Clinacanthus nutans: a review of the medicinal uses, pharmacology and phytochemistry
2016-11-14MdArifulAlamSahenaFerdoshKashifGhafoorMdAbdulHakimAbdulShukorJuraimiAlfiKhatibMdZaidulSarker
Md. Ariful Alam, Sahena Ferdosh, Kashif Ghafoor, Md. Abdul Hakim, Abdul Shukor Juraimi,Alfi Khatib, Md. Zaidul I. Sarker*
1Faculty of Pharmacy, International Islamic University Malaysia (IIUM), Kuantan Campus, 25200 Kuantan, Pahang, Malaysia
2Faculty of Science, International Islamic University Malaysia (IIUM), Kuantan Campus, 25200 Kuantan, Pahang, Malaysia
3Department of Food Science and Nutrition, King Saud University, Riyadh 11451, Saudi Arabia
4Institute of Tropical Agriculture, University Putra Malaysia, 43400 UPM Serdang, Selangor Darul Ehsan, Malaysia
5Department of Crop Science, University Putra Malaysia, 43400 UPM Serdang, Selangor Darul Ehsan, Malaysia
Clinacanthus nutans: a review of the medicinal uses, pharmacology and phytochemistry
Md. Ariful Alam1, Sahena Ferdosh2*, Kashif Ghafoor3, Md. Abdul Hakim4, Abdul Shukor Juraimi5,Alfi Khatib1, Md. Zaidul I. Sarker1*
1Faculty of Pharmacy, International Islamic University Malaysia (IIUM), Kuantan Campus, 25200 Kuantan, Pahang, Malaysia
2Faculty of Science, International Islamic University Malaysia (IIUM), Kuantan Campus, 25200 Kuantan, Pahang, Malaysia
3Department of Food Science and Nutrition, King Saud University, Riyadh 11451, Saudi Arabia
4Institute of Tropical Agriculture, University Putra Malaysia, 43400 UPM Serdang, Selangor Darul Ehsan, Malaysia
5Department of Crop Science, University Putra Malaysia, 43400 UPM Serdang, Selangor Darul Ehsan, Malaysia
Accepted 15 March 2016
Available online 20 April 2016
Clinacanthus nutans
Medicinal uses
Phytochemicals
Pharmacology
Therapeutic potential
Clinacanthus nutans Lindau is known as snake grass belonging to the Acanthaceae family. This plant has diverse and potential medicinal uses in traditional herbal medicine for treating skin rashes, insects and snake bites, lesions caused by herpes simplex virus, diabetes, and gout in Malaysia, Indonesia, Thailand and China. Phytochemical investigations documented the varied contents of bioactive compounds from this plant namely fl avonoids, glycosides,glycoglycerolipids, cerebrosides and monoacylmonogalatosylglycerol. The pharmacological experiment proved that various types of extracts and pure compounds from this species exhibited a broad range of biological properties such as anti-inflammatory, antiviral,antioxidant, and anti-diabetic activities. The fi ndings of toxicity study showed that extracts from this plant did not show any toxicity thus it can be used as strong therapeutic agents for specifi c diseased conditions. However, further experiments on chemical components and their mode of action showing biological activities are required to elucidate the complete phytochemical profi le and assess to confi rm their suitability for future drugs. This review summarizes the medicinal uses, phytochemistry and pharmacology of this plant in order to explore its therapeutic potential and gaps necessitating for prospected research work.
1. Introduction
Acanthaceae is one of the leading families of dicotyledonous flowering plant which included 250 genera and about 2 500 species[1]. Most of them are tropical herbs, shrubs, or twining vines; some are epiphytes. A few of them are spread intemperate regions. Species from this family mainly distributed in Indonesia and Malaysia, Africa, Brazil, and Central America[2]. Plants of this family can be grown in most of the habitat, comprising dense or open forest, bushes, damp fi elds and valleys, sea shores and marine regions, swamps, and mangrove areas[3]. Acanthaceae family is considered as one of the largest sources of medicinal plants providing effective traditional medicines against specific health impediments and those can be simply predictable morphologically by their simple, differing, decussate, whole leaves, zygomorph fl owers and their superior ovary.
Clinacanthus nutans (C. nutans) Lindau is one of the important species from this family and has been used as vital herbal medicines in tropical Asia (Figure 1). At the moment this plant attracts much attention of many researchers for its medicinal potency[4,5]. Thai ministry of public health has been considered this plant as a main remedy for the treatment of skin infl ammations and lesion caused by virus[6].
