Bioactive extracts of red seaweeds Pterocladiella capillacea and Osmundaria obtusiloba (Floridophyceae: Rhodophyta) with antioxidant and bacterial agglutination potential
2016-11-14DanielBarrosodeAlencartimaCristianeTelesdeCarvalhoRosaHelenaRebouasDanielRodriguesdosSantosKelmaMariadosSantosPiresCavalcanteRebecaLarangeiradeLimarbaraMendesBarachoRayssaMendesBezerraFranciscoArnaldoVianaRegineHelena
Daniel Barroso de Alencar, Fátima Cristiane Teles de Carvalho, Rosa Helena Rebouças, Daniel Rodrigues dos Santos, Kelma Maria dos Santos Pires-Cavalcante, Rebeca Larangeira de Lima,Bárbara Mendes Baracho, Rayssa Mendes Bezerra, Francisco Arnaldo Viana, Regine Helena Silva dos Fernandes Vieira, Alexandre Holanda Sampaio, Oscarina Viana de Sousa, Silvana Saker-Sampaio
1Laboratório de Produtos Naturais Marinhos, Departamento de Engenharia de Pesca, Universidade Federal do Ceará, Campus do Pici, Av. Mister Hull, s/n, Caixa Postal 6043, 60455-970 Fortaleza, CE, Brazil
2Laboratório de Microbiologia Ambiental e do Pescado, Instituto de Ciências do Mar, Universidade Federal do Ceará, Av. da Abolição 3207, 60165-081, Fortaleza, CE, Brazil
3Laboratório de Cromatografia, Departamento de Química, Universidade do Estado do Rio Grande do Norte, Campus Universitário Central, Setor Ⅲ,Rua Prof. Antônio Campos, 59633-010, Mossoró, RN, Brazil
Bioactive extracts of red seaweeds Pterocladiella capillacea and Osmundaria obtusiloba (Floridophyceae: Rhodophyta) with antioxidant and bacterial agglutination potential
Daniel Barroso de Alencar1*, Fátima Cristiane Teles de Carvalho2, Rosa Helena Rebouças2, Daniel Rodrigues dos Santos2, Kelma Maria dos Santos Pires-Cavalcante1, Rebeca Larangeira de Lima1,Bárbara Mendes Baracho1, Rayssa Mendes Bezerra1, Francisco Arnaldo Viana3, Regine Helena Silva dos Fernandes Vieira2, Alexandre Holanda Sampaio1, Oscarina Viana de Sousa2, Silvana Saker-Sampaio1
1Laboratório de Produtos Naturais Marinhos, Departamento de Engenharia de Pesca, Universidade Federal do Ceará, Campus do Pici, Av. Mister Hull, s/n, Caixa Postal 6043, 60455-970 Fortaleza, CE, Brazil
2Laboratório de Microbiologia Ambiental e do Pescado, Instituto de Ciências do Mar, Universidade Federal do Ceará, Av. da Abolição 3207, 60165-081, Fortaleza, CE, Brazil
3Laboratório de Cromatografia, Departamento de Química, Universidade do Estado do Rio Grande do Norte, Campus Universitário Central, Setor Ⅲ,Rua Prof. Antônio Campos, 59633-010, Mossoró, RN, Brazil
Accepted 15 March 2016
Available online 20 April 2016
Bioactive compounds
Antioxidant
Bacterial agglutination
Rhodophyta
Objective: To evaluate the antioxidant, antibacterial and bacterial cell agglutination activities of the hexane (Hex) and 70% ethanol (70% EtOH) extracts of two species of red seaweeds Pterocladiella capillacea (P. capillacea) and Osmundaria obtusiloba. Methods: In vitro antioxidant activity was determined by DPPH radical scavenging assay, ferric-reducing antioxidant power assay, ferrous ion chelating assay, β-carotene bleaching assay and total phenolic content quantifi cation. Antimicrobial activity was tested using the method of disc diff usion on Mueller-Hinton medium. The ability of algal extracts to agglutinate bacterial cells was also tested. Results: The 70% EtOH extract of the two algae showed the highest values of total phenolic content compared to the Hex extract. The results of DPPH for both extracts(Hex, 70% EtOH) of Osmundaria obtusiloba (43.46% and 99.47%) were higher than those of P. capillacea (33.04% and 40.81%) at a concentration of 1 000 μg/mL. As for the ferrous ion chelating, there was an opposite behavior, extracts of P. capillacea had a higher activity. The extracts showed a low ferric-reducing antioxidant power, with optical density ranging from 0.054 to 0.180. Antioxidant activities of all extracts evaluated for β-carotene bleaching were above 40%. There was no antibacterial activity against bacterial strains tested. However, the extracts of both species were able to agglutinate bacterial Gram positive cells of Staphylococcus aureus and Gram negative cells of Escherichia coli, multidrug-resistant Salmonella and Vibrio harveyi. Conclusions: This is the fi rst report of the interaction between these algal extracts,rich in natural compounds with antioxidant potential, and Gram positive and Gram negative bacterial cells.
1. Introduction
Seaweeds are renewable natural resources with great potential for application in the pharmaceutical, food, nutraceutical and cosmetics industries. These plants are rich in bioactive compounds and watersoluble (B-complex and C) and fat-soluble (provitamin A and vitamins D, E and K), dietary fibers, proteins, polyunsaturated fatty acids and minerals, such as calcium, magnesium, phosphorus,potassium, sodium, iron and iodine[1-5].
Red marine algae are macroscopic, multicellular, benthic organisms, commonly marketed as food in Asian countries in the form of spices, condiments, pasta, and also employed in the phycocolloids industry (agar, carrageenan) as a food additive acting as gelling agents, emulsifi ers, in water retention and other physical properties[6].
In the metabolism of a living organism, reactive oxygen species(ROS) are normally produced and eliminated by enzymatic and non-enzymatic defense systems. However, under stressful conditions, defense systems can fail, and the accumulation of ROS and other free radicals can cause irreversible damage to proteins,amino acids, lipids and DNA. Thus, oxidative stress has been associated with several diseases such as cancer, hypertension,diabetes, atherosclerosis, neurological disorders and infl ammatory disorders[7-9].
