Eff ect of temperature on corrosion behavior of 3003 aluminum alloy in ethylene glycol–water solution
2016-11-24ChenXinTianWenmingLiSongmeiYuMeiLiuJianhua
Chen Xin,Tian Wenming,Li Songmei,Yu Mei,Liu Jianhua
School of Material Science and Technology,Beihang University,Beijing 100083,China
Eff ect of temperature on corrosion behavior of 3003 aluminum alloy in ethylene glycol–water solution
Chen Xin,Tian Wenming,Li Songmei*,Yu Mei,Liu Jianhua
School of Material Science and Technology,Beihang University,Beijing 100083,China
The effect of temperature on the corrosion behavior of 3003 aluminum alloy in ethylene glycol–water solution was investigated by potentiodynamic polarization and electrochemical impedance spectroscopy(EIS)techniques.The surface characterization was observed and determined by scanning electron microscopy(SEM),atomic force microscopy(AFM)and energy dispersive spectrometer(EDS).The results demonstrate that the anodic aluminum dissolution and the cathodic oxygen reduction were accelerated by the increased temperature.However,as temperature was over 60°C,the solubility and concentration of oxygen decreased,resulting in the inhibition of cathodic reaction.The cathodic reaction rate of 3003 aluminum alloy rose to the maximum at 60°C.The Warburg impedance in Nyquist diagram diminished and then was replaced by a negative capacitance caused by the absorption of intermediate corrosion product on electrode.On the other hand,after potentiodynamic measurements,3003 aluminum alloy suffered pitting corrosion.The dissolution of aluminum alloy around secondary phase particles expanded both horizontally and vertically.
1.Introduction
Aluminum alloys are increasingly used in aerospace industries owing to their excellent properties of high strength-to-weight ratio and good formability.1–3For example,the heat exchangers on the aircraft’AC modules are made of the aluminum alloy rather than the other two types of commonly applied materials:the copper alloy and the stainless steel.However,aluminum alloy corrosion occurs and is a major problem in the cooling system of aircraft engine block.4–6It is due to a wide temperature range and complex components of coolant to which the aluminum alloy is exposed.7–9The appropriate physical and chemical properties of heat coolant have been required to prevent malfunction of cooling system.10–12Mixed with water,ethylene glycol is a popular coolant in heat exchanger13,because of its low cost and excellent freeze and heat-protection over a wide temperature range.14–17The main composition of a conventional coolant consists of 30%–70%(by volume)ethylene glycol and some corrosion inhibitors.18–20Common heat transfer fluids show a low aggressivity,unless pollution or high-temperature exposure (degradation)occurs.21Coolant in the aircraft heat exchanger is always contaminated with chloride ions.In the presence of Cl-,the corrosionresistanceof aluminum andaluminum alloys decreased significantly and severe pitting corrosion was observed.22
3003 aluminum alloy is considered as an advanced material for manufacturing aero heat exchangers.23–26It has complex microstructure containing intermetallic particles and impurity precipitation in the aluminum matrix.These particles and precipitation can be either cathodic or anodic with respect to the matrix phase.27,28Such complexities have made it challenging to understand the corrosion of 3003 aluminum alloy in cooling systems at different temperatures.
There were a few works in the study of corrosion of aluminum alloys in aqueous ethylene glycol solutions.29Liu and Cheng30found that the oxide film formed on aluminum depended on the dissolved oxygen in the solution.The cathodic reaction is dominated by the oxygen reduction in aerated solution.31In the absence of oxygen,the main cathodic reaction is either reduction of water or reduction of ethylene glycol.32However,few of them focused on the effect of temperature on the corrosion behavior of 3003 aluminum alloy in aero coolant systematically.It can provide useful data for material protection and design.In particular,the change in temperature may make a big difference to the inhibition effect of inhibitor.There fore,it is necessary to study the effect of temperature on the corrosion behavior of 3003 aluminum alloy in the solution.
The present work studies the corrosion and electrochemical behavior of 3003 aluminum alloy in chloride-containing ethylene glycol–water solution at different temperatures by various electrochemical measurements,including corrosion potential,potentiodynamic polarization,cathodic polarization,electrochemical impedance spectroscopy(EIS).The geometrical parameters of pits are measured and analyzed.The surface morphology of aluminum electrode is characterized by scanning electron microscopy(SEM)and atomic force microscopy(AFM).The chemical composition is measured by an energy disperse spectroscopy(EDS)combined.
2.Experimental procedures
2.1.Electrodes and solutions
Test specimens were cut from a 3003 aluminum plate into cylinders of 10 mm diameter,with the chemical composition(wt%):Cu 0.2,Mn 1.5,Fe 0.70,Si 0.60,Zn 0.10,Mg 0.05,Ti 0.05,Cr 0.05 and aluminum in balance.The aluminum electrode was embedded in phenolic resin,leaving a working area of 0.785 cm2.Electrical contact of the specimen was madefrom the rear by spot welding a cooper lead on to the back of the specimen.The non-working surface of the electrode was sealed in an epoxy resin.The exposed surface of each electrode was finished by wet-grinding with a series of emery papers from 400 grit to 5000 grit and then cleaned thoroughly with alcohol acetone and deionized water in turn.The base solution was a mixture of 50%ethylene glycol+50%deionized water+0.1 mol·L-1NaCl,simulating the aero coolant and the potential chloride contamination.Tests were per formed at temperatures ranging from 30–80 °C,consistent with the typical operating temperature in the aero cooling system.The test temperature was maintained through a water bath controlled by a thermoelectric couple.All solutions were madefrom analytic grade reagents and ultrapure deionized water.