In the botanical viewpoint C. nutans (Burm. f.) Lindau and Clinacanthus siamensis Bremek are two diff erent species and often been mistaken due to their alike morphology. These two species have diff erent pharmacological characteristics, molecular aspect and anti-herpes simplex virus (HSV) type 1 and type 2 activities[7]. This plant is well recognized as anti-snake venom activity used by the traditional curers in Thailand. This plant is traditionally used as natural medicine in Malaysia, Indonesia and Thailand for treating certain diseases such as skin rashes, scorpion and insect bites,diabetes mellitus, fever and diuretics[8,9]. Pharmacological studies proved that this plant possessed a broad range of antimicrobial activity especially treating genital herpes and varicella-zoster virus(VZV) lesions diagnosed in immunocompromised people[10].
This plant is economically very important owing to the medicinal uses and herbal products. Diff erent kinds of topical preparations such as cream, lotions, capsule, tablet, herbal tea, concentrated extract and secondary metabolites products are available in the market. Preparation of cream using alcoholic extract of dried leaves can relieve pain and heal herpes infection[11]. Most of the products have not much popularity among the consumers because of the lack of pharmacological information.

Figure 1. C. nutans (Burm. f.) landau.(a) Whole plant; (b) leaves; (c) leaves with stem.
2. Botany
The vernacular name of this plant in English is snake grass; Belalai gajah, Sabah snake grass in Malaysia; Dandang Gendis, Ki tajam(Sunda) in Indonesia; Phaya yo, Phaya plongtong in Thailand; twist of flowers, alligator flower, e zuihua in China[12-14]. Clinacanthus burmanni Nees, Clinacanthus burmanni var. robinsonii Benoist are the synonym of C. nutans (Burm. f.) Lindau. Taxonomically this plant can be classified by kingdom: Plantae; phylum: Magnoliophyta;class: Magnoliopsida; subclass: Asteridae; order: Lamiales; family: Acanthaceae; genus: Clinacanthus Lindau; species: C. nutans (Burm. f.) Lindau[12].
C. nutans is a perennial herb which can grow up to 1 m tall with pubescent branches and cylindrical, striate, and glabrescent stems. The leaves are simple, opposite, narrowly elliptic-oblong or lanceolate (2.5-13.0 cm long×0.5-1.5 cm wide)[15]. This shrub is about 1 m tall, and stems cylindrical, striate and glabrescent. The petiole is 0.3-2.0 cm, sulcate, bifariously pubescent and leaf blade lanceolate-ovate, lanceolate or linear-lanceolate[16]. The leaves areapex acute or acuminate and exsculptate; dentate or subentine margins. Both surfaces of leaves are pubescent when young then glabrescent. The leaf base are cuncate, obtuse rounded or truncate;often oblique. Petiole is 3-15 mm long[17].
The flowers are sordidly yellow or greenish yellow and dense cymes at the top of branches and branchlets; always covered with 5-alpha cymules[18]. The calyx of flower about 1 cm long with grandular-pubescent. Corolla is dull red with green base, about 3.0-4.2 cm. The stamen is exerted from the throat of corolla. The ovary is compressed into two cells and each cell has two ovules. The styles are fi liform with shortly bidentate. Capsule is oblong basally wrapped into 4-seeded short stalk[18].
3. Ethnomedicinal uses
C. nutans has been traditionally used for a long time in diff erent regions of Asia due to their different pharmacological effects. Usually the fresh leaves are boiled with water and consumed as herbal tea in Malaysia. In Thailand, an alcoholic extract of fresh leaves is used externally for treatment of skin rashes, snake and insect bite, HSV, and VZV lesions[19]. Sometimes the leaves are consumed as raw material or mixed with apple juice, sugarcane or green tea and provide as fresh drink. It is also prominent in Thailand as anti-snake venom amongst the traditional healers. The mode of action of this plant is attributed to be its anti-cell lysis property rather than an anti-neuromuscular transmission blocker. It has been also used to treat scorpion bites and nettle rash. In China the whole plant is used in various manners to treat infl ammatory conditions like haematoma, contusion, strains and sprains of injuries and rheumatism[16,20,21]. This plant is widely used for treating gastrointestinal complications. Indonesian and Thai traditional curers prepare herbal medicines from C. nutans to treat dysentery. In Indonesia they boil a handful of the fresh leaves and boil them in fi ve glasses of water till the water level recedes to about three glasses and the bouillon is given in a dose of 1 glass each time[13]. It is also used to treat diabetes by boiling 7-21 fresh leaves in two glasses of water until the water level comes to one glass and serve twice daily. The decoction is made by boiling of 15 gm of fresh leaves for 15 min and consumed daily for the treatment of dysuria. Likewise this plant is also used in the treatment of fever. Whereas Chinese healers deal with the plant as useful in controlling menstrual function, relieving pain, anemia, jaundice and setting of fractured bones[13,21].
