7.80×10-4S/cm提高到1.08×10-3S/cm.

图1 具有核-壳结构的SiO2-PMMA亚微球合成示意图Fig.1 Synthetic scheme of core-shell structured SiO2-PMMA sub-microspheres
尚昕[3]将改性后的SiO2分散在MMA和St(苯乙烯)单体中,采用自由基聚合反应制备出SiO2/P(MMA-S)交联型纳米复合物,随后利用相转化法制得多孔膜.该膜吸液率为310%,拉伸断裂强度高达40 MPa,以LiClO4-DMC/EC/EMC电解液组装的电池在0.1 C倍率下具有高达156.8 mAh/g的首次放电比容量.
3 增强型分层复合多孔膜
鉴于PMMA强力不足限制了实际应用,研究者采用溶液浇筑、涂覆、浸渍、化学接枝改性、静电纺丝[9,35-37]等方法将其与PVDF、PVDF-HFP等聚合物,无机颗粒,聚烯烃隔膜乃至非织造膜分层结合的方式,以利于PMMA凝胶电解质发挥出自身优势.
3.1 与非织造膜复合
Wu等[37]以PP非织造膜为基材制备了2种含PMMA的分层复合隔膜,分别为:①将PP非织造膜浸入溶有不同质量PMMA纳米颗粒的PVDF-HFP溶液中,烘干得到复合膜(CSs);②先将PP非织造膜浸入PVDF-HFP溶液中2 min后烘干,再将含固量10%的PMMA纳米颗粒水溶液涂覆在表面,烘干制得纳米涂层复合隔膜(nano-CS).其中,CS(0.2)和nano-CS 2种隔膜的吸液率分别为212%和202%,孔隙率分别为77.9%和75.3%,室温离子电导率分别为1.575×10-3S/cm和1.846×10-3S/cm,0.2 C倍率下充放电50次后的放电比容量分别为138 mAh/g和152 mAh/g,分别占首次放电比容量的97%和99%.
3.2 与聚烯烃膜复合
市售聚烯烃隔膜强力高但其材质属于疏水性高聚物,难以完全润湿且表面能较低,缺乏稳定的吸液能力,存在漏液风险影响使用安全[38-39].涂层、接枝共聚是此类隔膜常用的改性方法,其中引入MMA等极性基团接枝改性后的隔膜被称为活性膜[40],具有更好的吸液能力和界面稳定性.
Kim等[41]将PMMA、TiO2纳米颗粒与聚乙烯乙二醇二丙烯酸酯(PEGDA)以6∶1∶4的质量比混合,溶于1 mol/L LiPF6(EC∶EMC∶DMC质量比为1∶1∶1)电解液中并添加引发剂和催化剂,然后涂覆在PP隔膜上并采用紫外光照射3 h,最终制备出复合GPE.结果表明,加入TiO2纳米颗粒后的GPE界面阻抗下降,电化学性能显著增强,0.5 C下测试具有高达320 mAh/g的首次放电比容量.此外,卢雷[42]采用乳液聚合法将MMA单体与粘合性能优异的醋酸乙烯醋 (PVac)单体合成出聚甲基丙烯酸甲酯-醋酸乙烯酯(PMMAVac),然后通过浸泡将其附着在PE隔膜上,烘干后浸入电解液中得到GPE.
使用涂层法制备的隔膜体系机械性能好、吸液率高,但也存在操作条件控制不易、涂层厚度不均、容易掉落等缺陷,光引发接枝聚合法可以避免这些问题[43]. Swon等[44]利用光引发接枝聚合法在商业PE隔膜表面接枝PMMA,制备出不同接枝度的PE-g-PMMA膜.结果表明:随着接枝度的增加,隔膜耐热收缩性能稳步提升,这是因为PMMA逐渐覆盖了PE膜的孔隙,阻止其受热收缩;测得活性膜的电化学稳定窗口均超过5.0 V,其中接枝度为127%的隔膜在0.5 C倍率下第300次放电比容量是首次放电比容量的85%.
由于光引发接枝法需要使用高能射线,容易降解PMMA并对聚烯烃隔膜强力造成损伤,研究人员尝试其他方法改进.Shi等[45]利用聚多巴胺作为ATFR反应的引发剂,在相对温和条件下将MMA接枝在PE隔膜表面,成功制备出了PE-g-PMMA活性膜,其中PE作为骨架起到强力支撑作用,凝胶电解质用来增强润湿能力和保液能力.实验表明:接枝后隔膜孔径、孔隙率及拉伸断裂强度下降,最佳接枝度是22%,此时活性膜的热稳定性和吸液能力获得提升,室温下测试离子电导率和首次放电比容量分别达到1.19×10-3S/cm、163.1 mAh/g,电化学性能优于纯PE膜.