2.2.Electrochemical measurement
Electrochemical measurements were conducted on a three electrode cell with the test specimen as working electrode(WE),a platinum as counter electrode(CE)and a saturated calomel electrode(SCE)as reference electrode(RE).Prior to electrochemical measurement,the specimen was immersed in the static solution for 3 h in order to obtain a steady corrosion potential.Open-circuit potentials were monitored prior to any polarization or impedance measurements.
Potentiodynamic scans were per formed at a positive direction from-0.8 V(vs.SCE)to-0.45 V(vs.SCE)with a scan rate of 30 mV/min on Princeton 2273.Polarization stopped when the anodic current density exceeded 0.01 mA.The values of corrosion current density(icorr),corrosion potential(Ecorr),anodic and cathodic Tafel slops(ba,bc)werefitted and calculated through a Zview analytical sof tware.Cathodic polarization was carried out by sweeping from-0.6 V(vs.SCE)negatively to about-1.3 V(vs.SCE).
All the EIS measurements were conducted at open circuit potential on CHI 660E(Hua Chen instrument corporation,Shanghai).Impedances were characterized over the frequency rangefrom 100 kHz to 10 mHz,with the AC signal amplitude being±10 mV(vs.SCE).Equivalent circuit modeling was per formed using ZsimpWin sof tware.
For each temperature,at least three parallel valid samples were tested to ensure the reproducibility of the results.The sample data closest to the average level was the final data used in analysis.Al the tests were per formed open to air without agitation of the electrolyte.
2.3.Surface characterization
After potentiodynamic polarization,the 3003 aluminum electrode was removed from solution.Be fore AFM measurement the sample was cleaned by ultrasonic bath for 10 min to get rid of corrosion product,rinsed with deionized water and dried with cold air for atomic force microscopy(AFM)analysis.AFM analysis was conducted on a Veeco Dimension Icon operating in Tap Control mode under ambient conditions in air at a scan size of 30 μm × 30 μm to provide detailed topographic in formation.Images were analyzed by the AFM NanoScope Analysis sof tware,version 1.40.And then a layer of gold was sprayed on the surface of the electrode to enhance the electrical conductivity prior to SEM(scanning electron microscopy)characterization conducted on CamScan3400.The morphological features of different regions of the electrode were characterized by SEM at 2000×magnification.Then,the total number and physical dimensions of pits located in the microscopical view were recorded without repetition.The number of pits was counted on the visual field of the microscopy.We chose ten visual fields of microscopy randomly on each sample under the same magnification,recorded the number of pits on it,and then calculated the number of pits on per mm2Elemental composition was measured with a combined EDS(energy dispersive spectrometer)instrument.
3.Presentation of results
3.1.Polarization curve measurements
The corrosion potential of the electrode increased at first and then fluctuated around-0.58 V(vs.SCE)with the immersion time.
Fig.1 shows the polarization curves of 3003 aluminum electrode measured in the test solution at different temperatures,and T is temperature.It is seen that 3003 aluminum alloy would not be passivated in the ethylene glycol–water solution containing 0.1 mol·L-1NaCl.With the increase of temperature,the corrosion potential of aluminum decreased and anodic dissolution current density increased.Cathodic current density increased until the temperature was up to 60°C,and then decreased as the temperature reached 80°C.
The electrochemical parameters,including corrosion current density,corrosion potential,anodic and cathodic Tafel slopes arefitted and listed in Table 1.
Fig.2 shows the cathodic polarization curves at various temperatures.The characteristic of cathodic polarization curves are similar.The reductive current density decreased rapidly with the positive shift of potential be fore the curve revealed a knee point after which an approximate plateau showed.Cathodic polarization curve was divided into two parts by the knee point.The part of cathodic curve at potentials more positive than the knee point has been demonstrated in Fig.2.We deal with the part of cathodic curve at potentials more negative than the knee point.With temperature increasing,the reductive current density increased.Moreover,the knee point shifted positively as temperature increased.
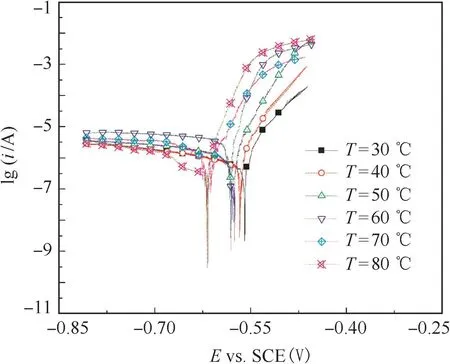
Figure 1 Polarization curves of 3003 aluminum alloy at different temperatures.

Table 1 Fitted electrochemical parameters at different temperatures.