4. Phytochemistry
Phytochemical investigation of this plant shows that C. nutans contains a wide range of bioactive compounds (Figure 2). The vital phytochemical are stigmasterol (1)[22], lupeol (2), b-sitosterol (3)[23],belutin[24] and myricyl alcohol[25,26] are found. There are six known C -glycosyl fl avones isolated from the n-BuOH and water soluble fractions of the methanolic extract of this plant in Thailand; they are vitexin (4), isovitexin (5), schaftoside (6), isomollupentin 7-O-bglucopyranoside (7), orientin (8) and isoorientin (9)[27]. The n-BuOH soluble fractions from methanolic extract of stems and leaves of this plant contain fi ve glucosides[27].
A mixture of cerebrosides (10) and a monoacylmonogalactosyl glycerol (2S)-1-O-linolenoyl- 3-O-b-dgalactopyranosylglycerol(11) were isolated from the EtOAc-soluble fraction of the ethanolic extract of the fresh leaves of C. nutans[28]. The hexane and chloroform extract of this plant were used for isolation of 13-hydroxy-(13-S)-phaeophytin b, pupurin-18-phytyl ester and phaeophorbide-a derived from the extracts[29]. Anti-HSV eff ective trigalactosyl and digalactosyl diglycerides (12) were isolated from leaves extract from this plant[30].
There were eight compounds related to chlorophyll a and chlorophyll b isolated from the chloroform extract of leaves 132-hydroxy-(132-S)-chlorophyll b, 132-hydroxy-(132-R)-chlorophyll b, 132-hydroxy-(132-S)-phaeophytin b[9], 132-hydroxy-(132-R)-phaeophytin b (13), 132-hydroxy-(132-S)-phaeophytin a (14), 132-hydroxy-(132-R)-phaeophytin a (15)[10], purpurin 18 phytyl ester and phaeophorbide a[9]. Four new sulfur-containing compounds,clinamides A-C (16-18) and 2-cis-entadamide A (19) and three known compounds, entadamide A (20)[31], entadamide C[32],and trans-3-methylsulfinyl-2-propenol[20] were isolated from the ethanolic extract of the aerial parts of C. nutans[33].

Figure 2. Structure of diff erent bioactive compounds from C. nutans.
5. Pharmacological effects
5.1. Anti-inflammatory activity
C. nutans has been used as anti-inflammatory agents for the treatment of insect bites and allergic responses and as remedies for herpes simplex and VZV lesions. Different pharmacological eff ects of plant extracts and isolated compounds are presented in the Table 1. The 80% ethanol extract of aerial part of this plant showed a signifi cant inhibition on the generation of superoxide anion and the elastase release by activated neutrophils. The inhibition was produced by 10 μg/mL ethanolic extract of C. nutans at 68.33%[33]. In this study MeO-Suc-Ala-Ala-Pro-Valp-nitroanilide was used as the elastase substrate for observing elastase release and superoxide anion formation examined by detecting the superoxide