3.3 与聚合物分层复合
Xiao等[9]采用静电纺丝的方法制备了PVDF/PM MA/PVDF三明治结构的自粘合复合多孔膜,如图2所示.

图2 PVDF/PMMA/PVDF三层复合凝胶电解质示意图Fig.2 SEM micrographs of cross-section of PVDF/PMMA/ PVDF trilayer membrane
结果表明:三层复合膜的拉伸断裂强度由PMMA单层膜的2.15 MPa提高到7.11 MPa,室温离子电导率高于单层复合膜(达到了1.93×10-3S/cm),电化学稳定窗口达4.5 V.赵剑蒙[19]制备出静电纺丝PVDF/PMMA/PVDF三层复合膜,测得层合后的隔膜平均孔径(3.683 μm)偏小但孔隙率较高(80.14%),且拉伸断裂强度由PMMA/PVDF直接混纺膜的11.21 MPa提升至13.7 MPa.程司辰[46]制备出静电纺丝PMMA/PVDF/ PMMA复合膜,该复合膜吸液率高达497%,室温下的离子电导率为3.63×10-3S/cm,界面阻抗仅为45 Ω,所组电池在0.2 C倍率下首次放电比容量则达到了147.7 mAh/g.
3.4 其他方式分层复合
Kim等[35]首先采用溶液浇筑法得到一层PMMA薄膜,然后将其浸入纳米Al2O3/PVDF-HFP(质量比90∶10,聚合物PVDF-HFP起粘结作用)混合液中保持1 h,得到具有无机涂层的Al2O3/PMMA/Al2O3三层复合隔膜,并得到相应GPE,如图3所示.测试结果表明:该隔膜的拉伸断裂强度为9.8 MPa,而吸液率接近500%,室温下的离子电导率为0.535×10-3S/cm.
图3 Al2O3/PMMA/Al2O3三层复合凝胶电解质示意图Fig.3 Schematic diagram of Al2O3/PMMA/Al2O3sandwiched GPE
Zhang等[36]在玻璃板表面依次涂上PVDF和PMMA溶液,聚合物质量比控制在0.5∶1∶0.5,制备出PVDF/PMMA/PVDF三层复合锂电隔膜,如图4所示.与Celgard 2 400隔膜相比,该隔膜组装电池的充放电循环表现优势显著.
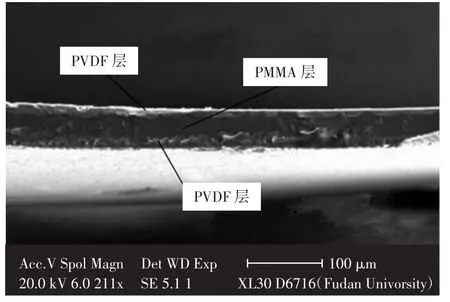
图4 PVDF/PMMA/PVDF三层复合隔膜示意图Fig.4 SEM micrograph of cross-section of sandwiched membrane of PVDF/PMMA/PVDF
4 结语
PMMA系凝胶电解质亲电解液能力强、界面阻抗低,表现出良好的电化学稳定性,在锂电池应用领域中具有很好的前景.由于PMMA是非结晶聚合物,机械强力偏弱,需要与其他高强力聚合物材料结合才能稳定发挥作用.通过分层结合,以PVDF、PAN等机械强力好的物质为基,有利于不同组份发挥出各自的性能优势.虽然PMMA在复合凝胶电解质体系中非支配地位(质量占比低于50%),但其对电解液亲和力高,对电化学性能的提升做出了巨大贡献.有鉴于此,笔者预测未来在PMMA基复合凝胶电解质的研究中将会有以下趋势:
(1)静电纺丝技术是目前制备多孔膜的理想方式,PMMA系GPE的制备将更多采用此法;
(2)PMMA有赖于结晶型聚合物提供强力支撑;
(3)分层复合的凝胶电解质因不同组分能够形成优势互补,是一个重要的研究趋势;
(4)无机纳米颗粒的添加有益于聚合物膜增强增韧、提高热稳定性等,将会广泛的使用.
[1] FERGUS Jeffrey W.Ceramic and polymeric solid electrolytes for lithium-ion batteries[J].Journal of Power Sources,2010,195(15):4554-4569.
