芹菜品种‘六合黄心芹’AgCCoAOMT基因的克隆及表达特性分析
2016-11-17苑笑阳吴雪君熊爱生
苑笑阳, 吴雪君, 聂 力, 许 珂, 熊爱生
(南京农业大学园艺学院 作物遗传与种质创新国家重点实验室 农业部华东地区园艺作物生物学与种质创制重点实验室, 江苏 南京 210095)
芹菜品种‘六合黄心芹’AgCCoAOMT基因的克隆及表达特性分析
苑笑阳, 吴雪君, 聂力, 许珂, 熊爱生①
(南京农业大学园艺学院 作物遗传与种质创新国家重点实验室 农业部华东地区园艺作物生物学与种质创制重点实验室, 江苏 南京 210095)
根据伞形科(Apiaceae)植物芹菜(ApiumgraveolensLinn.)的转录组数据库,从芹菜品种‘六合黄心芹’(‘Liuhe Huangxinqin’)叶片总RNA中克隆获得咖啡碱-辅酶A-O-甲基转移酶基因,命名为AgCCoAOMT基因。序列分析结果表明:AgCCoAOMT基因包含1个长度726 bp的开放阅读框(ORF),编码241个氨基酸;该基因编码的蛋白质为AgCCoAOMT,其理论相对分子质量为27 010,理论等电点pI 5.35;在AgCCoAOMT蛋白中,酸性氨基酸所占比例高于碱性氨基酸,脂肪族氨基酸所占比例约为芳香族氨基酸的3倍;该蛋白属于疏水性蛋白,并含有1个保守的AdoMet_MTases结构域,说明AgCCoAOMT蛋白属于AdoMet_MTases超级家族;AgCCoAOMT蛋白的三级结构包含多个α-螺旋和β-折叠,与紫花苜蓿(MedicagosativaLinn.)CCoAOMT蛋白三级结构的一致性为84.58%。多重比对和系统树分析结果表明:AgCCoAOMT蛋白有较高的保守性;该蛋白与同科植物大阿米芹(AmmimajusLinn.)和欧芹〔Petroselinumcrispum(Mill.) Nyman ex A. W. Hill〕CCoAOMT蛋白的进化关系最近,与睡莲科(Nymphaeaceae)植物莲(NelumbonuciferaGaertn.)CCoAOMT蛋白的进化关系较近。qRT-PCR检测结果表明:AgCCoAOMT基因能够在‘六合黄心芹’的根、茎、叶柄和叶片中表达,且在叶片中的相对表达量极显著(P<0.01)高于其他组织,说明该基因的表达具有明显的组织特异性;不同生长发育阶段该基因在叶片中的相对表达量有明显差异,其在商品期(播种后第65天)的相对表达量极显著高于幼苗期(播种后第25天)和生长旺盛期(播种后第45天)。研究结果显示:AgCCoAOMT基因与‘六合黄心芹’叶的老化和木质素含量升高有关,且在进化过程中具有较高的保守性。
芹菜;AgCCoAOMT基因; 序列分析; 多重比对; 表达特性
膳食纤维包括纤维素、木质素、低聚糖和果胶等[1],摄入适量的膳食纤维有益于人类的身体健康[2-3]。木质素可参与构成植物细胞壁,其积累能够增强细胞壁的硬度,使蔬菜组织木质化,进而影响蔬菜的口感和品质[4]。目前,有关木质素的研究及应用多涉及木材和造纸[5-6]、能源[7]和饲料[8-9]等方面,而对蔬菜中木质素的相关研究报道尚不多见。
咖啡碱-辅酶A-O-甲基转移酶(caffeoyl-CoA-O-methyltransferase,CCoAOMT)为木质素合成过程中起关键作用的一类甲基转移酶(methyltransferases,MTs)[10-11],参与植物的木质化进程[12]。在木质素生物合成过程中,CCoAOMT可催化咖啡酰CoA甲基化生成阿魏酰CoA[13],并促进复杂的甲基化次生代谢反应,参与不同的生物反应过程[14-16]。目前,已经从多种植物中分离获得CCoAOMT基因[17-20];而且反义抑制CCoAOMT基因后,细胞中的木质素含量明显降低,且S型和G型木质素含量同时降低,其中G型木质素含量降幅较大,导致S/G比值增加[21-23];此外,植物中的CCoAOMT还能够响应逆境胁迫[20,24]。
芹菜(ApiumgraveolensLinn.)为伞形科(Apiaceae)草本植物,是中国和全世界重要的叶菜类蔬菜作物之一,富含胡萝卜素、维生素和纤维素,兼具食用和药用价值[25]。与其他园艺作物相比,有关芹菜分子生物学方面的研究报道较少[26-28]。Pakusch等[29]和Schmitt等[30]均发现伞形科蔬菜作物的细胞培养液中存在CCoAOMT,但对芹菜中CCoAOMT基因及其与木质素合成的关系尚不清楚,在芹菜对逆境胁迫的响应过程中该基因的作用也未知。
产自江苏省南京市六合区的优良芹菜品种‘六合黄心芹’(‘Liuhe Huangxinqin’)具有喜湿、耐肥、耐热和耐寒等特点[31]。作者以该品种为实验材料,克隆获得其CCoAOMT基因,对该基因及其编码的氨基酸序列进行分析和比较;并采用qRT-PCR技术检测该基因在不同组织中和不同生长发育阶段叶片中的表达特性,以期为深入研究CCoAOMT基因在芹菜木质素代谢中的作用奠定研究基础,也为芹菜品质的改良提供基础数据。
1 材料和方法
1.1材料
于2015年5月,将经过催芽的‘六合黄心芹’种子播种到穴盘中,置于人工气候室内进行培养,培养条件为:光照时间16 h·d-1、昼温25 ℃、夜温18 ℃、光照强度300 μmol·m-2·s-1。在播种后第25天采集嫩叶,经液氮速冻后置于-80 ℃冰箱中保存,用于总RNA的提取。在播种后第25天(幼苗期)、第45天(生长旺盛期)和第65天(商品期),分别采集植株叶片,采用前述方法速冻和保存,用于不同发育阶段的基因表达分析;在播种后第65天分别采集植株的根、茎、叶柄和叶片,采用前述方法速冻和保存,用于不同组织的基因表达分析。