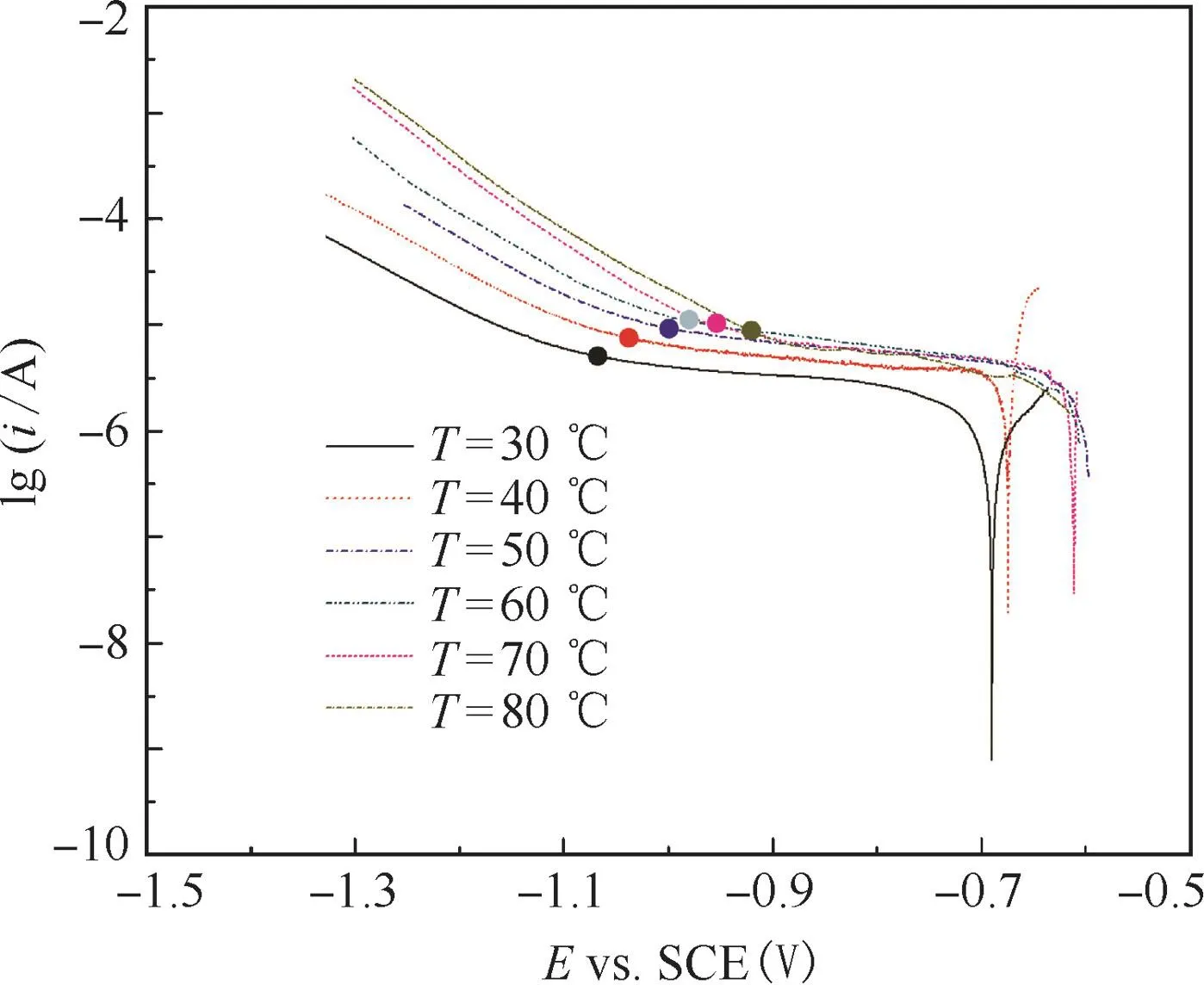
Figure 2 Cathodic polarization curves of 3003 aluminum alloy at different temperatures.
3.2.EIS measurements
Fig.3 shows the Nyquist diagrams of 3003 aluminum electrode in the aerated test solution at different temperatures.It is seen that in the high-frequency range,all the impedance spectra had an identical characteristic that a depressed semicircle showed and the diameter of the semicircle decreased with the increasing of temperature.In the low-frequency range at temperatures of 30,40,50°C,the measured EIS plots were typically featured with a diffusive tail,a relatively straight line,and became less straight at 60°C.The diffusive tail of the impedance spectra was replaced by a reversed semicircle at low frequency when the temperature rose to 70 °C and 80 °C.Furthermore,the diameter of the semicircle decreased with the increasing temperature.
3.3.Surface characterization
Figs.4 and 5 show the SEM images of the surface morphology for 3003 aluminum electrode be fore and after potentiodynamic polarization in the tested solution at different temperatures.
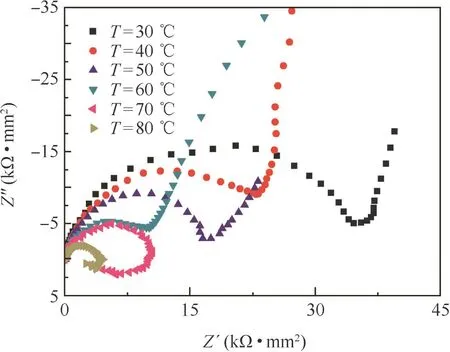
Figure 3 Nyquist diagrams of 3003 aluminum alloy at different temperatures.

Figure 4 SEM images of surface morphology for 3003 aluminum electrode be fore polarization(2000×).