dismutaseinhibitory reduction of ferricytochrome c. The petroleum extract of leaves produced highest cytotoxic eff ect as compared to the ethyl acetate and methanol extract. The increasing concentrations of the extracts showed variability on the percentage of cell viability of HeLa cells. On the other hand optimum cytotoxic activity of petroleum extract was found on HeLa cells at the incubation period 72 h. The inhibition of K-562 cells proliferation was observed at concentration of 20.0 μg/mL with an IC50of 20.0 μg/mL after 72 h incubation[34].
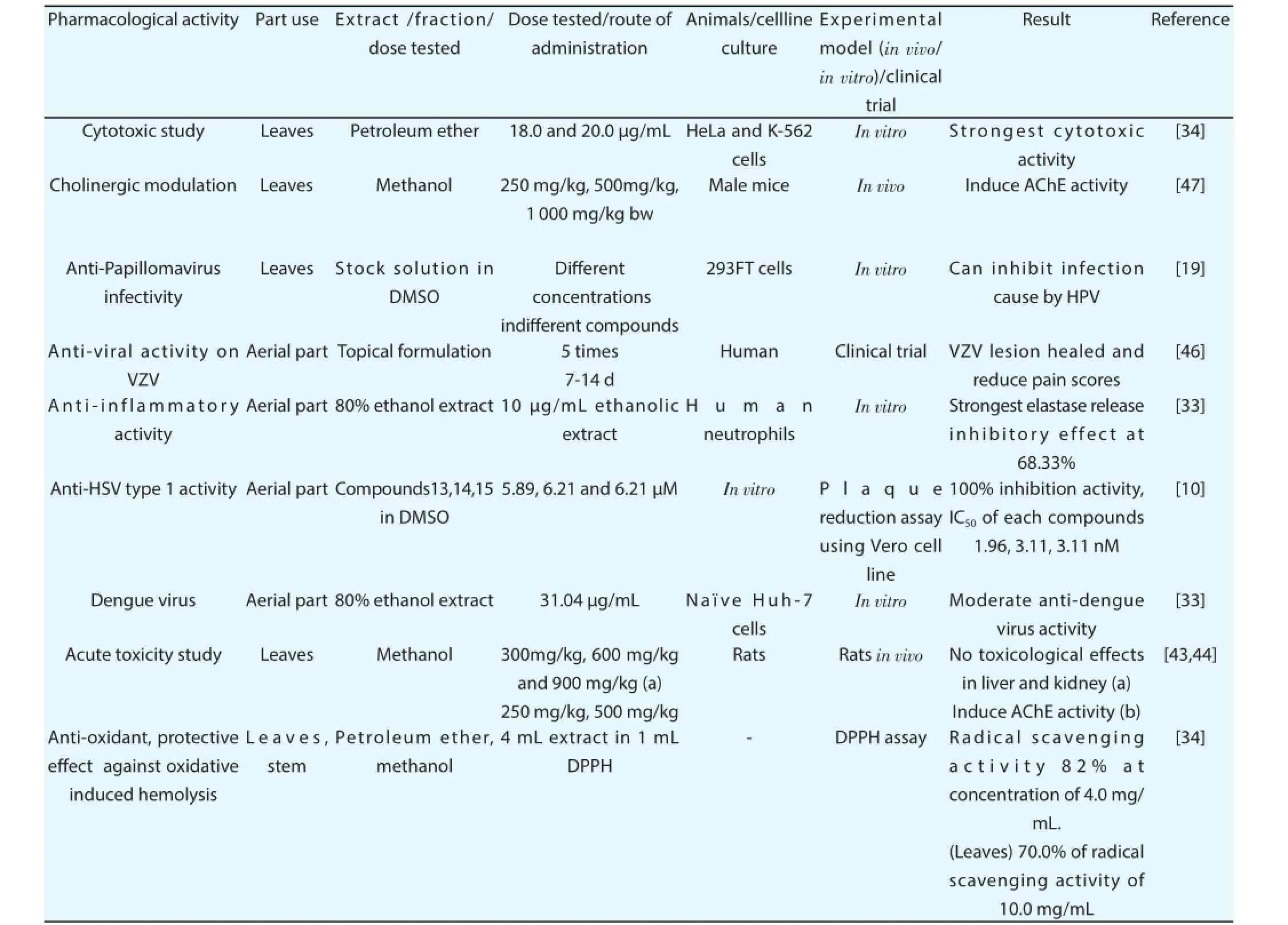
Table 1Pharmacological eff ects of C. nutans.
Methanol extract of the leaves were tested by topical application at the dosage of 3, 6, 9 mg/20 μL acetone on edema experimentally ethyl phenylpropiolate-induced rat. The extract at all concentrations showed the most potent inhibition at 15 min underlying prevention of the release and/or the effect of histamine and serotonin considered as initial infl ammatory mediators of this pathway[6]. Oral administration of methanolic extracts at the dosages of 50 100 and 200 mg/kg on carrageenan-induced edema in the hind paw ofmale Sprague-Dawley rats was observed. An equivalent volume of 0.5 mL/kg was administered 1 h before the animals were induced edema on the plantar side of the right hind paw by intradermal injection of carrageenan and results showed signifi cant reduction of foot volume by volume displacement technique[6]. Methanolic extracts of C. nutans were tested in four cancer cell lines NCI-23, HeLa, K-562,and Raji and no anti-proliferative activity was found. Whereas mild anti-proliferative activity found in IMR32, SNU-1, and LS-174T cell lines. A dosage of 100 μg/mL exhibited (41.88 ± 2.81)% inhibitions in HepG2 cell lines at 100 μg/mL. The dosage at a concentration of 100 μg/mL was tested onumbilical vein endothelial cells and lower percentage of inhibition found compared to the other cancer cells[35]. Treatment of HepA tumor-bearing mouse models with 30% ethanolic extract of aerial part (3 and 10 mg/kg) exhibited antitumor activity compared to the fl uorouracil-treated mice as positive control. The same study reported that upsurge of thymus indices, IL-2 and IFN-γ levels in serum which indicate the potential antitumor and immunomodulatory properties of C. nutans[36].
Phytosterols are soluble in polar and dipolar solvents while phytostanols, a derivative of phytosterols that are present in nonpolar solvent extraction like petroleum ether. In this plant, some of the phytosterols present are stigmasterol, lupeol and β-sitosterol. Phytosterols in combination with other bioactive compounds are able to exert cytotoxic eff ect against cancer cell lines[37].