[2] NUNES-PEREIRA J,LOPES A C,COSTA C M,et al.Microporous membranes of NaY zeolite/poly(vinylidene fluoridetrifluoroethylene)for Li-ion battery separators[J].Journal of Electroanalytical Chemistry,2013,689(2):223-232.
[3]尚昕.多相结构纳米SiO2类复合电解质的制备与应用[D].南昌:南昌大学,2015. SHANG X.Preparation and application of multiphase structure of nano-SiO2composite electrolyte system[D].Nanchang:Nanchang University,2015(in Chinese).
[4]ZHANG Jinqiang,CHEN Shuangqiang,XIE Xiuqiang,et al. Porous poly(vinylidene fluoride-co-hexafluoropropylene)polymer membrane with sandwich-like architecture for highly safe lithium ion batteries[J].Journal of Membrane Science,2014,472:133-140.
[5]RYOO Hee-Jin,KIM Hee-Tak,LEE Young-Gi,et al.Thermal and electrochemical characteristics of plasticized polymer electrolytes based on poly(acrylonitrile-co-methyl methacrylate)[J].Journal of Solid State Electrochemistry,1998,3(1):1-6.
[6]MA Ting,CUI Zhenyu,WU Ying,et al.Preparation of PVDF based blend microporous membranes for lithium ion batteries by thermally induced phase separation I:Effect of PMMA on the membrane formation process and the properties[J].Journal of Membrane Science,2013,444:213-222.
[7]IDRIS Nurul Hayati,RAHMAN Md Mokhlesur,WANG Jia-Zhao,et al.Microporous gel polymer electrolytes for lithium rechargeable battery application[J].Journal of Power Sources,2012,201:294-300.
[8]MAHANT Yogita P,KONDAWAR Subhash B,BHUTE Monali,et al.Electrospun poly(vinylidene fluoride)/poly(methyl methacrylate)composite nanofibers polymer electrolyte for Batteries[J].Procedia Materials Science,2015,10:595-602.
[9]XIAO Qizhen,LI Zhaohui,GAO Deshu,et al.A novel sandwiched membrane as polymer electrolyte for application in lithium-ion battery[J].Journal of Membrane Science,2009,326(2):260-264.
[10]RAO Mumin,GENG Xiuyu,LIAO Youhao,et al.Preparation and performance of gel polymer electrolyte based on electrospun polymer membrane and ionic liquid for lithium ion battery[J].Journal of Membrane Science,2012,399-400:37-42.
[11]GOPALAN Anantha Iyenger,SANTHOSH Padmanabhan,MANESH Kalayil Manian,et al.Development of electrospun PVdF-PAN membrane-based polymer electrolytes for lithium batteries[J].Journal of Membrane Science,2008,325(2):683-690.
[12]RAGHAVAN Prasanth,ZHAO Xiaohui,MANUEL James,et al.Electrochemical performance of electrospun poly(vinylidene fluoride-co-hexafluoropropylene)-based nanocomposite polymer electrolytes incorporating ceramic fillers and room temperature ionic liquid[J].Electrochimica Acta,2010,55(4):1347-1354.
[13]李景虹.先进电池材料[M].北京:化学工业出版社,2004:331,349-350. LI J H.Advanced Battery Materials[M].Beijing:Chemical Industry Press,2004:331,349-350(in Chinese).
[14]IIJIMA T,TOYOGUCHI Y,EDA N.Quasi-solid organic electrolytes gelatinized with poly methylmethacrylate and their applications for lithium batteries[J].Denki Kagaku,1985,53(8):619-623.
[15]倪冰选,焦晓宁,阮艳莉.聚合物锂离子电池用凝胶电解质的研究进展[J].天津工业大学学报,2009,28(3):48-52,57. NI B X,JIAO X N,RUAN Y L.Research progress of gel electrolyte for polymer lithium-ion battery[J].Journal of Tianjin Polytechnic University,2009,28(3):48-52,57(in Chinese).
[16]LIANG Yinzheng,CHENG Sichen,ZHAO Jianmeng,et al. Preparation and characterization of electrospun PVDF/PMMA composite fibrous membranes-based separator for lithium-ion batteries[J].Advanced Materials Research,2013,750/751/ 752:1914-1918.
[17]DING Yanhuai,ZHANG Ping,LONG Zhilin,et al.The ionic conductivity and mechanical property of electrospun P(VdFHFP)/PMMA membranes for lithium ion batteries[J].Journal of Membrane Science,2009,329(1/2):56-59.
[18]高虹,陈爱雨,王守兵.PVDF/PMMA/PEG型聚合物隔膜的制备[J].功能材料,2015(15):15138-15141,15147. GAO H,CHEN A Y,WANG S B.PVDF/PMMA/PEG polymer diaphragm preparation.[J].Journal of Functional Materials,2015(15):15138-15141,15147(in Chinese).