In addition to the damage to cellular components, ROS can also promote the degradation of oils and fats present in foods, leading to the appearance of odors and rancid fl avor, which contributes to decrease the quality and nutritional security, in view of the formation of potentially toxic secondary metabolites[8,10].
Dietary antioxidants are added to food to slow down the oxidative deterioration[8] and widely used in the prevention of chronic diseases[11]. Nevertheless, consumers are demanding new alternative natural antioxidants obtained from plant sources to replace the synthetic antioxidants currently used, such as butylhydroxyanisole(BHA), butylhydroxytoluene and tert butylhydroquinone, which could be toxic and exert carcinogenic eff ects[12].
During the life cycle of seaweeds, they are exposed to high concentrations of oxygen and light intensity, and this combination favors the formation of free radicals and other oxidizing agents. This suggests that the absence of oxidative damage to structural components of algae and their stability against adverse conditions is related to the presence of antioxidants[6,13,14].
The Brazilian coast has an extensive coastline with great biodiversity of marine organisms yet to be explored. Red seaweeds Pterocladiella capillacea (P. capillacea) and Osmundaria obtusiloba (O. obtusiloba) are abundant in the coast of the state of Ceará throughout the year. Although there are several studies on biological activity of extracts of seaweeds in recent decades, there is no information on the antioxidant, antimicrobial and bacterial agglutination potential of these species of the coast of the state of Ceará.
Considering the above, the purpose of this study was to evaluate the antioxidant, antimicrobial and bacterial agglutination activity of the hexane (Hex) and 70% ethanol (70% EtOH) extracts of the red seaweeds P. capillacea and O. obtusiloba.
2. Material and methods
2.1. Collection of seaweeds and species identification
Specimens of the red seaweed P. capillacea (S. G. Gmelin) Santelices & Hommersand were collected in March 2008, at Pacheco Beach, municipality of Caucaia, State of Ceará. Individuals of the red seaweed O. obtusiloba (C. Agardh) R. E. Norris were collected in September 2010, at Paracuru Beach, São Gonçalo do Amarante,State of Ceará. Samples were taken under low tide conditions,with the permission of the Brazilian Institute of Environment and Renewable Natural Resources (SISBIO 33913-1).
The collected algae were taken to the laboratory. Washed with distilled water to remove impurities and macroscopic epiphytes and then placed on absorbent paper to remove excess water and frozen at -24 ℃ until analysis.
Species were identified by Professor Kelma Maria dos Santos Pires-Cavalcante of the Department of Fisheries Engineering,Federal University of Ceará. The voucher specimens of P. capillacea and O. obtusiloba were deposited in the Herbarium Prisco Bezerra,Department of Biology at the same university, under the numbers 447310 and 56432, respectively.
2.2. Preparation of extracts
Fresh algae were dried in a recirculating air oven at 40 ℃ for 15 h, and then ground. Portions of dehydrated P. capillacea (134 g)and O. obtusiloba (120 g) were subjected, separately, to exhaustive extraction with cold hexane and then with 70% EtOH. The Hex and 70% EtOH extracts were concentrated in rotary evaporator.
2.3. Quantification of total phenolic content (TPC)
TPC was estimated by the Folin-Ciocalteu method described by Kumar et al[15]. To 200 μL of algal extracts at 1 000 μg/mL were added 1.4 mL distilled water, 100 μL Folin-Ciocalteu reagent and 300 μL sodium carbonate (20%). After 30 min incubation at room temperature in the dark, absorbance was read at 760 nm in a microplate reader (Biochrom Asys UVM 340).
TPC was calculated based on the standard curve of gallic acid at a concentration range of (0.005-0.500) mg/mL, and the results are expressed in milligrams (mg) of gallic acid equivalent/g extract.
2.4. DPPH radical scavenging capacity
DPPH radical scavenging capacity was determined according to Blois[16]. The sample consisted of a mixture of 0.5 mL aliquot of algal extracts at diff erent concentrations (7.81 to 1 000 μg/mL) and 2.5 mL methanol solution of DPPH at 75 μM. In the blank sample,the methanol solution of DPPH was replaced with methanol, and in the control, were used only 3 mL methanol solution of DPPH at 75 μM. The tubes (sample, blank sample and control) were incubated at room temperature in the dark for 30 min and the absorbance wasread at 517 nm in a microplate reader (Biochrom Asys UVM 340). Ascorbic acid was used as positive control at the same concentrations of algal extracts. The DPPH radical scavenging percentage was calculated according to equation 1.
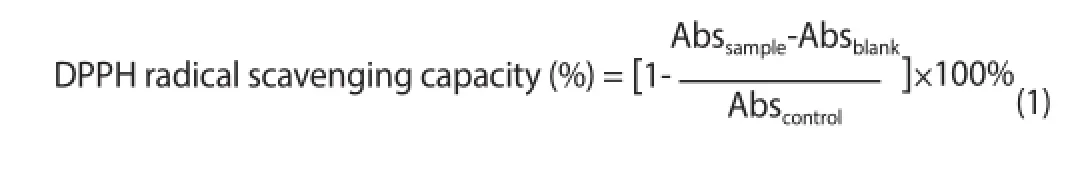
2.5. Ferrous ion chelating ability (FIC)
The FIC was determined according to the method of Wang et al[17]. The sample consisted of 1 mL of algal extracts at different concentrations (7.81 to 1 000 μg/L), 1.35 mL distilled water, 50 μL ferrous chloride (2 mM) and 100 μL ferrozine (5 mM). In the blank sample, 100 μL distilled water replaced ferrozine, while in the control, 1mL water was used instead of the algal extract. Sample,blank sample and control were incubated at room temperature for 10 min, and the absorbance was read at 562 nm in a microplate reader (Biochrom Asys UVM 340). The ethylenediaminetetraacetic acid was used as a positive control at the same concentrations of algal extracts. The percentage of ferrous ion chelating ability was calculated according to the equation 2.