5.2. Antioxidant activity
Petroleum ether (82.0%) extract of whole plant strongly scavenged 1, 1-diphenyl-2-picrylhydrazyl radical (DPPH) with a concentration of 4.0 mg/mL while methanolic extract of stem produced 70.0% radical scavenging activity at 10.0 mg/mL concentration. The crude ethanol extract within 1-300 μg/mL concentration demonstrated highest scavenging activity of (67.65±6.59)% and with IC50of (110.4±6.59) μg/mL[38]. The radical scavenging activity of chloroform, methanol and water extracts from leaves were examined using Trolox as a standard and highest antioxidant activity foundfor chloroform extract (7 852.63 ± 449.90) μg Troloxeq/g extract and the antioxidant activity declined in case of methanol and water extract[35]. Moreover nitric oxide scavenging activity observed only in water extract to the extent (32.33 ± 0.97)% using 100 μg/mL dosage and a relatively mild hydrogen peroxide scavenging activities recorded for chloroform, methanol and water extract[35]. The presence of alkaloids, fl avonoids and fl avones may be also a possible reason for higher radical scavenging activity[39].
5.3. Immune response activity
The eff ect of ethanolic extracts of leaves on modulating in vitro cell-mediated immune response was studied by observing human competent cells obtained from healthy human with no previous history of immune related complications and none were taking immunosuppressive drugs. Concentrations at a level of 0.5, 2.5, and 5.0 μg/mL could induce remarkable proliferation of lymphocytes but drastically decrease at 2.5 and 5.0 mg/mL of extracts. The activity of natural killer cells considerably decreased at the concentrations of 1 and 5 mg/mL but the level of interleukin-2 formation extract treated mononuclear cells was unobservable. In case of interleukin-4 it was induced by the extract to the concentration of 2.5 and 5.0 mg/mL[40].
5.4. Anti-herpes simplex viral activity assay
The activity of hexane, dichloromethane, and methanol extracts of leaves were examined on HSV type 1 (KOS), type 2 (Baylor 186)and Vero cells by plaque reduction assay. Every extracts showed HSV-1 and HSV-2 activities with more than 50% inhibition of plaque formation (30 PFU/25 μL) at a concentration of 100 μg/mL. Hexane extract showed moderate inhibition with lowest IC50values of HSV-1with selectivity index >50.36 and (65.13±2.22) μg/mL of methanol extract that inhibited HSV-2 with selectivity index >24.59. Three different bioactive compounds isolated from C. nutans(compounds 13, 14, 15) and their anti-herpes simplex viral activity was examined. For doing anti-HSV-1F activity, the cytotoxicity of all the compounds were investigated and found that 5.89, 6.21 and 6.21 μM of compound 1, 2, 3, respectively were the maximal concentration which were not toxic to Vero cells. The sub-toxic concentrations of each compound were used in anti-HSV-1F study and the concentration of 1, 2 3 compounds exhibited 100% inhibition activity and IC50of those compounds were 1.96, 3.11 and 3.11 nM,respectively. The experiment also assumed that the compounds 1-3 may interfere with the virion envelope structures or mask viral glycoproteins, which are necessary for adsorption and entry into host cell[10].
5.5. Antimicrobial activity
Ethyl acetate fractions from leaves of this plant were tested against Bacillus cereus, Escherichia coli, Salmonella enterica Typhimurium and Candida albicans using minimum inhibitory concentration and minimum bactericidal or fungicidal assays. The fractions and crude extracts demonstrated inhibition against all tested microorganisms within the range between 1.39 mg/mL and 6.31 mg/mL against Bacillus cereus and Candida albicans. Flavonoids and phenolic compounds that are synthesized universally in medicinal plants can induce antibacterial response due to the presence of carbonyl group[41].
5.6. Antivenom activity
This plant has been traditionally used for a long time as a remedy for envenomation snakes or venomous insects like scorpions and bees especially in the southern Thailand and North-Western Malaysia. There was no antivenin activity found by analyzing the ability of the extract to defuse the inhibitory eff ects of neurotoxins of Naja naja siamensis on neuromuscular transmission[42]. A report stated by Watson in botanical medicine that there is other components in the venom of the snake which can be neutralized by the plant extract[20].
5.7. Anti-dengue activity
The ethanolic extract from aerial part of the C. nutans showed moderate anti-dengue virus activity in the IC5031.04 μg/mL. Naïve Huh-7 cells and cultured in Dulbecco’s modifi ed Eagle’s medium were used in that study[33].
6. Toxicity
The Sprague Dawley female rats were subjected to 14 d oral administration of methanol leaves extract of C. nutans at a dose of 300 mg/kg, 600 mg/kg and 900 mg/kg and the result showed no toxicological eff ect on the liver and kidney that causes injury[43]. The eff ect of methanol extract of this plant on acetylcholinesterase enzyme activity which is responsible for terminating the cholinergic nerve transmission by hydrolyzing the acetylcholine into choline and acetatewas elucidated[44]. The experiment discovered that administration of 250 mg/kg, 500 mg/kg and 1 000 mg/kg dose of methanol leaves extract of C. nutans on the activity of AChE in Balb/C male was able to modulate cholinergic neurotransmission by activating AChE activity in mice kidney, liver and heart[44]. It was reported that ethanolic extract of C. nutans leaves at the highest dose of 1.3 g/kg given orally, subcutaneously or intraperitoneally did not produce any signs of acute toxicity in mice[45].