[19]赵剑蒙.锂离子电池用PVDF/PMMA静电纺复合隔膜的制备与改性研究[D].上海:东华大学,2014. ZHAO J M.Fabrication and modification of electrospun PVDF/ PMMA composite membrane as lithium-ion battery seperator[D].Shanghai:Donghua University,2014(in Chinese).
[20]FLORAT X Helan,ULAGANATHAN M,SHANKER Babu Ravi,et al.Evaluation of lithium ion conduction in PAN/PMMA-based polymer blend electrolytes for Li-ion battery applications[J].Ionics,2012,18(8):731-736.
[21]PRASANTH Raghavan,ARAVINDAN Vanchiappan,SRINIVASAN Madhavi.Novel polymer electrolyte based on cob-web electrospun multi component polymer blend of polyacrylonitrile/poly(methyl methacrylate)/polystyrene for lithium ion batteries—Preparation and electrochemical characterization[J]. Journal of Power Sources,2012,202:299-307.
[22]ZHONG Zheng,CAO Qi,WANG Xianyou,et al.PVC-PMMA composite electrospun membranes as polymer electrolytes for polymer lithium-ion batteries[J].Ionics,2011,18(1/2):47-53.
[23]JUNG Hong-Ryun,LEE Wan-Jin.Electrochemical characteristics of electrospun poly(methyl methacrylate)/polyvinyl chloride as gel polymer electrolytes for lithium ion battery[J]. Electrochimica Acta,2011,58:674-680.
[24]LEE Kyoung Hee,LEE Young Gi,PARK Jung Ki,et al.Effect of silica on the electrochemical characteristics of the plasticized polymer electrolytes based on the P(AN-co-MMA)copolymer[J].Solid State Ionics,2000,133(3):257-263.
[25]XU Jun John,YE Hui.Polymer gel electrolytes based on oligo-meric polyether/cross-linked PMMA blends prepared via in situ polymerization[J].Electrochemistry Communications,2005,7(8):829-835.
[26]LIU Y,J.LEE Y,HONG L.Synthesis,characterization and electrochemical properties of poly(methyl methacrylate)-grafted-poly(vinylidene fluoride-hexafluoropropylene)gel electrolytes[J].Solid State Ionics,2002,150(3/4):317-326.
[27]刘建生.锂离子电池新型凝胶聚合物电解质的改性研究[D].广州:华南理工大学,2013. LIU J S.Investigations on the preparation and performance of gel polymer electrolyte for lithium ion battery[D].Guangzhou:South China University of Technology,2013(in Chinese).
[28]ZHOU Ling,WU Na,CAO Qi,et al.A novel electrospun PVDF/PMMA gel polymer electrolyte with in situ TiO2 for Liion batteries[J].Solid State Ionics,2013,249/250:93-97.
[29]SONG Dayu,XU Chen,CHEN Yuanfu,et al.Enhanced thermal and electrochemical properties of PVDF-HFP/PMMA polymer electrolyte by TiO2nanoparticles[J].Solid State Ionics,2015,282:31-36.
[30]PARK S M,LEE Y S,KIM D W.High-performance lithium-Ion polymer cells assembled with composite polymer electrolytes based on core-shell structured SiO2particles contain-ing poly(lithium acrylate)in the shell[J].Journal of the Electrochemical Society,2014,162(2):A3071-A3076.
[31]YANG Pingting,ZHANG Peng,SHI Chuan,et al.The functional separator coated with core-shell structured silica-poly(methyl methacrylate)sub-microspheres for lithium-ion batteries[J].Journal of Membrane Science,2015,474:148-155.
[32]SHIN Won-Kyung,KIM Dong-Won.High performance ceramic-coated separators prepared with lithium ion-containing SiO2particles for lithium-ion batteries[J].Journal of Power Sources,2013,226:54-60.
[33]CUI Wei-Wei,TANG Dong-Yan,GONG Zai-Lin.Electrospun poly(vinylidene fluoride)/poly(methyl methacrylate)grafted TiO2composite nanofibrous membrane as polymer electrolyte for lithium-ion batteries[J].Journal of Power Sources,2013,223:206-213.
[34]ARRIAGADA F J,OSSEO-ASARE K.Synthesis of nanosize silica in a nonionic water-in-oil microemulsion:Effects of the water/surfactant molar ratio and ammonia concentration[J].J Colloid Interface Sci,1999,211(2):210-220.
