非手性的酞菁铜分子在Bi(111)表面上的手性特征
2016-11-22陶敏龙涂玉兵谢正波王亚利郝少杰肖华芳王俊忠
叶 娟 孙 凯 陶敏龙 涂玉兵 谢正波 王亚利郝少杰 肖华芳 王俊忠
(西南大学物理科学与技术学院,重庆400715)
非手性的酞菁铜分子在Bi(111)表面上的手性特征
叶娟孙凯陶敏龙涂玉兵谢正波王亚利郝少杰肖华芳王俊忠*
(西南大学物理科学与技术学院,重庆400715)
利用低温扫描隧道显微镜(LT-STM)研究了酞菁铜(CuPc)分子在Bi(111)表面上的吸附和手性自组装结构。由于较弱的分子-衬底相互作用,我们发现在液氮温度(78 K)下吸附在Bi(111)表面上的单个CuPc分子围绕着分子中心发生旋转,直到遇到其他分子形成团簇为止。随着分子覆盖度的增加,CuPc分子形成了自组装分子单层。高分辨STM图表明,非手性的CuPc分子出现了手性特征:两个相对的酞菁基团发生了弯曲。当覆盖度超过一个分子层,酞菁铜分子的吸附取向由“平躺”转变到“站立”姿态。我们认为,酞菁铜分子的手性起源是由两种因素共同导致的结果:一种是分子-衬底之间的非对称电荷转移,另一种是相邻分子间的非对称性的范德华力作用。
酞菁铜;扫描隧道显微镜;半金属Bi(111);手性特征;自组装
Significant progress has been achieved in the adsorption and self-assembly of various TMPcs on noble metal surfaces17-21. Because of high spatial resolution capability,scanning tunneling microscopy(STM)has proven a powerful technique for investigating the geometric conformations of TMPc molecules deposited on the substrates in the past few decades owning to the submolecular resolution22-25.This technique has greatly enhanced our understanding of the assemblies of phthalocyanines and their derivatives on various substrates26.The formation of densely packed monolayer on various metal substrates was investigated by many STM studies.However,so far,there have been few studies on the structural evolution of TMPcs on semi-metallic surface from isolated TMPc molecules to full monolayer,and finally to multilayer regime.The flat adsorption orientation of TMPc molecules facilitates the bonding of central magnetic ions with metallic substrate,making the systematic investigation of the molecule-substrate interaction possible27,28.
In this paper,STM study of CuPc on Bi(111)is presented for structural evolution and molecular orientational transition.Chirality of the self-assembly of the achiral CuPc molecules is attributed to the combined effect of asymmetric charge transfer between CuPc molecules and Bi(111)substrate and the intermolecular van der Waals(vdW)interactions.Furthermore,the chiral feature of filled-state STM images of CuPc molecules is more evident remarkable than the empty-state images,indicating that the chirality origin effect is an electronic effect,not the geometric modification of CuPc molecules.
2 Experimental
2.1Preparation of the Bi(111)substrate
Our experiments were conducted in a Japanese Unisoku ultrahigh vacuum low temperature STM system with a base pressure around 1.3×10-8Pa.The smooth Bi(111)substrate was obtained by depositing nominal 20 molecular layer(ML)of Bi atom onto the Si(111)-7×7 reconstructed surface at room temperature with subsequent annealing at 400 K for nearly 2.5 h29.
2.2Preparation of the CuPc assemblies
After overnight degassing,CuPc molecules(Sigma-Aldrich, 99%purity)were thermally sublimated from a Ta boat heated to about 480 K and then were deposited onto Bi(111)film at a rate of 0.45 monolayer per minute.In this paper one monolayer is defined as the amount of depositing CuPc molecules to cover the whole substrate surface.During the deposition,the Bi(111)substrate was kept at room temperature(RT).The constant-current mode and tungsten tips after e-beam heating in the molecular beam epitaxy(MBE)chamber were used for STM measurements. The calibrations of tip state were based on both Si(111)-7×7 reconstructed surface and the atomic resolution image of Bi(111) surface.The bias voltage between the sample and the tip is controlled between-3 and 3 V.All the STM images were obtained at liquid nitrogen temperature(78 K).