The pits were in rounded or elliptic shape.Some of the pits werefilled with a particle.A number of particles shaped of stretched ellipses were also observed in the aluminum alloy substrate.The element composition of the stretched-ellipse shaped particle was measured as shown in Table 2,demonstrating that they were secondary phase particles rich in copper and manganese.The number of pits and the size of pit mouth width at different temperatures are shown in Figs.6 and 7,where P is the percentage of pit mouth width at different temperatures.With temperature rising,pit number tended to decrease,while the pit mouth width increased and pit size distribution was inclined to be scattered.
Topographical changes were qualitatively characterized by AFM three-dimensional(3D)and two-dimensional(2D)images.The 3D and 2D AFM topography images and topographic line-scans through the second phase and the pit are depicted in Fig.8(a)–(c).Fig.8(a)depicts the 3D AFM topography image;Fig.8(c)shows the topographic line-scan results of Fig.8(b),where H is the coordinates of the vertical directionand L is the coordinates of the horizontal direction of the linescan.In the 3D image,small hills were present in the places of secondary phase and inverted hills were present in the places of pit.The depth of pits were recorded and 20 pits on the aluminum electrode were recorded at each temperature.The results are shown in Fig.9(a)–(f).The depth of pits increased with the increase of temperature.Pits showed a trend of expansion.
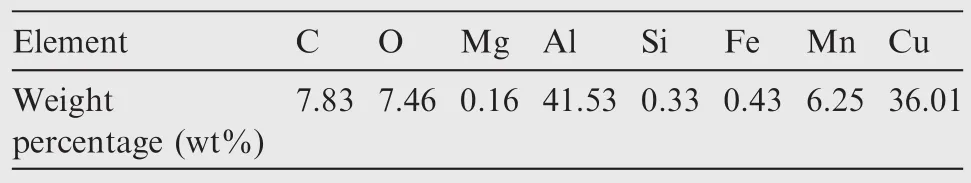
Table 2 Element composition of stretched-ellipse shaped particle.

Figure 6 Pit number on the surface at different temperatures.

Figure 7 Pit mouth width at different temperatures.

Figure 8 AFM topography images and topographic line-scan through the second phase and pit.
4.Discussions
4.1.Effect of temperature on corrosion reaction
Generally,the electrochemical corrosion of aluminum alloy in a near-neutral aqueous solution in the presence of oxygen is characterized by
Cathodic reaction:


The aluminum–alcohol is formed when the oxygen is insufficient.30In the absence of oxygen,the main cathodic reaction is either reduction of ethylene glycol by Eq.(4)or reduction of water:

There fore,the resultant film would be a mixture of aluminum oxide and aluminum–alcohol films.32
The positive shift of open circuit potential of 3003 aluminum electrode shows the oxidation of the electrode upon the immersion in the solution.The open circuit potential fluctuated around a specific value,indicating that thefilm formation and dissolution achieves an equilibrium state.33,34

Figure 9 Pit depth at different temperatures.
It is seen from Table 1 that the increased temperature shifted Ecorrnegatively.In general,a more negative corrosion potential indicates a more active state.The electrode became more vulnerable to the test solution with the increased temperature,which is associated with a higher anodic dissolution trend.With temperature rising,badecreased from 45.9 mV(vs.SCE)to 19.3 mV(vs.SCE).The decrement of bashowed that the increase in temperature lowered the anodic reaction resistance,and thus accelerated anodic dissolution.Apparently,the increase in temperature significantly activated the 3003 aluminum.bcdecreased from-317 mV(vs.SCE)to-656 mV(vs.SCE)and then increased to-287 mV(vs.SCE).It indicated that the cathodic reaction was accelerated with temperature rising until temperature was up to 60°C.And when temperature was higher than 60°C,the cathodic reaction rate slowed down.
With the increase of temperature,the anodic reaction rate of 3003 aluminum was accelerated while the cathodic reaction rate increased at first then decreased,which indicated that the corrosion mechanism of 3003 aluminum would be changed at higher temperature.
The effect of temperature on corrosion reaction basically complies with the Arrhenius equation:
2005年后,东营区先后投资200余万元,为庄户剧团购置了乐器、灯光、音响、服装等必要的演出设备和道具,定期组织专家对剧团演员进行艺术指导和业务培训,剧团演唱水平的提高带动了更多的群众学唱吕剧。

where k is reaction speed constant,A pre-exponential factor,Eaapparent activation energy,R molar gas constant and T thermodynamic temperature.
k determines the inherent reaction speed of a reaction.It can be seen from the Arrhenius equation,in the premise that Earemains unchanged and temperature range is limited,the reaction speed constant increases with the increase of temperature,and so is the reaction speed.The corrosion-related inter facial reaction process is accelerated with the elevated temperature.Moreover,an elevated temperature lowers the reaction activation energy at the same time.The anodic reaction,dissolution of aluminum tends to be easier to occur,and so does the cathodic reaction.Both the anodic and cathodic reaction accelerated due to the elevated temperature.There fore,the anodic and the cathodic current density increase with the increasing temperature to a certain extent.Thus,the corrosion reaction rate is accelerated which is identified by icorr.
On the other hand,the cathodic reaction is a purely diffusion-controlled process within a certain potential region in aerated ethylene glycol–water solution.35,36It is dominated by the diffusion and the reduction of dissolved oxygen.With temperature rising,the coefficient of diffusion oxygen molecules increases and the viscosity of ethylene glycol would decrease,37which resulted in the acceleration of oxygen diffusion and thus the increase of cathodic reductive current density.However,as the temperature of solution kept growing,the solubility of oxygen decreased,thus the concentration of dissolved oxygen decreased.There was not sufficient oxygen for cathodic reduction reaction.The decrease of the content of oxygen weakens the cathodic reaction,leading to the decrement of cathodic current density and the increase of bc,as shown in Fig.2 and Table 1.Here,the electrochemical dissolution of 3003 aluminum alloy is limited by the cathodic reduction of oxygen with diffusion control.The cathodic reaction rate of 3003 aluminum alloy rose to the maximum at 60°C.
