Dengue outbreak in Swat and Mansehra, Pakistan 2013; an epidemiological and diagnostic perspective
2016-11-14MuhammadSulemanRaniFaryalUzmaBashirAamirMuhammadMasroorAlamNadiaNisarSalmaanSharifShahzadShaukatAdnanKhurshidMeharAngezMassabUmairGhulamMujtabaMianMuhammadSufianYasirArshadLubnaRehmanSyedSohailZahoorZaidi
Muhammad Suleman, Rani Faryal, Uzma Bashir Aamir, Muhammad Masroor Alam, Nadia Nisar,Salmaan Sharif, Shahzad Shaukat, Adnan Khurshid, Mehar Angez, Massab Umair, Ghulam Mujtaba, Mian Muhammad Sufian, Yasir Arshad, Lubna Rehman, Syed Sohail Zahoor Zaidi*
1Department of Microbiology, Quaid-i-Azam University, Islamabad, Pakistan
2Department of Virology, National Institute of Health, Park Road, Chak Shahzad, Islamabad, Pakistan
Dengue outbreak in Swat and Mansehra, Pakistan 2013; an epidemiological and diagnostic perspective
Muhammad Suleman1,2, Rani Faryal1, Uzma Bashir Aamir2, Muhammad Masroor Alam2, Nadia Nisar2,Salmaan Sharif2, Shahzad Shaukat2, Adnan Khurshid2, Mehar Angez2, Massab Umair2, Ghulam Mujtaba2, Mian Muhammad Sufian2, Yasir Arshad2, Lubna Rehman2, Syed Sohail Zahoor Zaidi2*
1Department of Microbiology, Quaid-i-Azam University, Islamabad, Pakistan
2Department of Virology, National Institute of Health, Park Road, Chak Shahzad, Islamabad, Pakistan
Accepted 15 March 2016
Available online 20 April 2016
Dengue virus
NS1 Antigen
Pakistan
Diagnosis
Epidemiology
Khyber Pakhtunkhwa
Objective: To High light some epidemiological, clinical and diagnostic features of dengue fever during an outbreak and the role of diff erent diagnostic techniques to achieve the highest level of accuracy in results. Methods: Blood samples (n=323) were collected along with epidemiological and clinical data from suspected dengue patients who visited diff erent hospitals in Swat and Mansehra district of Pakistan between May-November 2013 during a dengue outbreak. Samples were tested for the detection of viral nucleic acid by real-time PCR, non structural protein-1 (NS1)antigen and IgM antibodies by ELISA. Results: Out of 323 cases with clinical dengue infection,304 were positive by one or more diagnostic parameter; 201 samples were positive by real-time PCR, 209 were positive by NS1 ELISA and 190 were positive by IgM antibodies. Sensitivities of real-time PCR and NS1 ELISA were comparable for early diagnosis of dengue virus infection,IgM antibody detection assay was found useful for the diagnosis in the samples collected later than day 5 of onset. Conclusions: The use of real-time PCR or detection of non structural protein NS1 by ELISA followed by IgM antibodies detection can be recommended for early diagnosis of dengue virus infection with a high level of accuracy.
1. Introduction
Dengue fever is an important emerging and re-emerging arboviral infection and major public health problem of tropical and subtropical regions of the world[1, 2]. According to the World Health Organization 2.5 billion people and 124 countries are at risk of dengue infection with over 100 million cases of dengue virus (DENV) infection and 30 000 estimated deaths. In most of the dengue endemic countries the DENV has caused regular cyclic epidemics after every 3-5 years[3].
Dengue fever is caused by the DENV which belongs to the genus flavivirus, family flaviviridae and has been classified into four(DENV-1-4) serotypes and ten genotypes on the basis of nucleotides differences in the sequence of envelope gene[4, 5]. The DENV infection is classified into three categories ranging from mild dengue fever to severe life threatening dengue hemorrhagic fever(DHF) and dengue shock syndrome (DSS)[6]. There are 500 000 cases of DHF and DSS reported annually which are related to severe disease manifestation characteristic of secondary infection[7, 8].
Pakistan has a temperate climate and the DENV has been endemic for many years[9-12]. However, since 2006 dengue outbreaks have been reported every year and co-circulation of multiple dengue serotypes has been reported[9]. The presence of rich fauna, vast agricultural land, open irrigation channels, artifi cial water reservoirs for power generation and floods from heavy rainfall provide ample breeding sites for the mosquito vector(s). The DENV vector activities diff er according to season in diff erent geographical areas of Pakistan and typically the incidence of cases increases after the rainy season[7].