实验用大肠杆菌菌株DH5α由南京农业大学作物遗传与种质创新国家重点实验室伞形科蔬菜课题组保存;质粒载体pMD19-T、TaKaRaExTaqMix、SYBR PremixExTaq、PrimeScript RT reagent Kit试剂盒和DL 2000 Marker等均购自宝生物工程(大连)有限公司;Axy Prep DNA凝胶回收试剂盒购自爱思进生物技术(杭州)有限公司;RNA Simple Total RNA Kit试剂盒购自天根生化科技(北京)有限公司。
1.2方法
1.2.1总RNA提取及cDNA合成取3份嫩叶样品,每份1 g,根据RNA Simple Total RNA Kit试剂盒说明书分别提取总RNA;采用Nanodrop 2000微量分光光度计(美国Thermo Scientific公司)检测总RNA浓度,并按照Prime Script RT reagent Kit试剂盒说明书将总RNA反转录成cDNA。
1.2.2目的基因克隆基于芹菜转录组数据库中的数据检索得到编码CCoAOMT的基因序列[32],根据该基因序列设计1对特异引物,正向引物序列为5′-ATGGCTTCTAATGCTGAATCC-3′, 反向引物序列为5′-TCAGCTGATACGACGGCACAG-3′。以前述cDNA为模板进行目的基因(即‘六合黄心芹’CCoAOMT基因)片段的PCR扩增。扩增体系总体积10.0 μL,包括0.30 ng·μL-1cDNA模板0.5 μL、2×TaKaRaExTaqMix (产品编号RR001A)5.0 μL、 双蒸水3.5 μL、 0.10 μmol·L-1正向引物和反向引物各0.5 μL。扩增程序为: 94 ℃预变性5 min; 94 ℃变性30 s、 54 ℃退火30 s, 72 ℃延伸105 s,共35个循环;最后72 ℃延伸10 min。扩增产物用质量浓度12 g·L-1琼脂糖凝胶进行电泳分离,切取含目的片段的胶块,采用DNA凝胶回收试剂盒回收目的片段;将回收产物连接到pMD19-T质粒载体上,并转化至大肠杆菌DH5α中。转化菌液经鉴定后,交由南京金斯瑞生物科技有限公司进行测序。
1.2.3序列分析采用BioXM 2.6软件对克隆获得的目的基因片段进行核苷酸和氨基酸序列分析;在NCBI网站(http:∥blast.ncbi.nlm.nih.gov/Blast.cgi)上,采用BLASTp程序对目的基因编码的氨基酸序列进行保守域预测及同源性分析;用DNAMAN 6.0软件分析目的基因编码的氨基酸序列的亲水性和疏水性;采用序列处理在线工具包(SMS)(http:∥www.bio-soft.net/sms)和BioXM 2.6软件对目的基因及与其同源性较高的其他植物的CCoAOMT基因编码的氨基酸序列的氨基酸组成和理化性质(相关数据来自NCBI数据库)进行分析和多重比对,并采用MEGA 5.2软件绘制系统树;运用SWISS-MODEL软件对目的基因编码的氨基酸序列的三级结构进行预测分析。
1.2.4基因表达特性分析采用qRT-PCR技术、用SYBR PremixExTaq试剂盒以及iQTM5 Software和iQTM5 Real-time PCR System分析植株不同组织中和不同生长发育阶段叶片中目的基因的表达特性。根据克隆获得的目的基因序列设计引物,正向引物序列为5′-GGCTTCTAATGCTGAATCCAAAC-3′,反向引物序列为5′-CTAAGCTCTTTCATTGCCTCTGG-3′;以芹菜的actin基因为内参基因[33],正向引物序列为5′-C
GGTATTGTGTTGGACTCTGGTGAT-3′,反向引物序列为 5′-CAGCAAGGTCAAGACGGAGTATGG-3′。扩增体系总体积20.0 μL,包括SYBR PremixExTaq(产品编号RR430S) 10.0 μL、 0.15 ng·μL-1cDNA模板2.0 μL、 双蒸水7.2 μL、 0.50 μmol-1正向引物和反向引物各0.4 μL。 扩增程序为: 95 ℃预变性30 s; 95 ℃变性10 s、54 ℃退火30 s,共40个循环;65 ℃保温15 s。绘制熔解曲线,并根据2-ΔΔCT计算基因的相对表达量,其中,ΔCT=CT目标基因-CTactin[34]。
2 结果和分析
2.1目的基因的克隆及序列分析结果
以芹菜品种‘六合黄心芹’嫩叶的cDNA为模板,扩增到1条长度约750 bp的特异片段(图1),命名为AgCCoAOMT基因,其编码的蛋白质为AgCCoAOMT;AgCCoAOMT基因包含1个长度为726 bp的开放阅读框(ORF)(图2),编码241个氨基酸;AgCCoAOMT蛋白在第80位至第160位氨基酸间含有1个保守的AdoMet_MTases结构域(图3),说明该蛋白属于AdoMet_MTases超级家族。亲水性和疏水性分析结果(图4)显示:在AgCCoAOMT蛋白中,疏水性氨基酸多于亲水性氨基酸,据此推测该蛋白属于疏水性蛋白;其中,位于第159位的丙氨酸的亲水性最强,位于第66位的赖氨酸、第115位的丙氨酸和第227位的酪氨酸的疏水性均很强。
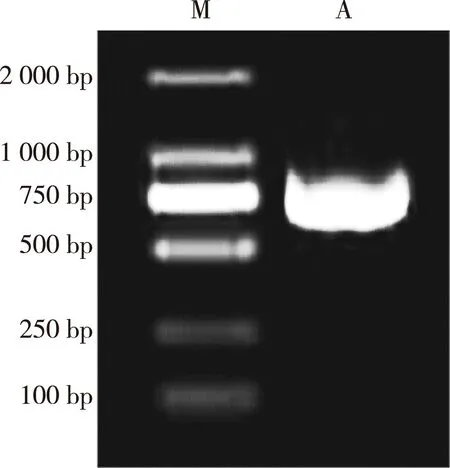
M: DL 2000 marker; A: AgCCoAOMT gene.