[35]KIM Min,HAN Gui Young,YOON Ki June,et al.Preparation of a trilayer separator and its application to lithium-ion batteries[J].Journal of Power Sources,2010,195(24):8302-8305.
[36]ZHANG H P,ZHANG P,LI Z H,et al.A novel sandwiched membrane as polymer electrolyte for lithium ion battery[J]. Electrochemistry Communications,2007,9(7):1700-1703.
[37]WU Dazhao,HE Jinlin,ZHANG Mingzu,et al.Fabrication of a novel sandwich-like composite separator with enhanced physical and electrochemical performances for lithium-ion battery[J].Journal of Power Sources,2015,290:53-60.
[38]KIM Jun Young,LEE Yongbeom,LIM Dae Young.Plasmamodified polyethylene membrane as a separator for lithium-ion polymer battery[J].Electrochimica Acta,2009,54(14):3714-3719.
[39]KIM Jun Young,LIM Dae Young.Surface-modified membrane as a separator for lithium-ion polymer battery[J].Energies,2010,3(4):866-885.
[40]LI Hao,MA Xiao-Ting,SHI Jun-Li,et al.Preparation and properties of poly(ethylene oxide)gel filled polypropylene separators and their corresponding gel polymer electrolytes for Li-ion batteries[J].Electrochimica Acta,2011,56(6):2641-2647.
[41]KIM Hyung-Sun,KUM Kyong-Soo,CHO Won-Il,et al. Electrochemical and physical properties of composite polymer electrolyte of poly(methyl methacrylate)and poly(ethylene glycol diacrylate)[J].Journal of Power Sources,2003,124(1):221-224.
[42]卢雷.一种锂离子电池新型聚合物电解质PMMA-Vac的制备及性能研究[D].广州:华南师范大学,2007. LU L.A study on the preparation and performances of pmmavac electrolyte for lithium ion battery use[D].Guangzhou:South China Normal Univesity,2007(in Chinese).
[43]LI Shudan,GAO Kun.The study on methyl methacrylate graft-copolymerized composite separator prepared by pre-irradiation method for Li-ion batteries[J].Surface and Coatings Technology,2010,204(16/17):2822-2828.
[44]GWON Sung-Jin,CHOI Jae-Hak,SOHN Joon-Yong,et al. Battery performance of PMMA-grafted PE separators prepared by pre-irradiation grafting technique[J].Journal of Industrial and Engineering Chemistry,2009,15(5):748-751.
[45]SHI Jun-Li,FANG Li-Feng,LI Hao,et al.Improved thermal and electrochemical performances of PMMA modified PE separator skeleton prepared via dopamine-initiated ATRP for lithium ion batteries[J].Journal of Membrane Science,2013,437:160-168.
[46]程司辰.基于静电纺丝法的PVDF基锂离子电池隔膜的制备与表征[D].上海:东华大学,2013. CHENG S C.Preparation and characterization of electrospun PVDF-based membrane as lithium battery separator[D]. Shanghai:Donghua University,2013(in Chinese).
Research progress of PMMA-based gel electrolyte for LIBS
JIAO Xiao-ning1,2,ZHOU Jin-tao1,CHEN Hong-li1
(1.Schoool of Textiles,Tianjin Polytechnic University,Tianjin 300387,China;2.Key Laboratory of Advanced Textile Composites of Ministry of Education,Tianjin Polytechnic University,Tianjin 300387,China)
The characteristics and application of polymethyl methacrylate (PMMA)used for lithium-ion batteries gel polymer electrolyte(GPE)are summarized.One of the advantages of PMMA-based GPE is the good interface stability with lithium metal electrode,and it also has high ionic conductivity of 10-3S/cm at room temperature,and its cycle performance is better than that of some other gel electrolyte.But the weakness of mechanical strength limits its application.But the weakness of mechanial strength limits its application.The mechanial property of PMMA-based GPE can be improved by combining with other polymers,inorganic nanoparticles,polyolefin films,even nanworens to form gel electrolytes through blending,coating,copolymerization,etc.The trend of research on PMMA-based GPE is to prepare multi-layered composite porous electrolytes by electrospinning.
PMMA;lithium-ion battery(LIB);gel electrolyte;multi-layered composite
TM911.3
A
1671-024X(2016)05-0046-07
10.3969/j.issn.1671-024x.2016.05.009
2016-07-04
国家科技支撑计划项目(2015BAE01B03)
焦晓宁(1958—),女,教授,硕士生导师,主要研究方向为功能非织造材料.E-mail:xiaoningj@tjpu.edu.cn