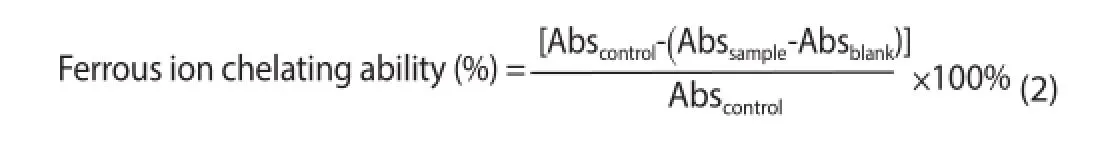
2.6. Ferric ion reducing power (FRAP)
FRAP was determined by the method described by Ganesan et al[18]. To 1 mL algal extracts at different concentrations (7.81 to 1 000 μg/L) were added 2.5 mL phosphate buff er (0.2 M, pH 6.6) and 2.5 mL potassium ferricyanide (1%). This mixture was incubated at 50 ℃ for 20 min, cooled in ice-water and then added of 2.5 mL trichloroacetic acid (10%). After stirring, 2.5 mL were taken and mixed with 2.5 mL distilled water and 0.5 mL ferric chloride (0.1%). After 10 min incubation at room temperature, the absorbance was read at 700 nm in a microplate reader (Biochrom Asys UVM 340). The BHA was used as a positive control at the same concentrations of algal extracts. The increase in absorbance indicated increase of FRAP, that is, the higher the absorbance, the greater the FRAP.
2.7. Degradation of β-carotene (BCB)
Degradation of β-carotene was determined by the method of Chew et al[19], with minor modifi cations. There were added 2.5 mg β-carotene and 40 mg linoleic acid, both solubilized in chloroform,to 400 mg Tween 40 emulsifier. In a rotary evaporator, the chloroform was evaporated and 100 mL oxygen-saturated ultrapure water (Milli-Q) was added. Then, the mixture was vigorously stirred to form an emulsion; 3 mL of this emulsion was mixed with 1 mL algal extracts at diff erent concentrations (7.81 to 1 000 μg/mL) and the initial absorbance was read at 470 nm. The tubes were incubated at 50 ℃ for 3 h and, after that, the absorbance was read again at the same wavelength; the two readings were made in a microplate reader(Biochrom Asys UVM 340). The BHA was used as positive control at the same concentrations of algal extracts. The antioxidant activity was calculated according to equation 3.
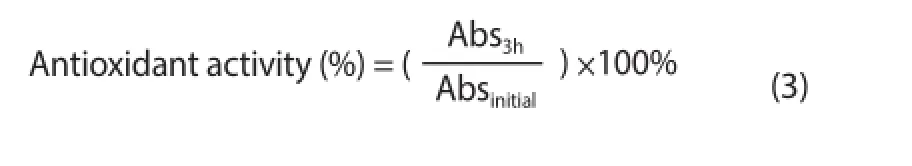
2.8. Antibacterial activity
The antibacterial activity of algal extracts was determined by antibiogram test using the well diffusion method, following recommendations of the Clinical and Laboratory Standard Institute[20]. In the tests, there were used standard strains of Escherichia coli (E. coli) ATCC 25922, Staphylococcus aureus (S. aureus) ATCC 25923 e Pseudomonas aeruginosa ATCC 27853. The inocula were prepared respectively in test tubes containing 0.85% NaCl saline solution and turbidity adjusted according to 0.5 McFarland scale in a spectrophotometer. Inocula were uniformly distributed on the surface of Petri dishes containing Muller-Hinton agar (Difco) with sterile swabs, and the wells were drilled after absorption of the inoculum in the culture medium. The algal extracts were grouped according to the solvent used for preparation and the diluents was used as a negative control. Each well received 50 μL algal extract at 1 000 μg/mL and, then, the plates were incubated at 35 ℃ for 24 h. The procedure was performed in duplicate.
2.9. Bacterial agglutination assay
The ability of algal extracts to agglutinate bacterial cells was tested according to the method described by Imamichi and Yokoyama[21],using as indicator strains E. coli (ATCC 25922), multidrug-resistant E. coli (Lamap collection) S. aureus (ATCC 25923), multidrugresistant Salmonella Infante (Lamap collection) and Vibrio harveyi(Lamap collection). Bacterial cultures were grown at 35 ℃ for 24 h and collected by centrifugation at 1 000 × g for 10 min. Cells were washed three times with saline phosphate buff er pH 7.2 and fi xed in the same buff er added with 4% formaldehyde and maintained at 4℃ for 16 h. Once fi xed, the bacteria were washed again with Tris-HCl buff er pH 7.5 and diluted to obtain at 600 nm an optical density corresponding to 0.5 McFarland scale. The bacterial inoculum was mixed with an equal volume (50 μL) of algal extract (1 000 μg/ mL) on a slide and kept at room temperature for 2 h. The negative control was done using the bacterial suspension in distilled water.Agglutination was analyzed by optical microscopy (Olympus CBB).
2.10. Statistical analysis
Results of CFT of extracts of the seaweed species P. capillacea and O. obtusiloba were subjected to one-way analysis of variance followed by Tukey’s test (P < 0.05).
To investigate the correlation between CFT and antioxidant activities of algal extracts, we used the Pearson correlation coefficient, where the CFT was considered the independent variable (x) and each In vitro antioxidant methodology, the dependent variable (y).
3. Results
3.1. TPC
Quantitation of TPC in the Hex and 70% EtOH extracts in the two species of red seaweeds P. capillacea and O. obtusiloba was based on the standard curve of gallic acid. The correlation (r = 0.999 2, P <0.05) between the absorbance (760 nm) and concentrations, varying in the range of (0.005-0.500) mg/mL, enabled its quantifi cation (y = 0.075 5 + 4.896x, n = 8). TPC values of 70% EtOH extracts of P. capillacea and O. obtusiloba were 1.48 and 2.55 higher than the Hex extracts, respectively. Extracts of O. obtusiloba showed CFT values signifi cantly higher than P. capillacea (Figure 1).