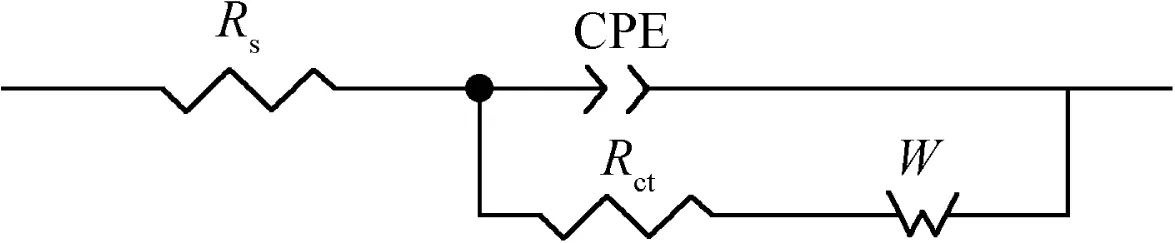
Figure 10 Equivalent circuits for fit of experimental data.
As the cathodic reaction went,the dissolved oxygen reacted and the oxidation of aluminum electrode persisted.As the content of dissolved oxygen became less and less,the reduction of ethylene glycol or water which refers to the curve at potentials more negative than the knee point in Fig.3,became prominent.The reduction of ethylene glycol and water was enhanced by the increasing temperature,since its reaction active energy was lower and its reaction speed constant was larger at higher temperature.As a result,the cathodic current density increased with the rising temperature monotonously.The knee point shifting positively as temperature increased provided evidence for the less content of oxygen at higher temperature.
4.2.Effect of temperature on EIS plots
To further determine the electrochemical corrosion mechanism of aluminum alloy in ethylene glycol–water solution at different temperatures,EIS measurements and analyses were per formed(see Fig.4).The observed ‘diffusive tail”in the low frequency range at lower temperature represents the Warburg impedance and is the typical characteristic of the corrosion process where diffusion of oxygen is involved.38,39It indicates that mass-transfer step plays an important role in electrode process.The diffusive Warburg impedance tended to diminish with the increasing temperature.When temperature was higher than 60°C,the Warburg impedance characteristics almost disappeared.This provides evidence to demonstrate that the corrosion mechanism of 3003 aluminum is already changed with the increase of temperature.Technically,the slopes of diffusive tails are not strict 45°,which is because of the intermediate reaction during corrosion parallel to the oxygen diffusion34,35or the non-stationary conditions under measurement.40
The electrochemical equivalent circuit shown in Fig.10 was used for fitting EIS data with diffusive impedance.To simulate the ‘dispersion effect” and compensate for the electrode nonhomogeneity such as the rough electrode surface,a constant phase element(CPE)is used in the circuit to replace the ideal double-layer capacitance.A CPE’s impedance(ZCPE)is given by

where ω is angular frequency and Y0constant.n is the frequency dispersion factor,which varies from 1 to 0 generally.When n=1,CPE can be considered an ideal capacitance;when n=0,it is a resistance.41
Simulations by means of the circuit provide a good agreement with the experimental data at different temperatures.Fig.11 shows a typical example to compare the fitted results with the experimental data by both Bode and phase angle diagrams,where Rsis solution resistance,CPE constant phase element,Rctcharge-transfer resistance and W Warburg impedance.It is seen that the selected equivalent circuit models fit the experimental data very well.The values of the fitted equivalent circuit elements are listed in Table 3.
In the Nyquist diagram,charge transfer resistance(Rct)is a characteristic quantity for an electrode reaction and has a critical influence on the inherent speed of interfacial charge transfer reaction.38,42The value of Rct,shown as Table 3,basically decreaseswith the elevated temperature,indicating an enhanced destabilization of electrode upon the increase of temperature.It is attributed to the enhanced oxygen diffusion and aluminum oxidation.The value of Warburg impedance decreases with the rising temperature,agreed with analyzed.It should be mentioned that the fitting results,values of fitted equivalent circuit elements should only be considered in a form of indirect changes of electric parameters,rather than direct values because of the possible non-stationarity of the system and high level of CHI2error during the fitting procedure.
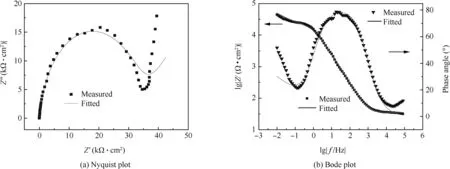
Figure 11 EIS Nyquist plot and Bode plot of aluminum electrode at 30°C.