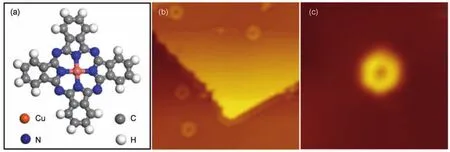
Fig.1 STM images of the isolated CuPc molecules on Bi(111)surface
3 Results and discussion
3.1Rotation of the isolated CuPc molecules
A small amount(0.03 ML)of CuPc molecules were deposited onto the Bi(111)surface at room-temperature and individual monomers were observed by LT-STM shown in Fig.1(b).Fig.1(c) is a typical topographic image of the isolated CuPc molecule obtained at low temperature(78 K).The individual CuPc molecules adsorbed on Bi(111)surface like a rotating-disc,which differs from the inherent cross-like molecular structure in Fig.1(a), indicating that the isolated CuPc molecules keep rotating,thus thefour lobes of the molecule cannot be distinguished.The central metal ion of the CuPc molecule appears as a hole in the STM image,which can be attributed to the occupied d-orbitals away from the Fermi energy of the copper ion.This phenomenon differs from the previous reports about the MnPc29and CoPc30on Bi(111), which were rotating around a bright protrusion located at the center.The strong d-orbital occupation-dependent STM images of TMPc on the apparent height of the central metal ion are demonstrated experimentally by Hipps et al.31.
Furthermore,high-resolution STM image reveals that the circular symmetry becomes an oval shape,which means an asymmetrical rotation for the four lobes of CuPc molecule.The observed rotation of the individual CuPc molecules demonstrates that a quite weak molecule-substrate interaction,thus the molecular rotation stems from the energy provided by inelastic tunnel effects.At high bias voltage,we can observe the rotation of CuPc molecules on Bi(111)surface at liquid nitrogen temperature(78 K);while at the low bias voltage,we noticed that the speed of molecular rotation decreases significantly or even becomes zero at very low bias voltage,as demonstrated in CoPc molecules on Bi(111)surface,whereas on noble metal substrates such as Pb (111)17and Au(111)18,the CuPc rotation is absent due to the considerable molecule-substrate mutual coupling,meanwhile the inelastic effects become less important(almost no tunneling in molecular states).
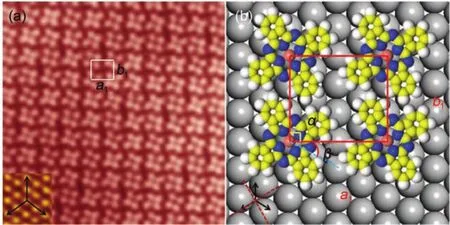
Fig.2 Self-assembled thin film of copper-phthalocyanine
3.2Assemblies of CuPc molecules
With the CuPc coverage increasing,individual CuPc molecules are preferentially assembled together forming the two-dimensional (2-D)domains with parallel arrangement.Fig.2(a)displays a STM image of self-assembled CuPc thin film in the coverage of 0.45 ML,each CuPc molecule exhibits a cross shape with four perpendicular lobes,consistent with the four-fold symmetry of CuPc molecule shown in Fig.1(a).This indicates that the CuPc molecules adopt a flat-lying adsorption orientation without any rotation on Bi(111)due to the increasing interaction between the neighboring molecules,causing that the degree of freedom of movements is greatly reduced,the original free rotation is completely limited32.Furthermore,the two opposing lobes of each CuPc molecule are aligned at one of the three principal axes of the Bi (111)surface,the atomic resolution image of the underlying Bi (111)surface is shown in the insert.The self-assembled CuPc thin films on Bi(111)surface have identical in-plane orientations, which can be attributed to the mutual coupling of the symmetry of Bi(111)and CuPc33,however the MnPc and CoPc on Bi(111)are aggregated into 2-D domains consisting of two different molecular orientations,denoted as‘A’and‘B’.The white quadrilateral in Fig.2(a)represents a unit cell(denoted as“a1”and“b1”)and the lattice constant are a1=(1.42±0.02)nm,b1=(1.35±0.02)nm, corresponding to a packing density of 0.52 nm-2,which is obviously smaller than the lattice constant(a1=(1.78±0.02)nm,b1= (1.34±0.02)nm)of CoPc on Bi(111).Fig.2(b)is the structural model of the unit cell,the angle α between a1and b1is 90°±2° and the angle β is 30°.The dashed and solid arrows represent the substrate and molecular symmetry at the bottom left corner,respectively.Based on the lattice parameters of Bi(111)substrate c1=c2=0.454 nm,we can deduce the relationship between the CuPc layer and the Bi(111)substrate expressed as the following matrixe:

Non-integers in the transformation matrix indicate that the same oriented CuPc domain is not commensurate with the Bi(111) substrate.It demonstrates that the intermolecular interaction is strong enough to dominate the quite weak interfacial interaction between the molecules and the substrate due to the semimetal nature of the Bi(111)surface.