Various diagnostic methods are currently available for the diagnosis of DENV infection, like virus isolation, conventional reverse transcriptase polymerase chain reaction (RT-PCR), real-time PCR, genomic sequencing, ELISA for detection of viral antigen and IgM, IgG antibodies[13]. Virus isolation and molecular techniques are highly sensitive assays but often need a specialized laboratory setting and highly trained staff as well. In a developing country like Pakistan, serology is the more commonly used method for diagnosis of dengue infections. Although RT-PCR and viral antigen non structural protein-1 (NS1) detection by ELISA are highly sensitive techniques for the early diagnosis of DENV, they are not routinely used in public health laboratories due to resource limitations.
The aim of the present study was to evaluate the diagnostic accuracy of diff erent diagnostic tests such as NS1 ELISA, real-time PCR and IgM ELISA for the detection of DENV infection during an outbreak and correlate with clinical data especially the date of onset of infection.
2. Materials and methods
A total of 323 blood specimens collected from the suspected DENV cases were transported to the Department of Virology National Institute of Health Islamabad maintaining cold chain. This study was approved by the Internal Review Board of National Institute of Health, Islamabad, Pakistan.
Sera were separated by centrifuging the whole blood at 3 000 rpm for 10 min. Each sample was analyzed for the detection of NS1 antigen and IgM antibodies by ELISA and viral RNA by real-time PCR. All serum samples were stored at -80 ℃ until further use.
2.1. Detection of NS1 antigen
NS1 antigen was detected by using Platelia dengue antigen detection kit (Biorad Laboratories, Marnes-la-Coquette, France)according to the instruction provided by the manufacturer. Briefl y,sample diluents buff er (50 μL) was added to each well of microplate followed by the addition of serum, positive and negative controls(50 μL) and conjugate (100 μL). After the addition of conjugate,microwell plate was covered with an adhesive plate sealer and incubated at 37 ℃ for 90 min. After incubation, plate was washed and 160 μL of substrate was added followed by incubation at room temperature for 30 min. The presence of NS1 antigen was demonstrated by a color development and reading of optical density(OD) at a wavelength of 450 nm. Test results were determined by comparing the OD values of sample to the OD values of cut-off controls.
2.2. Viral RNA detection by real-time PCR
RNA was extracted using 140 μL of serum samples with QIAamp Viral RNA Mini Kit (Qiagen, Germany) according to the protocol provided by manufacturer.
Serotype specific four-plex, real-time TaqMan RT-PCR was carried out according to the CDC protocol developed by Johnson et al[14]. Briefly, four-plex reaction mixtures of 25 μL were run for each DENV serotype (DENV1-4) in ABI7500 real-time thermocycler using the SuperScript Ⅲ Platinum one-step qRT-PCR kit (Invitrogen). Amplifi cations for each serotype was carried out separately in 25 μL reaction mixture containing 5 μL extracted RNA,12.5 μL of 2×reaction mixture, 0.5 μL enzyme mix, 2 μM of each primer and 1 μM of TaqMan probe. The cycling conditions were as follows: RT step 50 ℃ for 10 min, initial denaturation at 95 ℃ for 5 min and 45 cycles at 95 ℃ for 15 s and at 60 ℃ for 60 s. The data was analyzed using software SDS version 1.4.
2.3. Detection of IgM antibodies
DENV specific IgM antibodies were detected using DENV IgM Capture ELISA (Panbio Queenland Australia) according to the protocol provided by the manufacturer. Results were interpreted by the calculation of cut-off and index values.
2.4. Statistical analysis
Statistical analysis of data was performed by chi-square test and student t-test using SPSS version 18.0 (SPSS. Inc., Chicago, IL,USA). A P value of <0.05 was considered as signifi cant.
3. Results
A total of 323 serum samples were collected from suspected dengue fever cases during the month of May-November 2013 (Table 1). Gender distribution was signifi cant (P=0.006) with 65% male. Age was found to be a significant characteristic among dengue positive and dengue negative cases by independent sample t-test(P=0.025). Most effected 49.5% (160/323) age group was 16-30 years old. Mean days after illness was 4.78±2.06 for dengue positive cases which was found signifi cant compared to dengue negative group (P=0.001). Platelets count and TLC values were signifi cantly diff erent between dengue positive and negative groups.

Table 1Epidemiological characteristics of patients enrolled in the study (n=323).
3.1. Month wise distribution of cases
During the study period from May-November 2013 the highest number of dengue fever cases was reported in September (Figure 1).