图1芹菜品种‘六合黄心芹’AgCCoAOMT基因的PCR扩增结果
Fig. 1PCR amplification result ofAgCCoAOMTgene fromApiumgraveolens‘Liuhe Huangxinqin’
2.2AgCCoAOMT蛋白与不同植物CCoAOMT蛋白的比较
2.2.1氨基酸序列同源性分析将芹菜品种‘六合黄心芹’及NCBI数据库中茶树〔Camelliasinensis(Linn.) Kuntze〕和枇杷〔Eriobotryajaponica(Thunb.) Lindl.〕CCoAOMT蛋白的氨基酸序列(登录号分别为AFY97679.1和AFZ76980.1)进行比对,结果见图5。结果表明:虽然‘六合黄心芹’、茶树和枇杷CCoAOMT蛋白的氨基酸序列长度存在一定差异(分别为241、245和247个氨基酸),但相似度却很高(91.09%),说明CCoAOMT蛋白具有高度保守性。
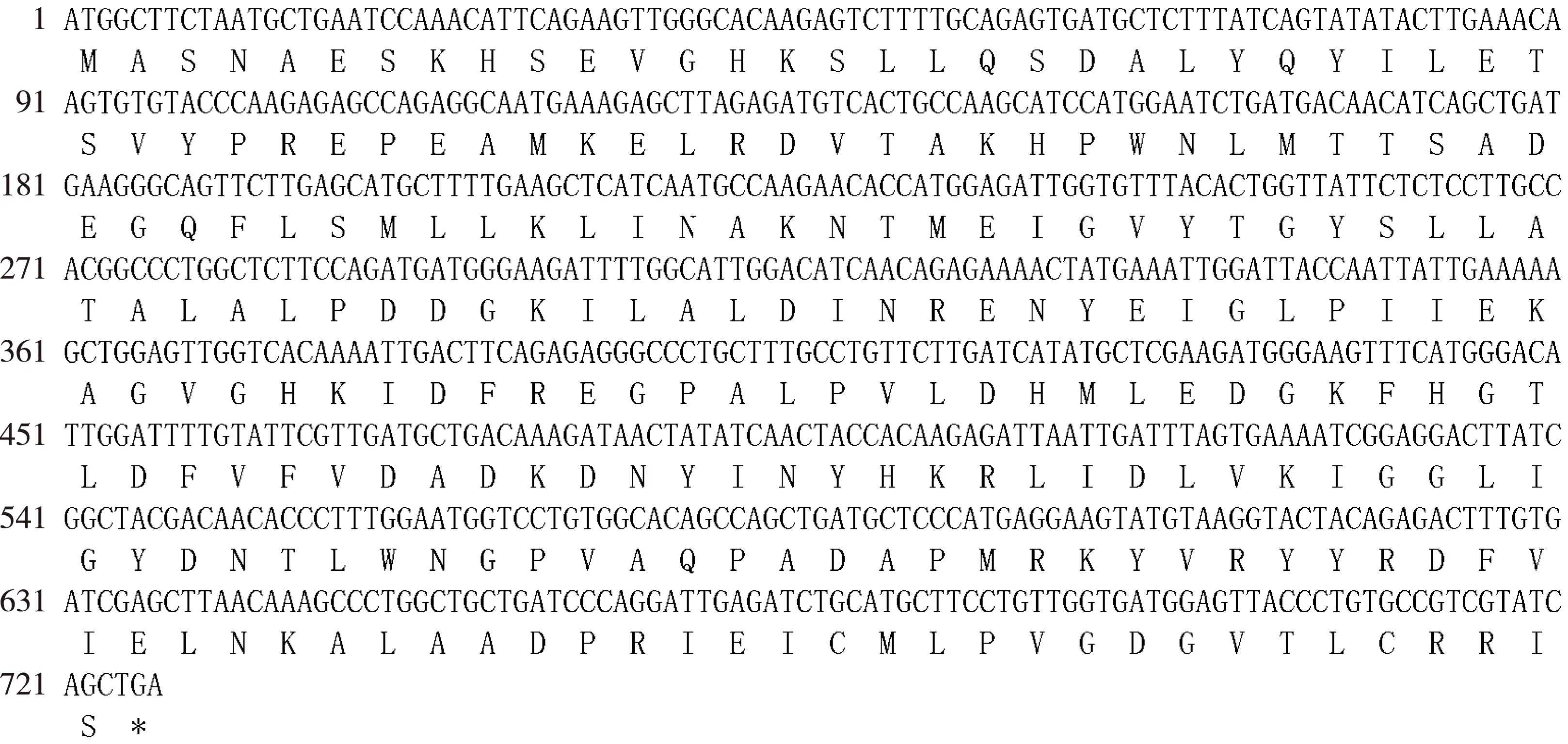
*: 终止密码子 Stop codon.图2 芹菜品种‘六合黄心芹’AgCCoAOMT基因的核苷酸序列及其编码的氨基酸序列Fig. 2 Nucleotide sequence of AgCCoAOMT gene from Apium graveolens ‘Liuhe Huangxinqin’ and its amino acid sequence encoded
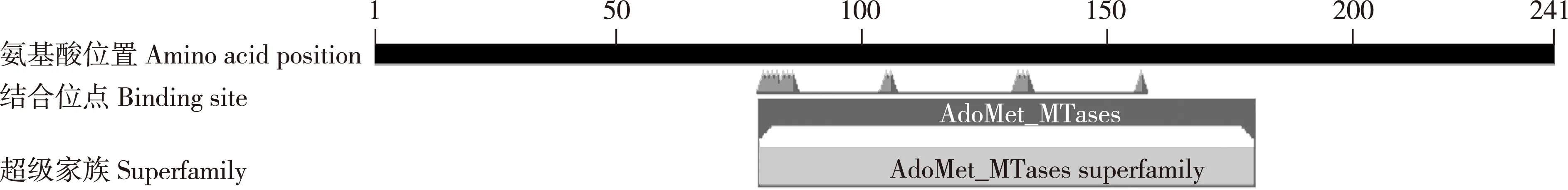
图3 芹菜品种‘六合黄心芹’AgCCoAOMT蛋白的保守域预测结果Fig. 3 Prediction result of conserved domain of AgCCoAOMT protein from Apium graveolens ‘Liuhe Huangxinqin’
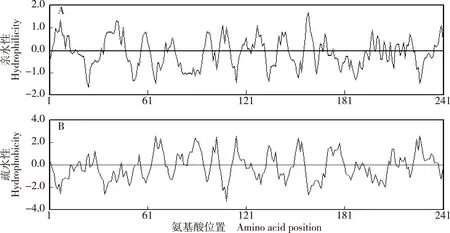
图4 芹菜品种‘六合黄心芹’AgCCoAOMT蛋白氨基酸序列的亲水性(A)和疏水性(B)分析结果Fig. 4 Analysis result of hydrophilicity (A) and hydrophobicity (B) of amino acid sequence of AgCCoAOMT protein from Apium graveolens ‘Liuhe Huangxinqin’
2.2.2蛋白质理化性质及氨基酸组成分析采用BLASTp程序从NCBI数据库中选取与AgCCoAOMT蛋白氨基酸序列相似度高于80%的19个种类,对芹菜品种‘六合黄心芹’与它们CCoAOMT蛋白的理化性质和氨基酸组成进行分析,结果见表1。

A: ‘六合黄心芹’‘Liuhe Huangxinqin’; B: 茶树 Camellia sinensis (Linn.) Kuntze; C: 枇杷 Eriobotrya japonica (Thunb.) Lindl.图5 芹菜品种‘六合黄心芹’、茶树和枇杷的CCoAOMT蛋白氨基酸序列的多重比对结果Fig. 5 Result of multiple alignment of amino acid sequences of CCoAOMT protein from Apium graveolens ‘Liuhe Huangxinqin’,Camellia sinensis (Linn.) Kuntze and Eriobotrya japonica (Thunb.) Lindl.