3.3Chiral feature of the achiral CuPc molecules in self-assembled monolayer
With the CuPc coverage approached to 0.86 ML,the highresolution STM image of self-assembled CuPc thin film in Fig.3 (a)reveals different feature,which is acquired at a negative bias voltage of-1.3 V.It can be observed that all the CuPc molecules are revealing a strong chiral feature,for MnPc and CoPc on Bi (111)surface appear achiral at both negative and positive bias voltage.The observed molecular chirality on Bi(111)surface originates from the asymmetric charge transfer between the CuPc molecules and Bi(111),as demonstrated in CuPc molecules onAg (100)surface.The asymmetry is due to the misalignment between the Bi(111)substrate and CuPc molecular symmetry axes.STM image of Fig.3(b)shows the same scanning position of 0.86 ML self-assembled CuPc thin film with the CuPc molecules appearing achiral at positive bias voltage.Fig.3(c)and Fig.3(d)are the enlarged images of the labeled molecules in the STM images of Fig.3 (a,b).By comparison from the images demonstrated that the chiral contrast is strong at negative bias voltage and disappears at the positive bias voltage,which is attributed to the asymmetric electronic interaction of the a1uorbital and of the partially occupied 2egorbital with the Bi(111)states,this effect is similar to CuPc/Ag (100)in Ref.12,leading to a voltage-dependent chiral appearance of CuPc molecules on Bi(111).This behavior suggests that chi-rality in this system can be manifest exclusively at the electronic level due to the asymmetry of electronic orbital occupation,indicating that the original effect of molecular chirality is an electronic effect,not related to the molecular geometric structure,as confirmed by Mugarza et al.16.

Fig.3 Chirality of the self-assembly of CuPc on Bi(111)and bias-dependent topographic images of the CuPc molecules
It was also found that the chirality of individual CuPc molecules is related to the intermolecular interactions,as CuPc coverage increases,the intermolecular interaction(that is the attractive van der Waals interactions)becomes stronger and thus the molecules showing chirality are closer to the neighboring molecules relative to the molecules in Fig.2(a).Furthermore,high-resolution STM image shows that the two opposing lobes of each CuPc molecule are twisted toward opposite directions,this differs from the previous reports about TMPc′s rotation in the same direction called a left-handed feature or a right-handed feature(denoted as R and L,respectively),revealing the asymmetric intermolecular vdW interactions.The formation mechanism is attributed to the combined effect of asymmetric charge transfer between CuPc molecules and Bi(111)substrate and the asymmetric intermolecular vdW interactions.
3.4Standing-up orientation of CuPc molecules
To investigate the growth behavior of CuPc molecules on Bi (111)surface with coverage-dependence,we continue to increase the molecular coverage.The STM image in Fig.4(a)shows the CuPc thin film of 1.6 ML,consisting of the upper-layer CuPc molecules adopted the on-top adsorption rather than the planar orientation on the underlying layer.The CuPc coverage calibration was achieved using a STM to count the number of surface features as a function of deposition time,thus we can define it as the second CuPc layer.By measurement of the unit cell,the lattice constants are a2=(1.18±0.02)nm,b2=(0.45±0.02)nm,corresponding to a packing density of 1.87 nm-2,which is 27.8% larger than that of the flat-lying CuPc domain of 0.52 nm-2in Fig.2 (a),because of the reduction of steric hindrance among the CuPc molecules in the standing-up domain relative to the flat-lying domain.Based on the fact that two-layered films of CuPc are very interesting,the growth process for standing-up CuPc configuration on Bi(111),is dominated by intramolecular interactions,is distinguished from the two-layer film of rubrene onAu(111)surface. A study of orientational transformation in films of copperphthalocyanine molecules on Bi(111)demonstrates that the molecule-substrate interaction can be ignored owing to the decoupling of the first CuPc layer,whereas the intermolecular interaction becomes predominant,similar behavior was observed for SnPc adsorption on NaCl,intermolecular interactions dominate over the molecule-NaCl coupling and result in a tilted adsorption configuration34,revealing that it is the energetically favorable adsorption configuration.