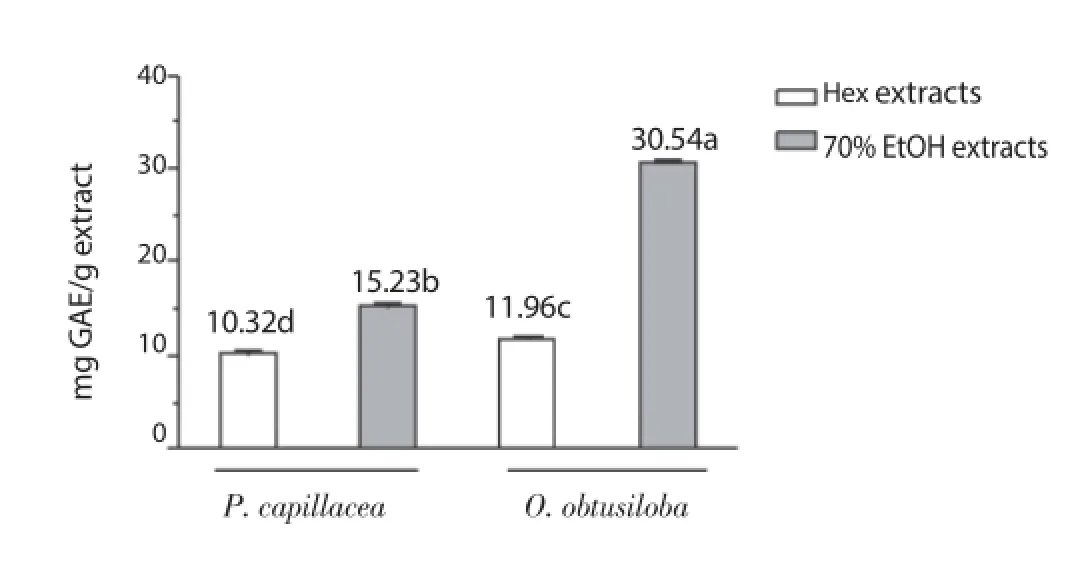
Figure 1. Total phenolic content of the Hex and 70% EtOH extracts of P. capillacea and O. obtusiloba.Diff erent letters indicate statistically signifi cant diff erences (Tukey’s test, P <0.05). GAE: gallic acid equivalent.
3.2. DPPH radical scavenging capacity
The DPPH radical scavenging capacity by Hex and 70% EtOH extracts of the algae P. capillacea and O. obtusiloba was evaluated at different concentrations of the extracts and the results are illustrated in Figure 2. The 70% EtOH extract of O. obtusiloba(99.47% ± 0.22%) exhibited a strong DPPH activity, followed by the Hex extract of O. obtusiloba (43.46% ± 0.19%), 70% EtOH of P. capillacea (40.81% ± 0.60%), Hex of P. capillacea (33.04% ± 2.08%) at a concentration of 1 000 μg/mL. Aside from the 70% EtOH extracts of O. obtusiloba at concentrations of 500 μg/mL and 1 000 μg/mL, no other showed activity similar to ascorbic acid.
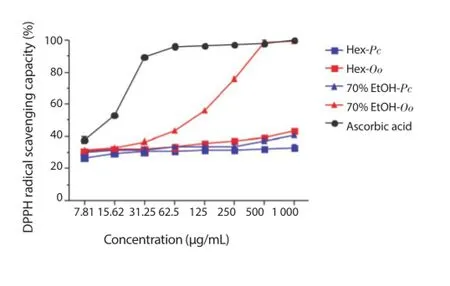
Figure 2. DPPH radical scavenging capacity of the Hex and 70% EtOH extracts of P. capillacea (Pc) and O. obtusiloba (Oo).
3.3. FIC
The results of FIC indicated that the 70% EtOH (54.70% ± 0.60%)and Hex (52.27% ± 1.01%) extracts of P. capillacea exhibited the best activities, followed by Hex (33.00% ± 0.24%) and 70% EtOH(27.70% ± 1.30%) extracts of O. obtusiloba at a concentration of 1 000 μg/mL. None of these extracts showed activity similar to ethylenediaminetetraacetic acid (Figure 3).
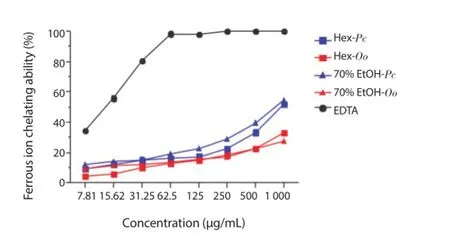
Figure 3. Ferrous ion chelating ability of the Hex and 70% EtOH extracts of P. capillacea (Pc) and O. obtusiloba (Oo).
3.4. FRAP
Increase in absorbance was found as the concentration of the extract increased from 7.81 to 1 000 μg/mL. Variations in optical density followed the decreasing order, as shown in Figure 4: BHA(0.158 - 2.375), 70% EtOH of O. obtusiloba (0.078 - 0.180), Hex of P. capillacea (0.109 - 0.167), 70% EtOH of P. capillacea (0.083 -0.136) and Hex of O. obtusiloba (0.054 - 0.101).
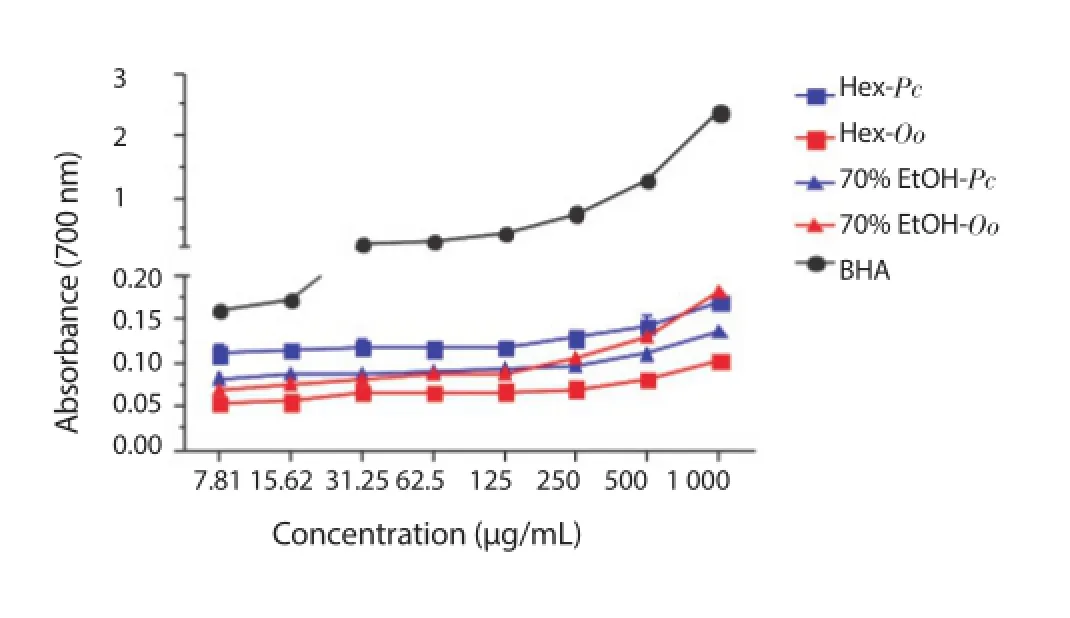
Figure 4. Ferric ion reducing power of the Hex and 70% EtOH extracts of P. capillacea (Pc) and O. obtusiloba (Oo).