表1芹菜品种‘六合黄心芹’及其他植物CCoAOMT蛋白的氨基酸组成和理化性质比较
Table 1Comparison on compositions of amino acids and physical and chemical property of CCoAOMT protein fromApiumgraveolens‘Liuhe Huangxinqin’ and other species
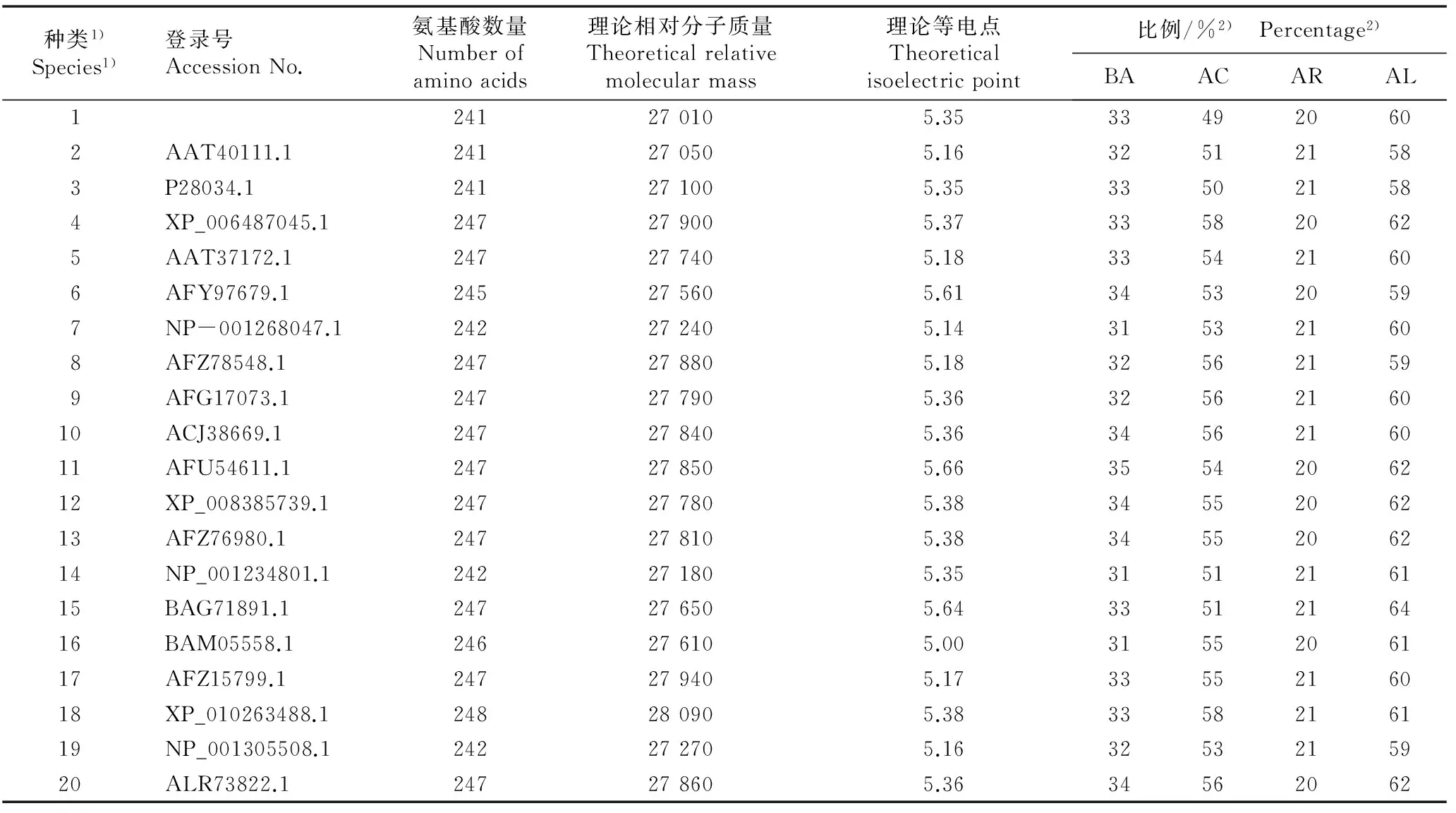
种类1)Species1)登录号AccessionNo.氨基酸数量Numberofaminoacids理论相对分子质量Theoreticalrelativemolecularmass理论等电点Theoreticalisoelectricpoint比例/%2) Percentage2)BAACARAL1241270105.35334920602AAT40111.1241270505.16325121583P28034.1241271005.35335021584XP_006487045.1247279005.37335820625AAT37172.1247277405.18335421606AFY97679.1245275605.61345320597NP-001268047.1242272405.14315321608AFZ78548.1247278805.18325621599AFG17073.1247277905.363256216010ACJ38669.1247278405.363456216011AFU54611.1247278505.663554206212XP_008385739.1247277805.383455206213AFZ76980.1247278105.383455206214NP_001234801.1242271805.353151216115BAG71891.1247276505.643351216416BAM05558.1246276105.003155206117AFZ15799.1247279405.173355216018XP_010263488.1248280905.383358216119NP_001305508.1242272705.163253215920ALR73822.1247278605.3634562062
1)1: ‘六合黄心芹’ ‘Liuhe Huangxinqin’; 2: 大阿米芹AmmimajusLinn.; 3: 欧芹Petroselinumcrispum(Mill.) Nyman ex A. W. Hill; 4: 甜橙Citrussinensis(Linn.) Osbeck; 5: 构树Broussonetiapapyrifera(Linn.) L’Hér ex Vent; 6: 茶树Camelliasinensis(Linn.) Kuntze; 7: 葡萄VitisviniferaLinn.; 8: 毛白杨PopulustomentosaCarr.; 9: 芍药PaeonialactifloraPall.; 10: 亮叶桦BetulaluminiferaH. Winkl.; 11: 白梨PyrusbretschneideriRehd.; 12: 苹果MalusdomesticaBorkh.; 13: 枇杷Eriobotryajaponica(Thunb.) Lindl.; 14: 番茄SolanumlycopersicumLinn.; 15: 红花CarthamustinctoriusLinn.; 16: 桉树EucalyptuspilularisSmith; 17: 忍冬LonicerajaponicaThunb.; 18: 莲NelumbonuciferaGaertn.; 19: 马铃薯SolanumtuberosumLinn.; 20: 白桦BetulaplatyphyllaSuk.