Fig.4 STM images of the second CuPc layer
The corresponding zoomed-in STM image of the standing-up CuPc chains is shown in Fig.4(b),all the CuPc molecules are arranged closely to the adjacent molecules within the CuPc chain. This arrangement is commonly called face-to-face alignment,marked by the superposed structural models.The pure domain are composed of a series of parallel CuPc chains,the appearance of the parallel chains reveals that the intermolecular interaction within the CuPc chain is obviously stronger than that in the neighboring chains because of a smaller intra-chain distance and a larger inter-chain distance.Fig.4(c)is the simulated structural model of the unit cell in Fig.4(a),which reveals the molecular adsorption orientation of the second CuPc layer onto the first CuPc layer.The colored atoms refer to the first CuPc layer,which is adopted a flat-lying adsorption orientation on Bi(111)surface.
4 Conclusions
In summary,we have studied the adsorption and chiral assembly of CuPc molecules on Bi(111)surface by using a LT-STM. Individual CuPc molecules keep rotating at 78 K due to the weak molecule-substrate interaction.As coverage increases,CuPc molecules show a structural evolution and molecular orientational transformation in films on Bi(111)surface.Most importantly,the self-assembled CuPc domains with each molecule revealing a chiral feature are observed.The chiral contrast is strong at negative bias voltage and disappears at positive bias voltage,leading to a voltage-dependent chiral appearance of CuPc molecules on Bi(111)surface.These findings provide insight into further studying the growth mechanism of transition-metal phthalocyanine molecules on semi-metallic surfaces.
References
(1) Jiang,P.;Ma,X.C.;Ning,Y.X.;Song,C.L.;Chen,X.;Jia,J. F.;Xue,Q.K.J.Am.Chem.Soc.2008,130,7790.doi:10.1021/ ja801255w
(2)Guo,Q.M.;Qin,Z.H.;Zang,K.;Liu,C.D.;Yu,Y.H.;Cao,G. Y.Langmuir 2010,26,11804.doi:10.1021/la1019907
(3)Rehman,R.A.;Dou,W.D.;Qian,H.Q.;Mao,H.Y.;Frederik, F.;Zhang,H.J.;Li,H.Y.;Pimo,H.;Bao,S.N.Surf.Sci.2012, 606,1749.doi:10.1016/j.susc.2012.07.021
(4) Atodiresei,N.;Brede,J.;Lazic,P.;Caciuc,V.;Hoffmann,G.; Wiesendanger,R.;Blugel,S.Phys.Rev.Lett.2010,105, 066601.doi:10.1103/PhysRevLett.105.066601
(5)Zhao,A.D.;Li,Q.X.;Chen,L.;Xiang,H.J.;Wang,W.H.; Pan,S.;Wang,B.;Xiao,X.D.;Yang,J.L.;Hou,J.G.;Zhu,Q. S.Science 2005,309,1542.doi:10.1126/science.1113449
(6)Liu,L.W.;Yang,K.;Jiang,Y.H.;Song,B.Q.;Xiao,W.D.;Li, L.F.;Zhou,H.T.;Wang,Y.L.;Du,S.X.;Ouyang,M.;Werner, A.H.;Antonio,H.C.N.;Gao,H.J.Scientific Reports 2013,3, 1210.doi:10.1038/srep01210
(7) Fu,Y.S.;Zhang,T.;Ji,S.H.;Chen,X.;Ma,X.C.;Jia,J.F.; Xue,Q.K.Phys.Rev.Lett.2009,103,257202.doi:10.1103/ PhysRevLett.103.257202
(8) Chen,X.;Fu,Y.S.;Ji,S.H.;Zhang,T.;Cheng,P.;Ma,X.C.; Zou,X.L.;Duan,W.H.;Jia,J.F.;Xue,Q.K.Phys.Rev.Lett. 2008,101,197208.doi:10.1103/PhysRevLett.101.197208