3.5. BCB
BCB in the Hex and 70% EtOH extracts of the species P. capillacea and O. obtusiloba was examined at diff erent concentrations, and the results are depicted in Figure 5. The results indicated that 70% EtOH extract of O. obtusiloba (91.02% ± 0.15%) showed a strong BCB activity followed by the Hex extract of the same species (52.81% ± 0.49%), 70% EtOH of P. capillacea (50.61% ± 0.13%) and Hex of P. capillacea (41.33% ± 0.32%) at a concentration of 1 000 μg/mL. Except for the 70% EtOH extract of O. obtusiloba at concentrations of 500 and 1 000 μg/mL, no other showed activity similar to that of BHA.
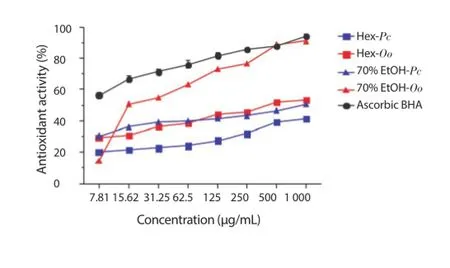
Figure 5. Degradation of β-carotene of the Hex and 70% EtOH extracts of P. capillacea (Pc) and O. obtusiloba (Oo).
3.6. Antibacterial activity
The Hex and 70% EtOH extracts of the marine algae P. capillacea and O. obtusiloba at a concentration of 1 000 μg/mL showed no antibacterial activity against standard strains of E. coli ATCC 25922,S. aureus ATCC 25923, and Pseudomonas aeruginosa ATCC 27853.
3.7. Bacterial agglutination assay
The Hex and 70% EtOH extracts of the red seaweeds P. capillacea and O. obtusiloba were able to agglutinate bacterial Gram positive cells of S. aureus and Gram negative cells of E. coli, multiresistant Salmonella and Vibrio harveyi, except the multiresistant strains of E. coli (Figure 6).
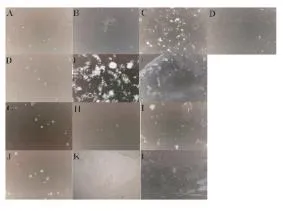
Figure 6. Bacterial agglutination prometed by the Hex and 70% EtOH extracts of P. capillacea (Pc) and O. obtusiloba (Oo).(A) E. coli negative control; (B) E. coli incubated with Hex-Pc; (C) E. coli incubated with Hex-Oo; (D) E. coli incubated with 70% EtOH-Oo; (E) S. aureus negative control; (F) S. aureus incubated with Hex-Oo;(G) S. aureus incubated with 70% EtOH-Oo; (H) multidrug-resistant Salmonella negative control; (I) multidrug-resistant Salmonella incubated with Hex-Pc; (J)multidrug-resistant Salmonella incubated with Hex-Oo; (K) Vibrio harveyi negative control; (L) Vibrio harveyi incubated with Hex-Pc; (M) Vibrio harveyi incubated with70% EtOH-Oo.
4. Discussion
Quantitation of TPC in extracts of the seaweeds P. capillacea and O. obtusiloba, measured by the spectrophotometric method of Folin-Ciocalteu, is based on the redox reaction of phenolic compounds present in algal extracts with metal ions. In this method, in alkaline medium, phenols reduce phosphomolybdate phosphotungstate,yellow in color, to molybdenum, of blue color[22,23].
The 70% EtOH was the most effective solvent for extracting phenolic compounds from red seaweeds. This because polar solvents effi ciently extract a series of polar compounds, such as polyphenols bound to sugars or proteins, phlorotannins, saponins, glycosides and organic acids[24,25]. Moreover, hexane will extract metabolites of low polarity like hydrocarbons, fatty acids, acetogenins, terpenes halogenated or not, carotenoids and tocopherols[26,27].
Variations in the CFT of marine macroalgae may be influenced by extrinsic factors, including herbivory pressure, irradiance,depth, salinity and nutrients; by intrinsic factors, such as, seaweed morphology, age and reproductive stage, but also by the type of solvent used in the extraction of phenolic compounds[19,28,29].
The DPPH radical scavenging method, among colorimetric antioxidant assays, has long been used in the search for new natural antioxidant compounds because of radical stability, reproducibility,simplicity, speed and ease of use[7,8,30,31].
The DPPH activity of (50%) dichloromethane: methanol extracts of P. capillacea e O. obtusiloba, collected in the State of Santa Catarina, at 1 000 μg/mL, were 57.51% and 30.59%[32]. In this study,considering the same concentration, the activities of the extracts(70% EtOH and Hex) of P. capillacea were lower, but those of O. obtusiloba, showed activities up to three times higher.
For the Hex extract, there was a negative correlation between the CFT and DPPH radical scavenging capacity (r = -0.715 4; P = 0.020 0), ie, the phenolic compounds were not the major responsible for DPPH activity, but apolar compounds like glycolipids,phospholipids, steroids, terpenes, fatty acids, carotenoids and tocopherols, which are present in these extracts[3-5,32,33].