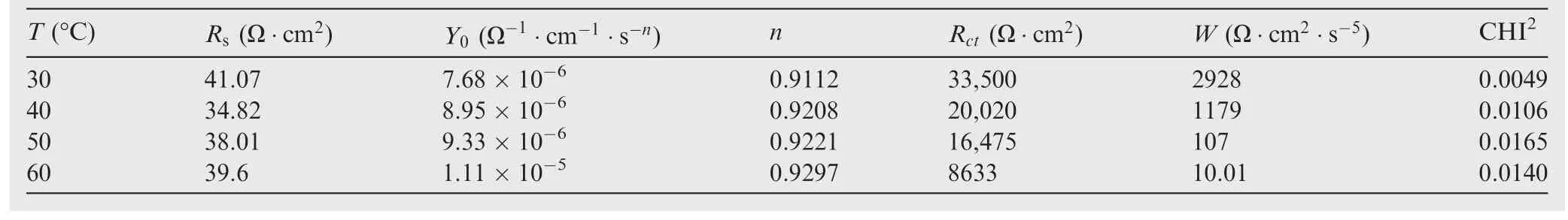
Table 3 Values of fitted equivalent circuit elements.
As temperature increased to 70°C,instead of the diffusive impedance,a reversed semicircle emerged in the low frequency range.The emergence of reversed semicircle is attributed to either a high electrode surface roughness or an absorption effect of intermediate corrosion product.43,44It is studied by Liu and Cheng32that when Cl-concentration is up to 0.01 M(0.1 mol·L-1)and 0.1 M(0.5 mol·L-1),the content of intermediate products adsorbing in aluminum–alcohol film is significant.There fore,the observed reversed semicircle mainly results from the absorption of intermediate corrosion product on electrode.Equivalent circuits used in calculating the impedance of electrochemical adsorption systems are better to be negative capacitance.The existing devices designed for impedance optimization lack equivalent circuits involving the negative capacitance,thus the equivalent circuit for impedance including reversed semicircle is not given.45
Aluminum corrosion reaction is mixed-controlled by activation step and mass-transfer step through the anodic film in ethylene glycol–water solution.44As temperature rose,the diffusive impedance feature faded away.There fore,mass-transfer step became less dominant in the electrode reaction process,resulting in the change of Nyquist diagram and electrochemical equivalent circuit.
4.3.Surface morphology
According to the surface characterization,after the potentiodynamic measurements the aluminum electrode suffered pitting corrosion.Pit nucleation was associated with the active points and the local dissolution of aluminum alloy substrate around the secondary phase particles.The stretched second phase particles are primarily intermetallics rich in copper and manganese.Corrosion is known to initiate preferentially at the particle–matrix interface.43There actually existing an effect which is called micro-galvanic coupling between secondary phase particles and the aluminum matrix resulting in preferential dissolution of aluminum alloy.3,43Intermetallic particles acted as local cathodes in the corrosion process.Pits formed when aluminum dissolved and intermetallic particles detached from the aluminum matrix.At higher temperature,aluminum alloy substrate had a higher dissolution activity,the active dissolution of aluminum was enhanced and the dissolved area of aluminum was larger.The micro-galvanic coupling effect was enhanced.The dissolution of aluminum alloy expanded both horizontally and vertically(see Figs.7 and 9).The adjacent pits converged to form a larger pit,which would cause the decrement of the amount of pits under different temperatures.As a consequence,the size of pit was enlarged and the aluminum electrode had a trend of uniform corrosion.
It is commonly acknowledged that the corrosion of aluminum under certain environment is determined by the specific corrosion process.41In the solution of ethylene glycol–water containing 0.1 mol·L-1NaCl,the elevated temperature can affect the corrosion process mainly from two aspects:one is that it accelerates the electrochemical reaction(both anodic and cathodic)and determines the diffusion process of reaction species towards the reaction sites;the other is that it affects the size and expansion of pits.Larger and deeper pits are formed at higher temperature,and a trend of uniform corrosion is presented.
5.Conclusions
(1)An increase of experimental temperature would accelerate the 3003 aluminum alloy corrosion rate in ethylene glycol–water solution containing 0.1 mol·L-1NaCl.The dissolution of aluminum shows a feature of accelerated with the rise of temperature.The elevated temperature enhances the diffusion of oxygen and accelerated cathodic reaction rate.However,as temperature kept rises,the solubility and concentration of oxygen decrease,resulting in the inhibition of cathodic reaction.The cathodic reaction rate of 3003 aluminum alloy rises to the maximum at 60°C.Overall,the elevated temperature accelerates the anodic and cathodic reaction speed and facilitates the corrosion reaction to a certain extent.
(2)On the other hand,with the increase in temperature,the Warburg impedance in Nyquist diagram diminishes and a reversed semicircle caused by the absorption of intermediate corrosion product on electrode emerges.The mass-transfer step of corrosion becomes less dominant in the electrode reaction process.
(3)After potentiodynamic measurements,3003 aluminum alloy suffers pitting corrosion.At higher temperature,the micro-galvanic coupling effect between secondary phase particles and the aluminum matrix is enhanced.The adjacent pits converge to form a larger pit,thus the active dissolution of aluminum is enhanced and the dissolved area of aluminum is larger.The pit corrosion process is promoted by both horizontal and vertical expansion of pit.
Acknowledgment
This study was co-supported by the National Natural Science Foundation of China(No.51271012).
1.Abiola OK,Otaigbe JOE.Effect of common water contaminants on the corrosion of aluminum alloys in ethylene glycol–water solution.Corros Sci 2008;50(5):242–7.
2.Yang B,Gershun AV,Woyciesjes PM.Controlled atmosphere brazing of aluminum heat exchangers and effects of flux residues on corrosion of the cooling system components in engine coolants.Fuel 2009;15(2):2099–115.