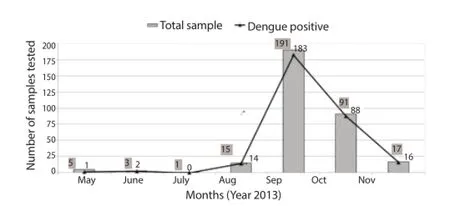
Figure 1. Month wise distribution of dengue fever cases in 2013 outbreak.
3.2. Symptoms correlation
The frequency of clinical symptoms was unequal in patients with acute dengue infection compared to dengue negative cases. Fever,body ache, retro-orbital pain, rash and bleeding were the most common symptoms among dengue positive cases (P>0.05) (Table 2).
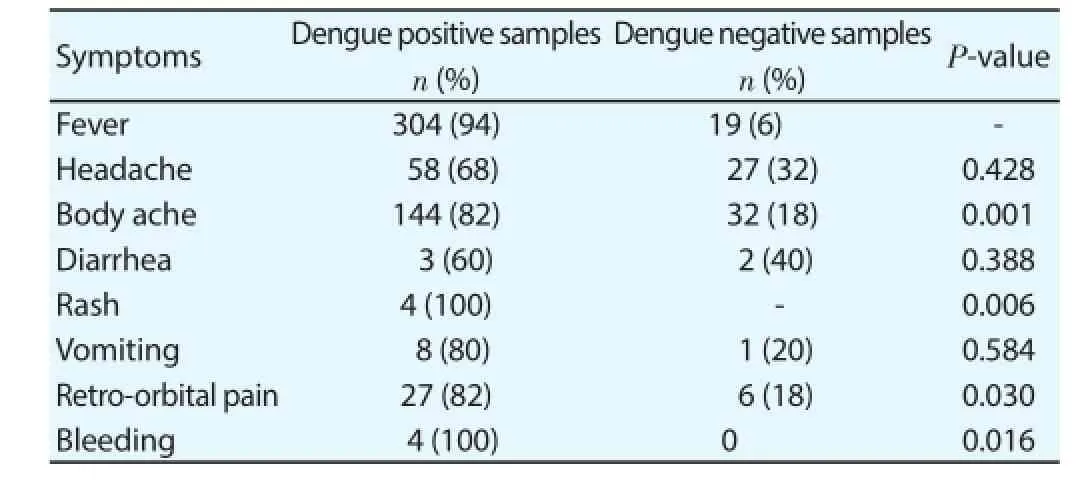
Table 2Association of the most frequently reported symptoms presented by dengue patients enrolled in the study (n=323).
3.3. NS1 antigen detection
The non-structural antigen assay was positive in 65% (209 out of 323) of tested samples. The highest percentage of NS1 positive samples were collected on day 1-3 after onset with 80% (57 out of 71) followed by 68% (115/170) on day 4-6, 48% ( 30 out of 63) on day 7-9 and 37% (7 out of 19) after day 9 of onset of infection.
3.4. Viral RNA detection by real-time PCR
A total of 62% (201 out of 323) serum samples were found positive by real-time PCR. The Ct values of positive cases varied from 22-36. DENV-3 was the dominant serotype detected from 34% (69 out of 201) patients followed by DENV-2 in 22% (45 out of 201) patients and DENV-1 found in 12% (24 out of 201) subjects. DENV-4 was not found in any of the study samples. Mixed infection with multiple dengue serotypes was detected in 31% (63 out of 201) samples. The proportion of positive samples in real-time PCR was the higest in samples collected on day 1-3 with 69% (49 out of 71) followed by 59.4% (107 out of 170) on day 4-6 and 55% (35 out of 63) on day 7-9 after onset and gradually decresed after day 9 of disease onset.
3.5. Detection of IgM antibodies
All 323 serum samples were tested for the detection of anti-DENV IgM antibody by ELISA. Out of 323 samples, IgM antibodies were detected in 59% (190/323) suspected cases. The IgM antibodies were found in 35% (25 out of 71) of samples collected on day 1-3 and increasing trend was found in samples collected on day 4-6 with 60% (102 out of 170), 76% (48 out of 63) on day 7-9 and 79% (15 out of 19) in samples collected after day 9 of symptoms onset.
3.6. Comparative results The results of real-time PCR, NS1 antigen and IgM antibodies were compared with reference to the time of sample collection. IgM ELISA in combination with NS1 antigen ELISA or real-time PCR were found suitable marker for the early diagnosis of dengue infection. Using the combination of NS1 and IgM, 87% (283 out of 323) samples were positive. Positivity of NS1 ELISA and real-time PCR was 84% (271 out of 323), IgM ELISA and real-time PCR was 83% (268 out of 323). Only 6% (19 out of 323) samples were found negative on all diagnostic parameters used during this study. The sensitivities of real-time PCR and NS1 ELISA were almost similar and combination of NS1 ELISA or real-time PCR with IgM ELISA increased diagnostic sensitivity (Table 3, 4, 5).