2)BA: 碱性氨基酸 Basic amino acids; AC: 酸性氨基酸 Acidic amino acids; AR: 芳香族氨基酸 Aromatic amino acids; AL: 脂肪族氨基酸 Aliphatic amino acids.
结果显示:不同植物CCoAOMT蛋白的氨基酸数量为241~248,其中,同为伞形科的‘六合黄心芹’、大阿米芹(AmmimajusLinn.)和欧芹〔Petroselinumcrispum(Mill.) Nyman ex A. W. Hill〕CCoAOMT蛋白的氨基酸数量最少,均为241;睡莲科(Nymphaeaceae)植物莲(NelumbonuciferaGaertn.)CCoAOMT蛋白的氨基酸数量最多,为248;另有11种植物CCoAOMT蛋白的氨基酸数量为247。从氨基酸组成看,除‘六合黄心芹’AgCCoAOMT蛋白外,其他19种植物的CCoAOMT蛋白中酸性氨基酸所占比例均在50%以上,明显高于碱性氨基酸的比例;而脂肪族氨基酸所占比例均在58%以上,约为芳香族氨基酸的3倍。
从蛋白质理化性质看,表1涉及的这些种类的CCoAOMT蛋白的理论相对分子质量差异较小,以‘六合黄心芹’CCoAOMT蛋白的理论相对分子质量最低(27 010)、莲CCoAOMT蛋白的理论相对分子质量最高(28 090)。这20种植物CCoAOMT蛋白的理论等电点为pI 5.00~ pI 5.66,变幅不大。
2.2.3系统进化分析采用MEGA 5.2软件绘制上述20种植物CCoAOMT蛋白的系统树,结果见图6。
结果显示:这20种植物被分成2支,其中,隶属于伞形科、睡莲科、山茶科(Theaceae)、葡萄科(Vitaceae)和茄科(Solanaceae)的8种植物聚为一支,另外9科12种植物聚为另一支。‘六合黄心芹’与同科植物大阿米芹和欧芹的进化关系最近,并且与睡莲科植物莲的进化关系较近;此外,其他同科不同植物的进化关系均较近,说明同科植物CCoAOMT蛋白的进化关系较近。

图6 芹菜品种‘六合黄心芹’及其他植物CCoAOMT蛋白的系统树Fig. 6 Phylogenetic tree of CCoAOMT protein from Apium graveolens ‘Liuhe Huangxinqin’ and other species
2.3AgCCoAOMT蛋白三级结构的预测分析
由于芹菜品种‘六合黄心芹’AgCCoAOMT蛋白与紫花苜蓿(MedicagosativaLinn.)CCoAOMT蛋白的氨基酸序列相似度很高,因此,以紫花苜蓿CCoAOMT(PDB ID:1sui.1)为模型[35],采用SWISS-MODEL软件对该蛋白的三级结构进行预测,结果见图7。‘六合黄心芹’AgCCoAOMT蛋白与紫花苜蓿CCoAOMT蛋白三级结构的一致性达84.58%,并且存在多个α-螺旋和β-折叠。
2.4AgCCoAOMT基因的表达特性分析
采用qRT-PCR技术对芹菜品种‘六合黄心芹’不同组织中以及不同生长发育阶段叶片中AgCCoAOMT基因的相对表达量进行检测,结果见图8。
2.4.1在不同组织中的表达特性分析由图8-A可见:播种后第65 天,AgCCoAOMT基因在‘六合黄心芹’根、茎、叶柄和叶片中均能够表达,但相对表达量存在明显差异;AgCCoAOMT基因的相对表达量在叶片中最高、根中次之、茎中较少、叶柄中最少。叶片中AgCCoAOMT基因的相对表达量分别为叶柄、茎和根的15.17、10.89和3.70倍,且差异极显著(P<0.01);而根、茎和叶柄中该基因的相对表达量则无显著差异(P>0.05)。