3.Sakairi M,Sasaki M,Kaneko A,Seki Y,Nagasawa D.Evaluation of metal cation effects on galvanic corrosion behavior of the A5052 aluminum alloy in low chloride ion containing solutions by electrochemical noise impedance.Electrochim Acta 2014;131(3):123–9.
4.Thomson JK,Pawel SJ,Wilson DF.Susceptibility of aluminum alloys to corrosion in simulated fuel blends containing ethanol.Fuel 2013;111(1):592–7.
5.David JK,Paul L.Evaluation of heat exchanger surface coatings.Appl Therm Eng 2010;30(2):2333–8.
6.Wang Q,Yan PG,Dong P,Feng GT,Wang ZQ.Application of coolant diffusion equation in numerical simulation of flow in aircooled turbine.Chin J Aeronaut 2012;25(4):566–74.
7.Himpel G,Herrmann M,Hohn S.Comparison of high-temperature corrosion of aluminum nitride,alumina,magnesia and zirconia ceramics by coal ashes.Ceram Int 2015;41(5):8288–98.
8.Niu L,Cheng YF.Erosion–corrosion of aluminum alloys in ethylene glycol–water solutions in absence and presence of sand particles.Corros Eng Sci Technol 2009;44(5):389–93.
9.Su JX,Ma MY,Wang TJ,Guo XM,Hou LG,Wang ZP.Fouling corrosion in aluminum heat exchanger.Chin J Aeronaut 2015;28(3):954–60.
10.Seifzadeh D,Bashranavaz H.Corrosion protection of AZ91 magnesium alloy in cooling systems.Trans Nonferrous Met Soc China 2013;23(1):2577–84.
11.Bustos ES,Rodriguez JGG,Uruchurtu J,Bravo VMS.Corrosion behavior of iron-based alloys in the LiBr+ethylene glycol+H2O mixture.Corros Sci 2009;51(3):1107–14.
12.Park IJ,Nam TH,Kim JH,Kim JG.Evaluation of corrosion characteristics of aluminum alloys in the bio-ethanol gasoline blended fuel by 2-electrode electrochemical impedance spectroscopy.Fuel 2014;126(5):26–31.
13.Ranjbar K,Abasi A.Failure assessment of crude oil preheating tubes in mono ethylene glycol–water mixture solution.Eng Fail Anal 2013;31:161–7.
14.Weon JI.Corrosion mechanism of aluminum alloy by ethylene glycol-based solution.Mater Corros 2013;64(1):50–9.
15.Tadjamoli M,Tzinmann M,Fiaudc C.Galvanic corrosion of the aluminum-copper couple in uninhibited aqueous solutions of glycols.Br Corros J 1984;19(1):36–40.
16.Monticelli C,Brunoro G,Zucchi F,Fagiolif F.Inhibition of localized attack on the aluminum alloy AA 6351 in glycol/water solutions.Mater Corros 1989;40(3):393–8.
17.Wang XY,Wang JM,Wang QL,Shao HB,Zhang JQ.Effect of polyethylene glycol(PEG)as an electrolyte additive on the corrosion behavior and electrochemical performances of pure aluminum in an alkaline zincate solution.Mater Corros 2011;62(2):1149–52.
18.Vigdorovivh VI,Tsygankova LE.Corrosion of aluminum in sulfate ethylene glycol solutions.Corros Sci 1993;13(2):215–22.
19.Khomami MN,Danaee I,Attar AA,Peykari M.Corrosion of alloy steel in 30%ethylene glycol solution and Cr O24-under hydrodynamic condition.J Iron Steel Res Int 2013;20(6):82–7.
20.Fekry AM,Fatayerji MZ.Electrochemical corrosion behavior of AZ91D alloy in ethylene glycol.Electrochim Acta 2009;54(4):22–8.
21.Monticelli C,Brunoro G,Frignani A.Corrosion behavior of the aluminum alloy AA 6351 in glycol/water solutions degraded at elevated temperature.Mater Corros 1988;39(4):379–84.
22.Wong D,Swette L.Aluminum corrosion in uninhibited ethylene glycol–water solutions.Electrochem Soc 1979;126(1):11–5.
23.Yazdzad AR,Shahrabi T,Hosseini MG.Inhibition of 3003 aluminum alloy corrosion by propargyl alcohol and tartrate ion and their synergistic effects in 0.5%NaCl solution.Mater Chem Phys 2008;109(2):199–205.
24.Dehof f RR,Babu SS.Characterization of interfacial microstructures in 3003 aluminum alloy blocks fabricated by ultrasonic additive manufacturing.Acta Mater 2010;58(2):4305–15.
25.Majed MR,Carlberg T.Solidification studies of 3003 aluminium alloys with Cu and Zr additions.J Mater Sci Technol 2011;27(7):615–27.
26.Solange Y,Dugarte P,Benjamin HP.Statistical analysis of the optical interferometry of pitting process in aluminum 3003 sheets exposed to saline environment.Proc Mater Sci 2015;8(6):82–90.
27.Zhou W,Aung NN,Choudhary A,Kanouni M.Heat-transfer corrosion behaviorof castAlalloy.CorrosSci2008;50(3):3308–13.
28.Hughes AE,Markley TA,Garcia SJ,Mol JMC.Comparative study of protection of AA 2024-T3 exposed to rare earth salts solutions.Corros Eng Sci Technol 2014;49(6):674–87.