Table 3Comparison of real-time PCR and NS1 ELISA.
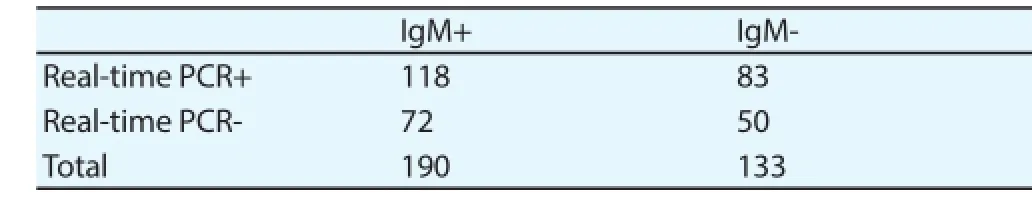
Table 4Comparison of real-time PCR and IgM ELISA.
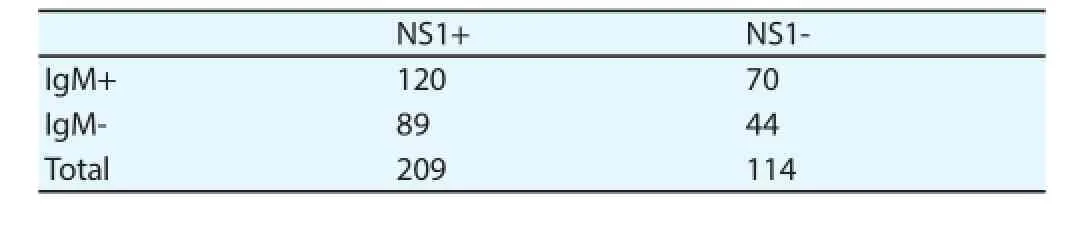
Table 5Comparison of IgM ELISA and NS1 ELISA.
4. Discussion
In Pakistan, the first outbreak of DENV was reported in 1982 in Punjab Province which was limited to district Lahore[15]. Since then, a number of outbreaks have been reported from KPK, Sindhand Balochistan Province, but no proper attention was given to establish a comprehensive laboratory based surveillance program in order to understand the burden of disease, and circulating viral serotypes, which may be helpful for early implementation of disease surveillance and control. As a consequence, in 2011 and 2013 two major DENV outbreaks occurred in district Lahore(Punjab Province) and Swat, Mansehra and adjacent areas of KPK Province[11].These outbreaks were relatively of short duration but high number of dengue fever cases and dengue related deaths were reported possibly due to two main factors: presence of a large immunologically susceptible/naive population and unavailability of accurate assay for early diagnosis of dengue fever which is important for the clinical management of patient and helpful in preventing the development of DHF and DSS[16].
Therefore in this study, the diagnostic accuracy for DENV infection was assessed for various assays, with respect to the date of onset of infection and sample collection. We analyzed of 323 serum samples collected from suspected dengue cases during 2013 outbreak in two districts of KPK using three different diagnostic tests: real-time PCR, NS1 ELISA and IgM ELISA and compared the results. The clinical and epidemiological data from the cases were also analyzed. The principally aff ected age group was 16-30 years old followed by 31-45 age groups as previously reported[17]. Demographic data analysis showed that the majority of the patients were male, with male to female ratio of 2:1. An observation also reported in previous studies from KPK Province[11, 18]. The most common symptoms observed in cases presented as dengue fever and DHF was, fever (100%) followed by body ache (54%) headache(26%) and retro-orbital pain (10%), an observation similar to other reports from South Asia[19, 20].The low platelet count reported earlier was observed in few cases[21, 22]. Similarly we found 51% cases with leukocyte count below 4 000 cells/μL while other studies have reported leucopenia in up to 90% cases with DENV infection[23].
Based on real-time PCR testing, we confi rmed the circulation of three dengue serotypes (DENV-1, 2 and 3) in contrast to Amjad Ali et al who reported the circulation of only two dengue serotypes DENV-2 and 3[18]. This may be attributed to the use of highly sensitive real-time PCR in our study compared to conventional RTPCR used in previous studies. The highest numbers of cases were detected in post monsoon period which might be due to increased humidity/precipitation and heavy rainfall which provide suitable environment for vector breeding. This observation is consistent with reports of dengue outbreaks in other provinces, as well as from diff erent studies conducted in India, Nepal and other South Asian Countries[17, 24, 25].