2.4.2在不同生长发育阶段的表达特性分析由图8-B可见:在幼苗期(播种后第25天)、生长旺盛期(播种后第45天)和商品期(播种后第65天),‘六合黄心芹’叶片中AgCCoAOMT基因的相对表达量存在明显差异,表现为商品期最高、幼苗期次之、生长旺盛期最低。商品期AgCCoAOMT基因的相对表达量分别为幼苗期和生长旺盛期的5.18和6.36倍,且差异极显著;而幼苗期和生长旺盛期该基因的相对表达量则无显著差异。
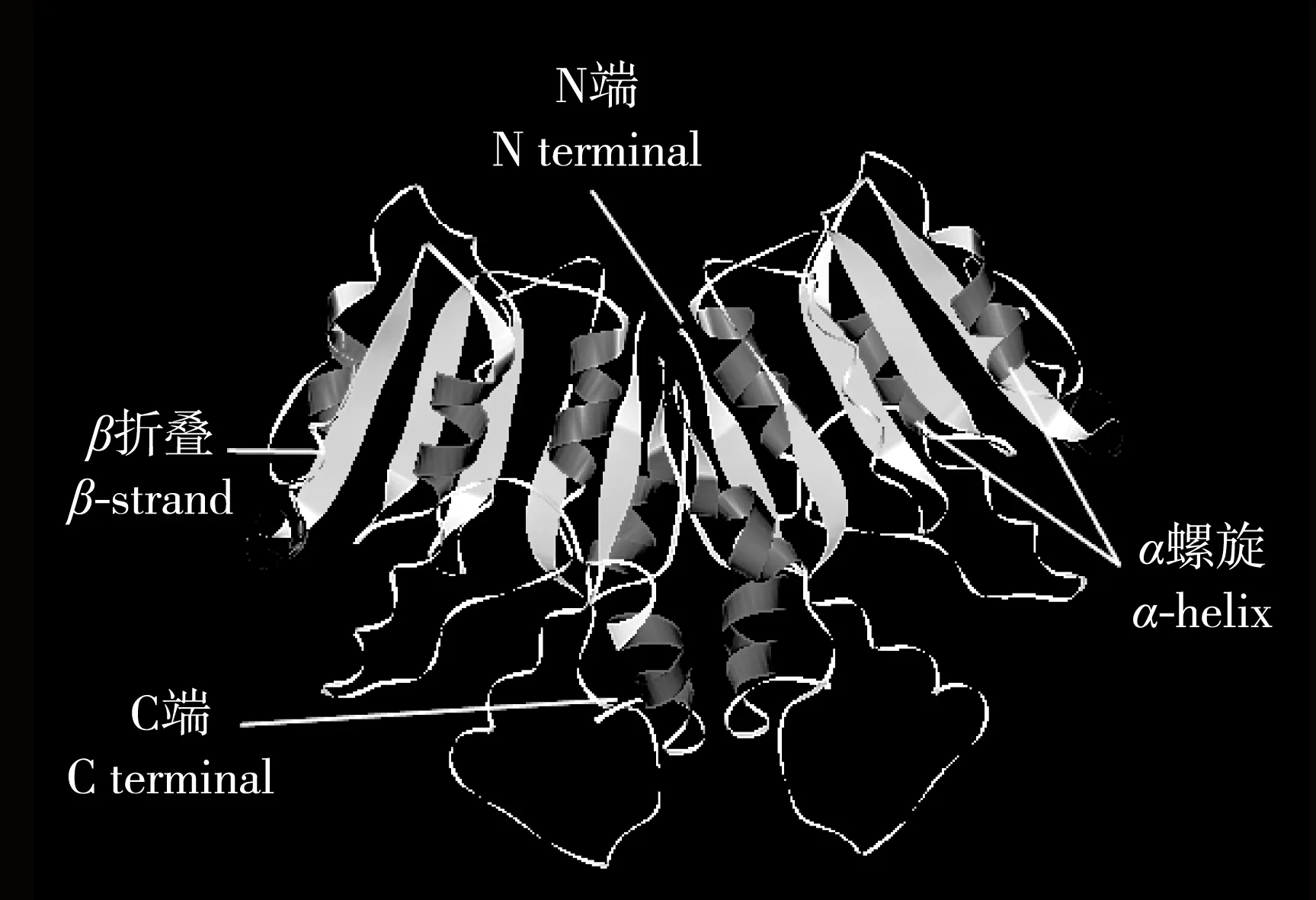
图7芹菜品种‘六合黄心芹’AgCCoAOMT蛋白的三级结构
Fig.7Tertiary structure of AgCCoAOMT protein fromApiumgraveolens‘Liuhe Huangxinqin’
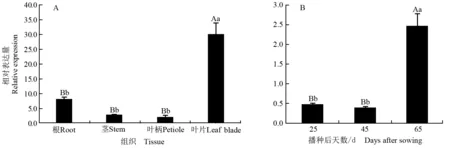
不同大写和小写字母分别表示差异极显著(P<0.01)或显著(P<0.05) Different capitals and small letters indicate the extremely significant (P<0.01) or significant (P<0.05) differences, respectively.
图8芹菜品种‘六合黄心芹’不同组织(A)和不同生长发育阶段叶片(B)中AgCCoAOMT基因相对表达量的比较
Fig. 8Comparison on relative expression ofAgCCoAOMTgene in different tissues (A) and in leaf blade at different growth and development stages (B) ofApiumgraveolens‘Liuhe Huangxinqin’
3 讨 论
氨基酸序列同源性分析结果显示:芹菜品种‘六合黄心芹’AgCCoAOMT蛋白与茶树和枇杷CCoAOMT蛋白的氨基酸序列相似度为91.09%,说明CCoAOMT蛋白的氨基酸序列具有高度保守性。而在基于AgCCoAOMT蛋白的氨基酸序列构建的系统树中,‘六合黄心芹’与同科植物欧芹和大阿米芹聚在一起,也表明在植物进化过程中CCoAOMT蛋白的氨基酸序列具有一定的保守性。
qRT-PCR检测结果表明:在芹菜品种‘六合黄心芹’的根、茎、叶柄和叶片中,AgCCoAOMT基因均能够表达但其相对表达量有明显差异,说明该基因的表达存在明显的组织特异性,推测这可能与芹菜不同部位木质素的积累和分布差异有关。在不同生长发育阶段,‘六合黄心芹’AgCCoAOMT基因的相对表达量也存在明显差异,其中,幼苗期和生长旺盛期该基因的相对表达量较低,而商品期该基因的相对表达量则显著提高,推测这可能与商品期植株叶的老化和木质素含量升高有关。
植物的木质素代谢是一个复杂的生理过程,受到多种酶的综合调控[36-37]。本研究仅对芹菜品种‘六合黄心芹’的AgCCoAOMT基因进行了相关的初步研究,但AgCCoAOMT基因对芹菜尤其是‘六合黄心芹’体内木质素代谢的作用机制尚待进一步研究。
[1]DHINGRA D, MICHAEL M, RAJPUT H, et al. Dietary fibre in foods: a review[J]. Journal of Food Science and Technology, 2012, 49: 255-266.