29.Zaharieva J,Milanova M,Mitov M,Lutov L,Manev S,Todorovsky D.Corrosion of aluminum and aluminum alloy in ethylene glycol–water mixtures.J Alloy Compd 2009;470(1):397–403.
30.Liu Y,Cheng YF.Characterization of passivity and pitting corrosion of 3003 aluminum alloy in ethylene glycol–water solutions.J Appl Electrochem 2011;41(1):151–9.
31.Trombetta F,Souza FR,Souza MO,Borges BC,Panno AF,Martini EMA.Stability of aluminum in 1-butyl-3-methylimidazolium tetra fluoroborate ionic liquid and ethylene glycol mixtures.Corros Sci 2011;53(1):51–8.
32.Liu Y,Cheng YF.Effects of coolant chemistry on corrosion of 3003 aluminum alloy in automotive cooling system.Mater Corros 2010;61(7):574–8.
33.Bazeleva NA,Herasymenko YS.Corrosion-electrochemical behavior of aluminum alloys in aqueous ethylene glycol media.Mater Sci 2007;43(6):851–60.
34.Niu L,Cheng YF.Electrochemical characterization of metastable pitting of 3003 aluminum alloy in ethylene glycol–water solution.Mater Sci 2007;42(2):8613–7.
35.Niu L,Cheng YF.Cathodic reaction kinetics and its implication on flow-assisted corrosion of aluminum alloy in aqueous ethylene glycol solution.J Appl Electrochem 2009;39(6):1267–72.
36.Zhang GA,Xu YL,Cheng YF.Investigation of erosion–corrosion of 3003 aluminum alloy in ethylene glycol–water solution by impingement jet system.Corros Sci 2009;51(1):283–90.
37.Xu LY,Cheng YF.Electrochemical characterization and CFD simulation of flow-assisted corrosion of aluminum alloy in ethylene glycol–water solution.Corros Sci 2008;50(3):2004–10.
38.Martin FJ,Cheek GT,Grady WE,Natishan PM.Impedance studies of the passivefilm on aluminum.Corros Sci 2005;47(7):3187–201.
39.Conde A,Damborenea JD.An electrochemical impedance study of a natural aged Al–Cu–Mg alloy in NaCl.Corros Sci 1997;39(1):295–303.
40.Orlikowski J,Ryl J,Jarzynka M,Krakowwiak S,Darowicki K.Instantaneous impedance monitoring of aluminum alloy 7075 corrosion in borate buffer with admixed chloride ions.Corrosion 2015;71(7):828–38.
41.ScullyJR,Silverman DC,KengdingMW.Electrochemical impedance:analysis and interpretation.CorrosSci1993;21(3):128–32.
42.Zhang GA,Xu YL,Cheng YF.Mechanistic aspects of electrochemical corrosion of aluminum alloy in ethylene glycol–water solution.Electrochim Acta 2008;53(5):8245–52.
43.Feriky AM,Fatayerji MZ.Electrochemical corrosion behavior of AZ91D alloy in ethylene glycol.Electrochim Acta 2009;54(1):6522–8.
44.Niu L,Cheng YF.Synergistic effects of fluid flow and sand particles on erosion–corrosion of aluminum in ethylene glycol–water solutions.Wear 2008;265(1):367–74.
45.Elkin VV,Marshako AI,Rybkina AA,Maleeva MA.Interpretation of the impedance comprising negative capacitance and constant phase elements on iron electrode in weakly acidic media.Russ J Electrochem 2011;47(2):147–58.
Chen Xin is a postgraduate at school of material science and engineering,Beihang University.His main research interests are material science and engineering and material corrosion.
Li Songmei is a prof essor and Ph.D.supervisor at school of material science and engineering,Beihang University.She received the Ph.D.degree from the same university.Her current research interests are material science and engineering and material corrosion.
12 August 2015;revised 12 September 2015;accepted 29 September 2015 Available online 23 December 2015
3003 aluminum alloy;
Corrosion behavior;
Electrochemical measurements;
Ethylene glycol;
Temperature
©2016 The Authors.Production and hosting by Elsevier Ltd.on behalf of Chinese Society of Aeronautics and Astronautics.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
*Corresponding author.Tel./fax:+86 10 82317103.
E-mail address:songmei_li@buaa.edu.cn(S.Li).
Peer review under responsibility of Editorial Committee of CJA.
Production and hosting by Elsevier
http://dx.doi.org/10.1016/j.cja.2015.12.017
1000-9361©2016 The Authors.Production and hosting by Elsevier Ltd.on behalf of Chinese Society of Aeronautics and Astronautics.
This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
猜你喜欢
杂志排行
CHINESE JOURNAL OF AERONAUTICS的其它文章
- E ff ect of sodium tartrate concentrations on morphology and characteristics of anodic oxidefilm on titanium alloy Ti–10V–2Fe–3Al
- New design of a compact aero-robotic drilling end eff ector:An experimental analysis
- Coupling behavior between adhesive and abrasive wear mechanism of aero-hydraulic spool valves
- Cathode design investigation based on iterative correction of predicted profile errors in electrochemical machining of compressor blades
- Eff ect of tube-electrode inner diameter on electrochemical discharge machining of nickel-based superalloy
- Effects of process parameters on mechanical properties of abrasive-assisted electroforming nickel