(9) Gao,L.;Ji,W.;Hu,Y.B.;Cheng,Z.H.;Deng,Z.T.;Liu,Q.; Jiang,N.;Lin,X.;Guo,W.;Du,S.X.;Hofer,W.A.;Xie,X.C.; Gao,H.J.Phys.Rev.Lett.2007,99,106402.doi:10.1103/ PhysRevLett.99.106402
(10) Liu,J.;Chen,T.;Deng,X.;Wang,D.;Pei,J.;Wan,L.J.J.Am. Chem.Soc.2011,133,21010.doi:10.1021/ja209469d
(11) Sun,R.R.;Wang,L.;Tian,J.;Zhang,X.M.;Jiang,J.Z. Nanoscale 2012,4,6990.doi:10.1039/c2nr31525d
(12) Mugarza,A.;Lorente,N.;Ordejion,P.;Krull,C.;Stepanow,S.; Bocquet,M.L.;Fraxedas,J.;Ceballos,G.;Gambardella,P. Phys.Rev.Lett.2010,105,115702.doi:10.1103/ PhysRevLett.105.115702
(13) Barlow,S.M.;Raval,R.Surf.Sci.Rep.2003,50,201. doi:10.1016/s0167-5729(03)00015-3
(14) Schock,M.;Otero,R.;Stojkovic,S.;Hummelink,F.;Gourdon, A.;Laegsgaard,E.;Stensgaard,I.;Joachim,C.;Besenbacher,F. J.Phys.Chem.B 2006,110,12835.doi:10.1021/jp0619437
(16) Mugarza,A.;Krull,C.;Korytar,R.;Lorente,N.;Gambardella, P.Phys.Rev.B 2012,85,155437.doi:10.1103/ PhysRevB.85.155437
(17)Hao,D.;Song,C.;Ning,Y.;Wang,Y.;Wang,L.;Ma,X.C.; Chen,X.;Xue,Q.K.J.Chem.Phys.2011,134,154703. doi:10.1063/1.3579493
(18) Jiang,Y.H.;Xiao,W.D.;Liu,L.W.;Zhang,L.Z.;Yang,C.K.; Du,S.X.;Gao,H.J.J.Phys.Chem.C 2011,115,21750. doi:10.1021/jp203462f
(19)Hipps,K.W.;Lu,X.;Wang,X.D.;Mazur,U.J.Phys.Chem. 1996,100,11207.doi:10.1021/jp960422o
(20) Li,Q.X.;Yang,J.L.;Yuan,L.F.;Hou,J.G.;Zhu,Q.S.Chin. Phys.Lett.2001,18,1234.
(21) Lippel,P.H.;Miller,M.D.;Wilson,R.J.Phys.Rev.Lett.1989, 62,171.doi:10.1103/PhysRevLett.62.171
(22) Berner,S.;Wild,M.D.;Ramoino,L.;Ivan,S.;Baratoff,A.; Guntherodt,H.J.;Suzuki,H.;Schlettwein,D.;Jung,T.A.Phys. Rev.B 2003,68,115410.doi:10.1103/PhysRevB.68.115410
(23) Takada,M.;Tada,H.Chem.Phys.Lett.2004,392,265. doi:10.1016/j.cplett.2004.04.121
(24) Mannsfeld,S.C.B.;Fritz,T.Phys.Rev.B 2005,71,235405. doi:10.1103/PhysRevB.71.235405
(25) Koudia,M.;Abel,M.;Maurel,C.;Bliek,A.;Catalin,D.; Mossoyan,M.;Mossoyan,J.C.;Porte,L.J.Phys.Chem.B 2006,110,10058.doi:10.1021/jp0571980
(26)Wang,Y.F.;Zhang,X.R.;Ye,Y.C.;Liang,D.J.;Wang,Y.;Wu, K.Acta Phys.-Chim.Sin.2010,26,933.[王永峰,张鑫然,叶迎春,梁德建,王远,吴凯.物理化学学报,2010,26,933.] doi:10.3866/PKU.WHXB20100419
(27) Du,S.X.;Zhang,Y.Y.;Gao,H.J.Phys.Rev.B 2011,84, 125446.doi:10.1103/PhysRevB.84.125446
(28) Larsson,J.A.;Baran,J.D.;Cafolla.A.A.;Schulte,K.;Dhanak, V.R.Phys.Rev.B 2010,81,075413.doi:10.1103/PhysRevB.81.075413
(29) Zhang,T.T.;Wang,C,J.;Sun,K.;Yuan,H.K.;Wang,J.Z. Appl.Surf.Sci.2014,317,1047.doi:10.1016/j. apsusc.2014.08.198
(30) Tao,M.L.;Tu,Y.B.;Sun,K.;Zhang,Y.;Zhang,X.;Li,Z.B.; Hao,S.J.;Xiao,H.F.;Ye,J.;Wang,J.Z.J.Phys.D:Appl. Phys.2016,49,015307.doi:10.1088/0022-3727/49/1/015307
(31)Hipps,K.W.;Lu,X.;Wang,X.D.;Mazur,U.J.Phys.Chem. 1996,100,11207.doi:10.1021/jp960422o
(32) Du,X.Q.;Li,H.Q.;Zhu,Q.R.;Zou,Z.Q.;Liang,Q.Acta Phys.-Chim.Sin.2011,27,2457.[杜晓清,李慧琴,朱齐荣,邹志强,梁齐.物理化学学报,2011,27,2457.]doi:10.3866/ PKU.WHXB20111010
(33)Huang,H.;Wong,S.L.;Chen,W.;Wee,A.T.S.J.Phys.D: Appl.Phys.2011,44,464005.doi:10.1088/0022-3727/44/46/ 464005