7. Clinical trial
7.1. Treatment of recurrent aphthous ulcer
A double blind controlled trial was conducted to assess the effi cacy of C. nutans in orabase for the treatment of recurrent aphthous stomatitis. Total forty-three subjects with aphthous stomatitis complain were hired for the trial and the eff ect of extract tested against triamcinolone acetonide in orabase and placebo. The result showed that C. nutans in orabase provide better healing of the ulcer as compared to placebo but was less so when compared totriamcinolone acetonide in orabase[46].
7.2. Anti-VZV infection
A topical formulation of C. nutans extract was prepared and its effect on 51 patients with VZV infection examined through randomized, placebo-controlled trial. The result was promising crusting on lesion formed within 3 d and healing process occurred within 7 d. The patients were treated 5 times with the preparations per day for 7-14 d until lesion healed and the pain scores also reduced signifi cantly. There were no side eff ects detected during the progress of treatment[46].
8. Conclusion
C. nutans has been broadaly used as traditional medicine in several countries in Asia. All parts of this plant have been used in the treatment and prevention of several coplications especially for viral infection, cancer and skin infl ammation caused by insect bites. Flavonoids are the main bioactive compounds in this plant and diff erent extracts have been found to posses biological activity. Less toxicity of this plant represent the possible uses as therapeutic remedy for several ailments.
This review has presented a comprehensive view about the phytochemistry and pharmacology of C. nutans. However the research is very limited in some areas and futher study on phytochemicals and their mode of actions revealing pharmacological eff ects are required to fully understand in concern with the traditional uses. In addition majority of medicinal studies were conducted using crude and poorly other solvent extracts. In such case more bioactive compounds should be identifi ed through bioassay guided isolation. More clinical studies on the toxicity of extracts from diff erent parts and the isolated compounds from this plant need to be assessed for ensuring the safe application as modern medicines.
Conflict of interest statement
We declare that we have no confl ict of interest.
Acknowledgements
The authors would like to acknowledge Kulliyyah of Pharmacy,International Islamic University Malaysia and the visiting professor program at King Saud University, Riyadh, Saudi Arabia, for supporting this study.
[1] Sharma SK. Plant taxonomy. 2nd ed. New Delhi: Pacific Book International; 2011. p. 414.
[2] Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JK,Tannenbaum SR. Analysis of nitrate, nitrite and [15N]nitrate in biological fl uids. Anal Biochem 1982; 126(1): 131-138.
[3] Meyer JY, Lavergne C. Beautés fatales: Acanthaceae species as invasive alien plants on tropical Indo-Pacific Islands. Diversity Distrib 2004;10(5-6): 333-347.
[4] Putwatana P, Sanmanowong P, Oonprasertpong L, Junda T, Pitiporn S,NarkwongL. Relief of radiation-induced oral mucositis in head and neck cancer. Cancer Nurs 2009; 32(1): 82-87.
[5] Yong YK, Tan JJ, Teh SS, Mah SH, Ee GCL, Chiong HS, et al. Clinacanthus nutans-extracts are antioxidant with antiproliferative eff ect on cultured human cancer cell lines. J Evid Based Complementry Altern Med 2013; 2013: 462751.
[6] Wanikiat P, Panthong A, Sujayanon P, Yoosook C, Rossi AG, Reutrakul V. The anti-inflammatory effects and the inhibition of neutrophil responsiveness by Barlerialupulina and Clinacanthus nutans extracts. J Ethnopharmacol 2008; 116(2): 234-244.
[7] Kunsorn P, Ruangrungsi N, Lipipun V, Khanboon A, Rungsihirunrat K. The identities and anti-herpes simplex virus activity of Clinacanthus nutans and Clinacanthus siamensis. Asian Pac J Trop Biomed 2013; 3(4): 284-290.
[8] Tuntiwachwuttikul P, Pootaeng-on Y, Pansa P, Srisanpang T, Taylor WC. Sulfur-containing compounds from Clinacanthus siamensis. Chem Pharm Bulle 2003; 51(12): 1423-1425.
[9] Sakdarat S, Shuyprom A, Ayudhya TDN, Waterman PG, Karagianis G. Chemical composition investigation of Clinacanthus nutans Lindau leaves. Thai J Phytopharmacol 2006; 13(2): 13-24.
[10] Sakdarat S, Shuyprom A, Pientong C, Ekalaksananan T, Thongchai S. Bioactive constituents from the leaves of Clinacanthus nutans Lindau. Bioorgan Med Chem 2009; 17(5): 1857-1860.
[11] Sangkitpporn S, Polchan K, Thawatsupa P, Bunchob M, Chawalitumrong P. Treatment of recurrent genital herpes simplex virus infection with Clinacanthus nutans extract. Bulle Depart Medical Serv 1993; 18(5): 226-231.