Unlike the Hex extract, 70% EtOH extract demonstrated a strong correlation between the CFT and the DPPH radical scavenging capacity (r = 0.881 7, P = 0.000 7). Red seaweeds, especially the Order Ceramiales, which includes O. obtusiloba, are the main producers of secondary metabolites; the main characteristic of these algae is their great ability to synthesize phenolic compounds and derivatives, as sulfated or non-sulfated bromophenols, which have arelevant role in protecting tissues exposed to oxidative stress,against diff erent diseases including cancer, diabetes, cardiovascular and neurodegenerative diseases[34-36].
Souza et al[14] also reported a correlation between the CFT and the DPPH scavenging (r = 0.959 0) for methanol and ethanol extracts of two red seaweed species, Gracilaria birdiae and Gracilaria cornea of the coast of the State of Ceará.
The ability to chelate metals is very important, since the transition metals catalyze the peroxidation of lipids forming undesirable compounds that attack several types of molecules[15]. The binding of antioxidant compounds with metal ions can be assessed by the ferrous ion chelating assay. An extract with high binding ability with metal ions can prevent or inhibit various reactions capable of producing reactive hydroxyl radicals[19].
Hex and 70% EtOH extracts of the algae studied presented moderate (r = 0.597 6; P = 0.068 0) and good (r = 0.714 6; P = 0.020 2) correlation between the TPC and FIC, respectively. According to Wang et al[17], phenolic compounds are not strong metal chelating agents. Other compounds, such as dietary fi bers(agar, carrageenan, and alginate), present in algal extracts are also known for their ability to chelate metal and there is evidence of their inhibitory eff ects on the absorption of ferrous ion[37].
In the FRAP assay, antioxidant activity is determined based on the ability of antioxidant compounds, present in algal extracts, to reduce ferric iron (Ⅲ) to ferrous iron (Ⅱ) in a colorimetric redox reaction that simply involves the transfer of electrons[19]. Reducing agents present in solution promote the reduction of the Fe3+/ferrocyanide complex to the ferrous form (Fe2+), which can be measured at the absorbance of 700 nm[28]. The greater the absorbance of the mixture,the greater the antioxidant activity of iron reduction[38].
Extracts of P. capillacea and O. obtusiloba at a concentration of 1 000 μg/mL have presented low values, FRAP values were greater than those of 70% EtOH extracts of the Rhodophyta Amansia multifida and Meristiella echinocarpa[39]. Farvin and Jacobsen[40], in turn, observed high optical densities, from 0.2 to 0.4, in the ethanolic extract of the Rhodophyta Palmaria palmata, Porphyra purpurea,Chondrus crispus and Mastocarpus stellatus.
In Hex extracts of the two species of algae it was registered a low correlation between TPC and FRAP (r= 0.318 5, P = 0.369 7),suggesting that phenolic compounds cannot be the main ferric ion reducing agent. Conversely, the 70% EtOH extracts of both algal species showed a very high correlation (r = 0.942 1; P < 0.000 1), as previously reported in the literature[9,41].
Oxidation of unsaturated fatty acids present in biological membranes causes the formation of lipid free radicals and destruction of the lipid membrane. Antioxidants can stop the chain reaction by donating hydrogen atoms[42]. The BCB method is simple, sensitive and enables to evaluate the ability of a substance to neutralize the free radicals generated during the oxidation of linoleic acid, thereby preventing the degradation of β-carotene. Once the methodology does not employ high temperatures, it is possible to determine heat sensitive substances[19,43].
Antioxidant activities measured by BCB in the 50% dichloromethane: methanol extract at 1 000 μg/mL, of P. capillacea and O. obtusiloba collected in the State of Santa Catarina, were around 43%[32]. These results are lower than those for the activities of the extracts (70% EtOH and Hex) of P. capillacea and O. obtusiloba detected herein.
High correlations between CFT and BCB were verifi ed for the Hex(r = 0.936 4; P < 0.000 1) and 70% EtOH (r = 0.998 8; P < 0.000 1)extracts of the red algae analyzed. This result was also reported by Souza et al[14], but O’ Sullivan et al[8] failed to establish a correlation between these two variables.
Concerning antibacterial activity, El Kassis and Attia[44] found no antibacterial activity of the aqueous extract of P. capillacea collected in Abur Thursday, Egypt, compared to strains of S. aureus,Pseudomonas aeruginosa, E. coli and Bacillus subtilis. Abou Zeid et al[45] observed that the aqueous extract of the seaweed P. capillacea collected in the same region showed antibacterial activity against Bacillus cereus, S. aureus, Streptococcus pyogen and Pseudomonas flouresens.
Compounds isolated from other species of the genus Osmundaria exhibit antibacterial activity, as is the case of halogenated lactones,halogenated volatile metabolites and lanosol ethyl ether, isolated from Osmundaria fimbriata, Osmundaria volubilis and Osmundaria serrata, respectively[36].
This is the first report on the ability of secondary metabolites of the red seaweeds P. capillacea and O. obtusiloba to agglutinate Gram positive and Gram negative bacteria. Among the possible mechanisms of action of these bioactive molecules are damage to the plasma membrane, the inhibition of enzymes and microbial aggregation. It has been suggested that terpenes can promote disruption of the cytoplasmic membrane of the microorganisms,as well as tannins can interact with polysaccharides, inactivating enzymes[47-49].
In conclusion, the evaluation of antioxidant activity using five diff erent methodologies evidenced that extracts of the red seaweeds P. capillacea and O. obtusiloba have antioxidant potential, except for the FRAP. Although the algal extracts have not presented antimicrobial activity, the possible interaction of these extracts with bacterial Gram positive cells of S. aureus and Gram negative cells of E. coli, multidrug-resistant Salmonella and Vibrio harveyi was detected and reported for the fi rst time in this work.
Conflict of interest statement
We declare that we have no confl ict of interest.
Acknowledgments
The authors would like to express their gratitude for grants and fi nancial support from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico (FUNCAP) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior(CAPES) of the Brazilian Government. A.H. Sampaio and R.H.S.F Vieira are senior investigators of CNPq (Brazil).
[1] Matanjun P, Mohamed S, Mustapha NM, Muhammad K. Nutrient content of tropical edible seaweeds, Euchema cottonii, Caulerpa lentillifera and Sargassum polycystum. J Appl Phycol 2009; 21: 75-80.