(34) Wang,Y.F.;Kroger,J.;Berndt,R.;Tang,H.J.Am.Chem.Soc. 2010,132,12546.doi:10.1021/ja105110d
Chiral Features of the Achiral Copper Phthalocyanine on a Bi(111)Surface
YE JuanSUN KaiTAO Min-LongTU Yu-BingXIE Zheng-BoWANG Ya-Li HAO Shao-JieXIAO Hua-FangWANG Jun-Zhong*
(School of Physical Science and Technology,Southwest University,Chongqing 400715,P.R.China)
The adsorption and chiral features of a self-assembled CuPc monolayer on a semi-metallic Bi(111) surface have been evaluated using the low temperature scanning tunneling microscopy(LT-STM).Under low coverage conditions,the individual CuPc molecules rotated around the molecular center at 78 K until they interacted with other molecule to form clusters,because of the relatively weak interfacial interactions between the CuPc molecules and the Bi(111)surface.As the level of molecular coverage increased,the CuPc molecules self-assembled into two-dimensional domains with each molecule exhibiting chiral features.Beyond one monolayer,the CuPc molecules underwent an orientational transition from a flat-lying to a standing-up configuration.The chiral features of the CuPc molecules were attributed to the combined effect of asymmetric charge transfer between the CuPc molecules and the Bi(111)substrate and the formation of asymmetric intermolecular van der Waals interactions.
CuPc;Scanning tunneling microscopy;Semi-metallic Bi(111);Chiral feature;Self-assembly
1 Introduction
In the past decades,transition-metal phthalocyanines(TMPc) molecules,have received considerable interest because of their unprecedented ability of self-assembly of ordered nanostructures on various metals1-3.Due to their relatively simple,stable,and symmetrical cross-like structure in Fig.1(a),TMPcs are consideredas a prototype of the single molecule magnet4.The magnetic properties of transition metal atoms in a host molecule can be detected by controlling charge states,spin states,and the Kondo effect at the single molecule level in cryogenic scanning tunneling5-9.The interaction of molecules with the corresponding substrate governs the self-assembly of TMPc molecules.In particular, chirality of the self-assembly of the achiral TMPc molecules deposited on metallic surfaces has been a hot research topic in recent years10-16.Chirality plays a fundamental role in molecular adsorption and self-assembly13.Highly symmetric molecules adsorbed on a substrate with dissimilar point-group symmetry have also been shown to develop chiral feature through asymmetric intermolecular interactions14,15.
June 1,2016;Revised:July 7,2016;Published online:July 7,2016.
.Email:jzwangcn@swu.edu.cn;Tel:+86-13883734915.
O647
10.3866/PKU.WHXB201607071
The project was supported by the National Natural Science Foundation of China(10974156,21173170,91121013,11574253).国家自然科学资金(10974156,21173170,91121013,11574253)资助项目©Editorial office ofActa Physico-Chimica Sinica
(15) Richardson,N.V.New J.Phys.2007,9,395.10.1088/1367-2630/9/10/395