[12] Global access to knowledge about life on Earth 1994. [Online]. Available from: http://www.eol.org [Accessed on 15 Jan2014].
[13] Ailiah MR. Rawatan Alternatif moden bagi penyakit kanser 2011.[Online]. Available from:http://b17nitrilosides.blogspot.com/2011/12/ sabah-snake-grass-extract.html?m=0 [Accessed on Dec 2011].
[14] Floral of China Editorial Committee. Flora of China, Vol.19,Curcurbitaceae through Valerianaceae with Annonaceae and Berberidaceae. Beijing: Science Press & Missouri Botanical Garden Press and St. Louis;2011. p. 1-884
[15] Fong SY, Piva T, Urban S, Huynh T. Genetic homogeneity of vegetatively propagated Clinacanthus nutans (Acanthaceae). J Med Plants Res 2014;8(25): 903-914.
[16] South China botanical garden: Clinacanthus nutans (Burm.) Lindau 2008.[Online]. Available from: http://www.efl oras.org/fl orataxon.aspx?fl ora_ id=610&taxon_id=200021997 [Accessed on 22 February 2008].
[17] Deng Y, Hu J, Daniel TF, Wood J, Wood JRI. Acanthaceae. In: Wu ZY,Raven PH, Hong DY, editors. Floral of China, Vol. 19, Cucurbitaceae-Valerianaceae with Annonaceae and Berberidaceae. Beijing: Science Press & Missouri Botanical Garden Press and St. Louis; 2011. p. 369-477.
[18] Panyakom K. Strcutural elucidation of bioactive compounds of Clinacanthus nutans (Burm. F.) Lindau leaves. Thailand: Suranaree University of Technology; 2012.
[19] Sookmai W, Ekalaksananan T, Pientong C, Sakdarat S, Kongyingyoes B. The anti-papillomavirus infectivity of Clinacanthus nutans compounds. Srinagarind Med J 2011; 26: 240-243.
[20] Watson RR, Preedy VR. Botabical medicine in clinical practice. Cambridge: CAB International Cambridge; 2008. p. 819.
[21] Andrea P, Vanderbroek I. The ethnobiology and ethnopharmacy of migrations. New York: Berghahn Books; 2007. p. 112.
[22] Charuwichitratana S, Wongrattanapasson N, Timpatanapong P, Bunjob M. Herpes zoster: treatment with Clinacanthus nutans cream. Int J Dermatol 1996; 35: 665-666.
[23] Dampawan P. Studies of the chemical constituents of Clinacanthus nutans(Acanthaceae) and Zingiber cassumunar Roxb [Master thesis]. Thailand: Mahidol University; 1976.
[24] Lin J, Li HM, Yu JG. Studies on the chemical constituents of niu xu hua(Clinacanthus nutans). Zhongcaoyao 1983; 14: 337-338.
[25] Boongerd K. The chemical constituents of Clinacanthus burmanii [M.Sc. Thesis]. Thailand: Chulalongkorn University; 1967.
[26] Dampawan P, Huntrakul C, Reutrakul V, Raston CL, White AH. Constituents of Clinacanthus-nutans and crystal-structure of Lup-20(29)-Ene-3-One. J Sci Soc Thailand 1977; 3(1): 14-26.
[27] Teshima KI, Kaneko T, Ohtani K, Kasai R, Lhieochaiphant S,Picheansoonthon C, et al. Sulfur-containing glucosides from Clinacanthus nutans. Phytochem 1998; 48(5): 831-835.
[28] Tuntiwachwuttikul P, Pootaeng-On Y, Phansa P, Taylor WC. Cerebrosides and a monoacylmonogalactosylglycerol from Clinacanthus nutans. Chem Pharm Bull (Tokyo) 2004; 52(1): 27-32.
[29] Ayudhya TDN, Sakdarat S,Shuyprom A, Du an gpen Pattaraadilok JB,Waterman PG, Karagianis G. Chemical constituents of the leaves of Clinacanthus nutans Lin-dau. Thai J Phytopharm 2001; 8(1): 1.
[30] Janwitayanuchit W, Suwanborirux K, Patarapanich C, Pummangura S,Lipipun V, Vilaivan T. Synthesis and anti-herpes simplex viral activity of monoglycosyl diglycerides. Phytochem 2003; 64(7): 1253-1264.
[31] Ikegami F, ShibasakiI, Ohmiya S, Ruangrungsi N, Murakoshi I. Entadamide A, a new sulphur-containing amide from Entada phaseoloides seeds. Chem Pharm Bull 1985; 33(11): 5153-5154.
[32] Ikegami F, Sekine T, Duangteraprecha S, Matsushita N, Matsuda N,Ruangrungsi N, et al. Entadamide C, a sulphur-containing amide from Entada phaseoloides. Phytochem 1989; 28(3): 881-882.