[2] Patarra RF, Paiva L, Neto AI, Lima E, Baptista J. Nutritional value of selected macroalgae. J Appl Phycol 2011; 23: 205-208.
[3] Pires KMS, Alencar DB, Sousa MB, Sampaio AH, Saker-Sampaio S. Teores de α- e β-caroteno em macroalgas marinhas desidratadas. Rev Cienc Agron 2008; 39(2): 257-262.
[4] Pires-Cavalcante KMS, Alencar DB, Sousa MB, Sampaio AH, Saker-Sampaio S. Seasonal changes of α-tocopherol in green marine algae(Caulerpa genus). J Food Sci 2011; 76(5): 775-781.
[5] Sousa MB, Pires KMS, Alencar DB, Sampaio AH, Saker-Sampaio S. α-, β-caroteno e α-tocoferol em algas marinhas in natura. Ciencia Tecnol Aliment 2008; 28(4): 953-958.
[6] Chakraborty K, Joseph D, Praveen NK. Antioxidant activities and phenolic contents of three red seaweeds (Division: Rhodophyta) harvest from Gulf of Mannar of Peninsular India. J Food Sci Tech Mys 2015;52(4): 1924-1935.
[7] Boisvert C, Beaulieu L, Bonnet C, Pelletier E. Assessment of the antioxidant and antibacterial activities of three species of edible seaweeds. J Food Biochem 2015; 39(4): 377-387.
[8] O’ Sullivan AM, O’Callaghan YC, O’Grady MN, Queguineur B, Hanniffy D, Troy DJ, et al. In vitro and cellular antioxidant activities of seaweed extracts prepared from fi ve brown seaweeds harvested in spring from the west coast of Ireland. Food Chem 2011; 126(3): 1064-1070.
[9] Tierney MS, Smyth TJ, Hayes M, Soler-Vila A, Croft AK, Brunton N. Influence of pressurised liquid extraction and solid-liquid extraction methods on the phenolic content and antioxidant activities of Irish macroalgae. Int J Food Sci Technol 2013; 48(4): 860-869.
[10] Ngo DH, Vo TS, Ngo DN, Wijesekara I, Kim SK. Biological activities and potential health benefi ts of bioactive peptides derived from marine organisms. Int J Biol Macromol 2012; 51(4): 378-383.
[11] Wojcik M, Burzynska-Pedziwiatr I, Wozniak LA. A review of natural and synthetic antioxidants important for health and longevity. Curr Med Chem 2010; 17(28): 3262-3288.
[12] Cox S, Abu-Ghannam N, Gupta S. An assessment of the antioxidant and antimicrobial activity of six species of edible Irish seaweeds. Int Food Res J 2010; 17: 205- 220.
[13] Gupta S, Abu-Ghannam N. Bioactive potential and possible health eff ects of edible brown seaweeds. Trends Food Sci Tech 2011; 22(6): 315-326.
[14] Souza BWS, Cerqueira MA, Martins JT, Quintais MAC, Ferreira ACS,Teixeira JA, et al. Antioxidant potential of two red seaweeds from the Brazilian coast. J Agric Food Chem 2011; 59: 5589-5594.
[15] Kumar KS, Ganesan K, Rao PVS. Antioxidant potential of solvent extracts of Kappaphycus alvarezii (Doty) Doty-An edible seaweed. Food Chem 2008; 107(1): 289-295.
[16] Blois MS. Antioxidant determinations by the use of a stable free radical. Nature 1958; 181(4617): 1199-1200.
[17] Wang T, Jónsdóttir R, Ólafsdóttir G. Total phenolic compounds, radical scavenging and metal chelation of extracts from Icelandic seaweeds. Food Chem 2009; 116(1): 240-248.
[18] Ganesan P, Kumar CS, B N. Antioxidant properties of methanol extract and its solvent fractions obtained from selected Indian red seaweeds. Bioresource Technol 2008; 99(8): 2717-2723.
[19] Chew YL, Lim YY, Omar M, Khoo KS. Antioxidant activity of three edible seaweeds from two areas in South East Asia. LWT-Food Sci Technol 2008; 41(6): 1067-1072.
[20] CLSI/NCCLS - Clinical and Laboratory Standards Institute 2010. Performance standards for antimicrobial susceptibility testing: nineteenthinformational supplement. 19 ed. M100-S19. Wayne, PA: Clinical and Laboratory Standards Institute; 29(3): 149.
[21] Imamichi Y, Yokoyama Y. Purification, characterization and cDNA cloning of a novel lectin from the jellyfi sh Nemopilema nomurai. Comp Biochem Physiol B 2010; 156(1): 12-18.
[22] Silva MLC, Costa RS, Santana AS, Koblitz MGB. Compostos fenólicos,carotenóides e atividade antioxidante em produtos vegetais. Semina 2010;31(3): 669-682.
[23] Thanigaivel S, Hindu SV, Vijayakumar S, Mukherjee A, Chandrasekaran N, Thomas J. Diff erential solvent extraction of two seaweeds and their effi cacy in controlling Aeromonas salmonicida infection in Oreochromis mossambicus: A novel therapeutic approach. Aquaculture 2015; 443: 54-64.
[24] Cho ML, Lee HS, Kang IJ, Won MH, You SG. Antioxidant properties of extract and fractions from Enteromorpha prolifera, a type of green seaweed. Food Chem 2011; 127(3): 999-1006.
[25] Ignat I, Volf I, Popa VI. A critical review of methods for characterisation of polyphenolic compounds in fruit and vegetables. Food Chem 2011;126(4): 1821-1835.
[26] Riguera R. Isolating bioactive compounds from marine organisms. J Mar Biotechnol 1997; 5(4): 187-193.
[27] Kelman D, Posner EK MCDermid KJ, Tabandera NK, Wright PR,Wright AD. Antioxidant activity of Hawaiian algae. Mar Drugs 2012;10(2): 403-416.
[28] Ganesan K, Kumar CS, Rao PVS. Comparative assessment of antioxidant activity in three edible species of green seaweed, Enteromorpha from Okha, Northwest coast of India. Innov Food Sci Emerg Technol 2011;12(1): 73-78.