[2]SLAVIN J. Fiber and prebiotics: mechanisms and health benefits[J]. Nutrients, 2013, 5: 1417-1435.
[4]THIMMJ,BURRITTD,SIMSIM,etal.Celery(Apiumgraveolens) parenchyma cell walls: cell walls with minimal xyloglucan[J]. Physiologia Plantarum, 2002, 116: 164-171.
[5]CHEN C Y, BAUCHER M, CHRISTENSEN J H, et al. Biotech-nology in trees: towards improved paper pulping by lignin engineering[J]. Euphytica, 2001, 118: 185-195.
[6]RONG H Y, GAO B Y, ZHAO Y X, et al. Advanced lignin-acrylamide water treatment agent by pulp and paper industrial sludge: synthesis, properties and application[J]. Journal of Environmental Sciences, 2013, 25: 2367-2377.
[7]周强, 於丙军. 潜在木质纤维素能源植物香根草的初步研究[J]. 植物资源与环境学报, 2012, 21(1): 98-103.
[8]KNUDSENKEB.Carbohydrateandlignincontentsofplant materials used in animal feeding[J]. Animal Feed Science and Technology, 1997, 67: 319-338.
[9]PETERSSON A, DOMIG K J, SCHEDLE K, et al. Comparison of three methods to enumerate gut microbiota of weanling piglets fed insoluble dietary fibre differing in lignin content[J]. Journal of Agricultural Science, 2010, 148: 225-232.
[10]ZHONG R Q, MORRISON Ⅲ W H, HIMMELSBACH D S, et al. Essential role of caffeoyl coenzyme AO-methyltransferase in lignin biosynthesis in woody poplar plants[J]. Plant Physiology, 2000, 124: 563-577.
[11]YE Z H, KNEUSEL R E, MATERN U, et al. An alternative methylation pathway in lignin biosynthesis inZinnia[J]. The Plant Cell, 1994, 6: 1427-1439.
[12]LI X Y, CHEN W J, ZHAO Y, et al. Downregulation of caffeoyl-CoAO-methyltransferase (CCoAOMT) by RNA interference leads to reduced lignin production in maize straw[J]. Genetics and Molecular Biology, 2013, 36: 540-546.
[13]GRIENENBERGERE,BESSEAU S,GEOFFROYP, et al. A BAHD acyltransferase is expressed in the tapetum ofArabidopsisanthers and is involved in the synthesis of hydroxycinnamoyl spermidines[J]. The Plant Journal, 2009, 58: 246-259.
[14]ZHANG G Y, ZHANG Y J, XU J T, et al. TheCCoAOMT3 gene from jute (CorchoruscapsularisL.) is involved in lignin biosynthesis inArabidopsisthaliana[J]. Gene, 2014, 546: 398-402.
[15]ZHAO H Y, SHENG Q X, LÜ S Y, et al. Characterization of three riceCCoAOMTgenes[J]. Chinese Science Bulletin, 2004, 49: 1602-1606.
[16]LIU X G, LUO Y, WU H K, et al. Systematic analysis ofO-methyltransferase gene family and identification of potential members involved in the formation ofO-methylated flavonoids inCitrus[J]. Gene, 2016, 575: 458-472.
[17]DO C T, POLLET B, THÉVENIN J, et al. Both caffeoyl Coenzyme A 3-O-methyltransferase 1 and caffeic acidO-methyltransferase 1 are involved in redundant functions for lignin, flavonoids and sinapoyl malate biosynthesis inArabidopsis[J]. Planta, 2007, 226: 1117-1129.
[18]BHUIYAN N H, SELVARAJ G, WEI Y D, et al. Gene expression profiling and silencing reveal that monolignol biosynthesis plays a critical role in penetration defence in wheat against powdery mildew invasion[J]. Journal of Experimental Botany, 2009, 60: 509-521.
[19]WEI J H, ZHAO H Y, ZHANG J Y, et al. Cloning of cDNA encoding CCoAOMT fromPopulustomentosaand down-regulation of lignin content in transgenic plant expressing antisense gene[J]. Acta Botanica Sinica, 2001, 43: 1179-1183.
[20]LIU Y X, ZOU D M, WU B S, et al. Cloning and expression analysis of aCCoAOMThomolog in loquat fruit in response to low-temperature storage[J]. Postharvest Biology and Technology, 2015, 105: 45-50.
[21]ZHONGRQ,MORRISONⅢWH,NEGRELJ,et al. Dual methylation pathways in lignin biosynthesis[J]. The Plant Cell, 1998, 10: 2033-2045.
[22]MEYERMANS H, MORREEL K, LAPIERRE C, et al. Modifica-tions in lignin and accumulation of phenolic glucosides in poplar xylem upon down-regulation of caffeoyl-coenzyme AO-methyl-transferase, an enzyme involved in lignin biosynthesis[J]. Journal of Biological Chemistry, 2000, 275: 36899-36909.
[23]ZHAO H Y, WEI J H, ZHANG J Y, et al. Lignin biosynthesis by suppression of twoO-methyl-transferases[J]. Chinese Science Bulletin, 2002, 47: 1092-1095.
[24]陈虎, 何新华, 罗聪, 等. 龙眼咖啡酰辅酶A-O-甲基转移酶(DLCCoAOMT)基因的克隆和表达分析[J]. 中国农业科学, 2012, 45(1): 118-126.