[33] Tu SF, Liu RH, Cheng YB, Hsu YM, Du YC, El-Shazly M, et al. Chemical constituents and bioactivities of Clinacanthus nutans aerial parts. Molecules 2014; 19(12): 20382-20390.
[34] Arullappan S, Rajamanickam P, Thevar N, Kodimani CC. In vitro screening of cytotoxic, antimicrobial and antioxidant activities of Clinacanthus nutans (Acanthaceae) leaf extracts. Trop J Pharm Res 2014;13(9): 1455-1461.
[35] Yong YK, Tan JJ, Teh SS, Mah SH, Ee GCL, Chiong HS, et al. Clinacanthus nutans extracts are antioxidant with antiproliferative eff ect on cultured human cancer celllines. Evid Based Complement Alternat Med 2013; 2013: 462751.
[36] Huang D, Guo W, Gao J, Chen J, Olatunji JO. Clinacanthus nutans(Burm. f.) Lindau ethanol extract inhibits hepatoma in mice through upregulation of the immune response. Molecules 2015; 20(9): 17405-17428.
[37] De Brabander HF, Verheyden K, Mortier V, Le Bizec B, Verbeke W,Courtheyn D, et al. Phytosterols and anabolic agents versus designer drugs. Anal Chim Acta 2007; 586(1-2): 49-56.
[38] Pannangpetch P, Laupattarakasem P, Kukongviriyapan V,Kukongviriyapan U, Kongyingyoes B, Aromdee C. Antioxidant activity and protective eff ect against oxidative hemolysis of Clinacanthus nutans(Burm.f) Lindau. J Sci Technol 2007; 29(Suppl 1): 1-9.
[39] Teshima K, Kaneko T, Ohtani K, Kasai R, Lhieochaiphant S,Picheansoonthon C, et al. C-glycosyl fl avones from Clinacanthus nutans. Natural Med 1997; 51(6): 557.
[40] Sriwanthana B, Chavalittumrong P, Chompuk L. Eff ect of Clinacanthus nutans on human cell-mediated immune response in vitro. Thai J Pharm Sci 1996; 20(4): 261-267.
[41] Rathee P, ChaudharyH, Rathee S, Rathee D, Kumar V, Kohli K. Mechanism of action of fl avonoids as antiinfl ammatory agents. Inflamm Allergy Drug Targets 2009; 8(3): 229-235.
[42] Cherdchu C, Poopyruchpong N, Adchariyasucha R, Ratanabanangkoon K. The absence of antagonism between extracts of Clinacanthus nutans Burm. and Naja naja siamensis venom. Southeast Asian J Trop Med Public Health 1977; 8(2): 249-254.
[43] P'ng XW, Akowuah GA, Chin JH. Evaluation of the sub-acute oral toxic eff ect of methanol extract of Clinacanthus nutans leaves in rats. J Acute Dis 2013; 2(1): 29-32.
[44] Zhang XJ,Yang L, Zhao Q, Caen JP, He HY, Jin QH, et al. Induction of acetylcholinesterase expression during apoptosis in various cell types. Cell Death Differ 2002; 9(8): 790-800.
[45] Chavalittumrong P, Attawish, A, Rugsamon P, Chuntapet P. Toxicological study of Clinacanthus nutans (Burm. f.) Lindau. Bull Dep Med Sci 2013;37(4): 323-338.
[46] Siriporn Timpawat LoV. Clinical evaluation of Clinacanthus nutans Lindau in orabase in the treatment of recurrent aphthous stomatitis. Mahidol Dental J 2013; 14(1): 10-16.
[47] Lau KW, Lee SK, Chin JH. Effect of the methanol leaves extract of Clinacanthus nutans on the activity of acetylcholinesterase in male mice. J Acute Dis 2014; 3(1): 22-25.
ent heading
10.1016/j.apjtm.2016.03.011
15 January 2016
Md. Zaidul I. Sarker and Sahena Ferdosh, International Islamic University Malaysia (IIUM), Kuantan Campus, 25200 Kuantan, Pahang,Malaysia.
E-mail: zaidul@iium.edu.my; sahena@iium.edu.my
Tel: +609 5704841
Fax: +6095716775
in revised form 20 February 2016
ARTICLE INFO
Article history:
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Determination of ligand cluster and binding site within VP40 of Ebola virus: clue for drug development
- Current perspectives on dengue episode in Malaysia
- Etiological agents causing leptospirosis in Sri Lanka: A review
- Phylogeny of Murray Valley encephalitis virus in Australia and Papua New Guinea
- Dengue outbreak in Swat and Mansehra, Pakistan 2013; an epidemiological and diagnostic perspective
- Bioactive extracts of red seaweeds Pterocladiella capillacea and Osmundaria obtusiloba (Floridophyceae: Rhodophyta) with antioxidant and bacterial agglutination potential