[29] Lann KL, Ferret C, Vanmee E, Spagnol C, Lhuillery M, Payri C, et al. Total phenolic, size-fractionated phenolics and fucoxanthin content of tropical Sargassaceae (Fucales, Phaeophyceae) from the South Pacifi c Ocean: Spatial and specifi c variability. Phycol Res 2012; 60(1): 37-50.
[30] Mishra K, Ojha H, Chaudhury NK. Estimation of antiradical properties of antioxidants using DPPH assay: A critical review and results. Food Chem 2012; 130(4): 1036-1043.
[31] Rajauria G, Jaiswal AK, Abu-Gannam N, Gupta S. Antimicrobial,antioxidant and free radical-scavenging capacity of brown seaweed Himanthalia elongata from western coast of Ireland. J Food Biochem 2013; 37(3): 322-335.
[32] Martins CDL, Ramlov F, Carneiro NPN, Gestinari LM, Santos BF, Bento LM, et al. Antioxidant properties and total phenolic contents of some tropical seaweeds of the Brazilian coast. J Appl Phycol 2013; 25: 1179-1187.
[33] Guaratini T, Lopes NP, Marinho-Soriano E, Colepicolo O, Pinto E. Antioxidant activity and chemical composition of the non polar fraction of Gracilaria domingensis (Kützing) Sonder ex Dickie and Gracilaria birdiae (Plastino & Oliveira). Rev Bras Farmacogn 2012; 22(4): 724-729.
[34] Liu M, Hansen PE, Lin X. Bromophenols in marine algae and their bioactivities. Mar Drugs 2011; 9(7): 1273-1292.
[35] Nogueira CCR, Paixão ICNP, Teixeira VL. Antioxidant activity of natural products isolated from red seaweeds. Nat Prod Commun 2014; 9(7): 1031-1036.
[36] Osako K, Teixeira VL. Natural products from marine algae of the genus Osmundaria (Rhodophyceae, Cearamiales). Nat Prod Commun 2013;8(4): 533-538.
[37] Vinayak RC, Sudha SA, Chatterji A. Bio-screening of a few green seaweeds from India for their cytotoxic and antioxidant potential. J Sci Food Agric 2011; 91(13): 2471-2476.
[38] Soltani SS, Saadatmand S, Khavarinejad R, Nejadsattari T. Antioxidant and antibacterial activities of Cladophora glomerata (L.) Kutz. in Caspian sea coast, Iran. Afr J Biotechnol 2011; 10(39): 7684-7689.
[39] Alencar DB, Silva SR, Pires-Cavalcante KMS, Lima RL, Pereira Júnior FN,Sousa, MB, et al. Antioxidant potential and cytotoxic activity of two red seaweed species, Amansia multifida and Meristiella echinocarpa, from the coast of Northeastern Brazil. An Acad Bras Cienc 2014; 86(1): 251-263.
[40] Farvin KHS, Jacobsen C. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem 2013;138(2-3): 1670-1681.
[41] Matanjun P, Mohamed S, Mustapha NM, Muhammad K, Ming C. Antioxidant activities and phenolics content of eight species of seaweed from north Borneo. J Appl Phycol 2008; 20(4): 367-373.
[42] Zubia M, Robledo D, Freile-Pelegrin Y. Antioxidant activities in tropical marine macroalgae from the Yucatán Penísula, Mexico. J Appl Phycol 2007; 19: 449-458.
[43] Alves CQ, David JM, David JP, Bahia MV, Aguiar RM. Métodos para determinação de atividade antioxidante In vitro em substratos orgânicos. Quim Nova 2010; 33(10): 2202-2210.
[44] El Kassas HY, Attia AA. Bacterial application and cytotoxic activity of biosynthesized silver nanoparticles with an extract of red seaweed Pterocladiella capillacea on the HepG2cell line. Asian Pac J Cancer Prev 2014; 15(3): 1299-1306.
[45] Abou Zeid AH, Aboutabl EA, Sleem AA, El-Rafie HM. Water soluble polysaccharides extracted from Pterocladia capillacea and Dictyopteris membranacea and their biological activities. Carbohyd Polym 2014; 113: 62-66.
[46] Pinteus S, Alves C, Monteiro H, Araújo E, Horta A, Pedrosa R. Asparagopsis armata and Sphaerococcus coronopifolius as a natural source of antimicrobial compounds. World J Microbiol Biotechnol 2015; 31(3): 445-451.
[47] Cushnie TPT, Lamb AJ. Recent advances in understanding the antibacterial properties of fl avonoids. Int J Antimicrob Agents 2011; 38(2): 99-107.
[48] Vasconcelos MA, Arruda FVS, Alencar DB, Saker-Sampaio S,Albuquerque MRJR, Santos HS, et al. Antibacterial and antioxidant activities of derriobtusone A isolated from Lonchocarpus obtusus. Biomed Res Int 2014; 2014: 1-10.
[49] Urzúa A, Rezende MC, Mascayano C, Vásquez L. A structure activity study of antibacterial diterpenoids. Molecules 2008; 13(4): 882-891.
ent heading
10.1016/j.apjtm.2016.03.015
15 January 2016
Daniel Barroso de Alencar, Laboratório de Produtos Naturais Marinhos, Departamento de Engenharia de Pesca, Universidade Federal do Ceará,Campus do Pici, Av. Mister Hull, s/n, Caixa Postal 6043, 60455-970 Fortaleza, CE,Brazil.
Tel: +558533669728
Fax: +558533669420
E-mail: daniellpesca@gmail.com
in revised form 20 February 2016
ARTICLE INFO
Article history:
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Determination of ligand cluster and binding site within VP40 of Ebola virus: clue for drug development
- Clinacanthus nutans: a review of the medicinal uses, pharmacology and phytochemistry
- Current perspectives on dengue episode in Malaysia
- Etiological agents causing leptospirosis in Sri Lanka: A review
- Phylogeny of Murray Valley encephalitis virus in Australia and Papua New Guinea
- Dengue outbreak in Swat and Mansehra, Pakistan 2013; an epidemiological and diagnostic perspective