[25]李梦瑶, 王枫, 侯喜林, 等. 芹菜过敏原蛋白Apig1基因的克隆与表达分析[J]. 南京农业大学学报, 2013, 36(2): 13-19.
[26]李梦瑶, 王枫, 侯喜林, 等. 2个芹菜品种泛变应原Apig4基因的克隆与分析[J]. 植物资源与环境学报, 2013, 22(1): 1-7.
[27]田畅, 蒋倩, 王枫, 等. 3个芹菜品种Agnp-G3PDH基因的克隆及其序列和表达特性分析[J]. 植物资源与环境学报, 2014, 23(2): 1-10.
[28]JIA X L, WANG G L, WANG F, et al. Anatomic structure and expression profiles of related genes: novel insights into leaf development in celery[J]. Journal of Plant Growth Regulation, 2015, 34: 519-531.
[29]PAKUSCH A E, KNEUSEL R E, MATERN U. S-adenosyl-L-methionine:trans-caffeoyl-coenzyme A 3-O-methyltransferase from elicitor-treated parsley cell suspension cultures[J]. Archives of Biochemistry and Biophysics, 1989, 271: 488-494.
[30]SCHMITT D, PAKUSCH A E, MATERN U. Molecular cloning, induction and taxonomic distribution of caffeoyl-CoA 3-O-methyltransferase, an enzyme involved in disease resistance[J]. The Journal of Biological Chemistry, 1991, 266: 17416-17423.
[31]王海峰, 张德琴, 刘长勇, 等. 六合黄心芹周年生产技术[J]. 长江蔬菜, 1999(1): 16-18.
[32]LI M Y, WANG F, JIANG Q, et al. Identification of SSRs and differentially expressed genes in two cultivars of celery (ApiumgraveolensL.) by deep transcriptome sequencing[J]. Horticulture Research, 2014, 1: 1-9.
[33]LI M Y, WANG F, JIANG Q, et al. Validation and comparison of reference genes for qPCR normalizationof celery(Apium
graveolens) at different development stages[J]. Frontiers in Plant Science, 2016, 7: 1-12.
[34]PFAFFL M W. A new mathematical model for relative quantification in real-time RT-PCR[J]. Nucleic Acids Research, 2001, 29: 2002-2007.
[35]FERRER J L, ZUBIETA C, DIXON R A, et al. Crystal structures of alfalfa caffeoyl coenzyme A 3-O-methyltransferase[J]. Plant Physiology, 2005, 137: 1009-1017.
[36]JIA X L, WANG G L, XIONG F, et al.Denovoassembly, transcriptome characterization, lignin accumulation, and anatomic characteristics: novel insights into lignin biosynthesis during celery leaf development[J]. Scientific Reports, 2015, 5: 8259.
[37]JIA X L, LI M Y, JIANG Q, et al. High-throughput sequencing of small RNAs and anatomical characteristics associated with leaf development in celery[J]. Scientific Reports, 2015, 5: 11093.
(责任编辑: 佟金凤)
Analyses on cloning and expression characteristics ofAgCCoAOMTgene fromApiumgraveolens‘Liuhe Huangxinqin’
YUAN Xiaoyang, WU Xuejun, NIE Li, XU Ke, XIONG Aisheng①
(State Key Laboratory of Crop Genetics and Germplasm Enhancement, Ministry of Agriculture Key Laboratory of Biology and Germplasm Enhancement of Horticultural Crops in East China, College of Horticulture, Nanjing Agricultural University, Nanjing 210095, China),J.PlantResour. &Environ., 2016, 25(3): 19-27
According to transcriptome database ofApiumgraveolensLinn. in Apiaceae, caffeoyl-CoA-O-methyltransferase gene was cloned from total RNA in leaf ofA.graveolens‘Liuhe Huangxinqin’, which was named asAgCCoAOMTgene. Sequence analysis result shows thatAgCCoAOMTgene contains an open reading frame (ORF) with length of 726 bp, 241 amino acids are encoded. The protein encoded by this gene is AgCCoAOMT, its theoretical relative molecular mass is 27 010, and theoretical isoelectric point is pI 5.35. In AgCCoAOMT protein, percentage of acidic amino acids is higher than that of basic amino acids, and percentage of aliphatic amino acids is about three times of that of aromatic amino acids. This protein belongs to hydrophobic protein and contains a conserved AdoMet_MTases domain, meaning that AgCCoAOMT protein belongs to AdoMet_MTases superfamily. Tertiary structure of AgCCoAOMT protein includes manyα-helix andβ-turn, its consistency with that of CCoAOMT protein fromMedicagosativaLinn. is 84.58%. Analysis results of multiple alignment and phylogenetic tree show that AgCCoAOMT protein has high conservation. And it has the nearest phylogeny with CCoAOMT protein from the same family speciesAmmimajusLinn. andPetroselinumcrispum(Mill.) Nyman ex A. W. Hill, also, has nearer phylogeny with CCoAOMT protein fromNelumbonuciferaGaertn. in Nymphaeaceae. qRT-PCR determination result shows thatAgCCoAOMTgene can express in root, stem, petiole and leaf blade of ‘Liuhe Huangxinqin’, and its relative expression in leaf blade is extremely significantly higher than that of other tissues (P<0.01), meaning that expression of this gene has obvious tissue specificity. There is obvious difference in relative expression of this gene in leaf blade at different growth and development stages, its relative expression at commodity stage (the sixty-fifth day after sowing) is extremely significantly higher than that at seedling stage (the twenty-fifth day after sowing) and vigorous growth stage (the forty-fifth day after sowing). It is suggested thatAgCCoAOMTgene is related to leaf senescence and lignin content increasing of ‘Liuhe Huangxinqin’, and has high conservation during evolutionary process.
ApiumgraveolensLinn.;AgCCoAOMTgene; sequence analysis; multiple alignment; expression characteristics
2016-04-21
国家大学生创新创业训练计划(201510307028); 国家自然科学基金资助项目(31200520)
苑笑阳(1994—),女,山东菏泽人,本科,主要从事蔬菜分子生物学方面的研究工作。
E-mail: xiongaisheng@njau.edu.cn
Q785; Q943.2; S636.3
A
1674-7895(2016)03-0019-09
10.3969/j.issn.1674-7895.2016.03.03