Comparison of serotype specific real-time PCR[14] and NS1 antigen ELISA showed almost similar results in early disease period, thus the use of NS1 ELISA is arguably an appropriate and reliable method for early diagnosis in laboratory setting where the facility of real-time PCR is not available. However for well equipped laboratories the use of real-time PCR is a rapid, sensitive and suitable diagnostic test which can not only detect viral RNA but also specify the viral serotypes at the same time[13]. The detection of DENV by serotype specifi c real-time PCR is very important, because the coinfection with multiple dengue serotypes and secondary dengue infection with diff erent serotypes can lead to DHF and DSS[26]. On the other hand, the detection of IgM antibodies by serologic methods can give excellent results in later stages (after day 5 of infection)when high concentrations of immunoglobulin are detectable in blood.
The accurate and exact date of onset of DENV infection needs to be taken into account before choosing which diagnostic tool to be use.
Defi nitive diagnosis of dengue fever cannot be based on clinical judgment alone as the disease presentation is indistinguishable from many febrile illnesses like malaria, measles, rubella, chikungunya and other bacterial and rickettsial diseases. In the presence of limited resources, this can lead to gross mismatch between estimated and actual number of cases[27]. Therefore pro-active laboratory based support is absolutely required in dengue surveillance, not only in diagnosis of suspected cases of dengue fever and DHF but also in monitoring the serotypes and strains circulating in the population and to provide an early warning of impending dengue epidemics. At governmental level, it should be important to introduce public awareness programs to minimize the spread of outbreak in the country. In addition the identification of risk factors like vector breeding sites and control strategies is another responsibility of the strong and fully functional and highly optimized surveillance system which is still lacking or under developmental process in Pakistan.
Conflict of Interest statement
We declare that we have no confi ct of interests.
Acknowledgements
We are highly thankful to Dr. Bilal Bahrawar Khan, Senior Epidemiologist, Khyber Pakhtunkhwa; Mian Sufian Mr. Umer Daraz, and Mr. Yasir Arshad from Department of Virology, National Institute of Health for their technical support in sample collectionand transportation.
[1] World Health Organization. Dengue and Severe dengue. [Online]Available at: http://www.who.int/mediacentre/factsheets/fs117/en/.[Accessed on May 2015].
[2] Guzman MG, Kouri G. Dengue: an update. Lancet Infect Dis 2002; 2(1): 33-42.
[3] Ferguson N, Anderson R, Gupta S. The eff ect of antibody-dependent enhancement on the transmission dynamics and persistence of multiplestrain pathogens. Proc Natl Acad Sci USA 1999; 96(2): 790-794.
[4] Vaughn DW, Green S, Kalayanarooj S, Innis BL, Nimmannitya S,Suntayakorn S, et al. Dengue viremia titer, antibody response pattern,and virus serotype correlate with disease severity. J Infect Dis 2000;181(1): 2-9.
[5] Murphy BR, Whitehead SS. Immune response to dengue virus and prospects for a vaccine. Annu Rev Immunol 2011; 29: 587-619.
[6] Halstead SB. Dengue. Lancet 2007; 370(9599): 1644-1652.
[7] A joint publication of the World Health Organization (WHO) and the Special Programme for Research and Training in Tropical Diseases. Dengue Guidelines for Diagnosis, Treatment, Prevention and Control.[Online] Available at: http://www.who.int/tdr/publications/documents/ dengue-diagnosis.pdf.
[8] Gubler DJ. The global pandemic of dengue/dengue haemorrhagic fever: current status and prospects for the future. Ann Acad Med Singapore 1998; 27(2):227-234.
[9] World Health Organization Weekly Epidemiological Monitor. Dengue in Pakistan. [Online] Available at: http://reliefweb.int/sites/reliefweb.int/ fi les/resources/Epi_Monitor_2013_6_52.pdf. [Accessed on 29 December 2013].
[10] Akram DS, Igarashi A, Takasu T. Dengue virus infection among children with undifferentiated fever in Karachi. Indian J Pediatr 1998; 65(5): 735-740.
[11] A joint report of World Health Organization and Health Department,Government of Khyber Pakhtunkhwa. Swat: Dengue fever snapshot (07 August-20 October 2013). [Online] Available at: http://reliefweb.int/ sites/reliefweb.int/fi les/resources/Swat_dengue_fever_snapshot_211013. pdf. [Accessed on 20 October 2013].
[12] Rasheed SB, Butlin RK, Boots M. A review of dengue as an emerging disease in Pakistan. Public Health 2013; 127(1): 11-17.
[13] De Paula SO, Fonseca BA. Dengue: a review of the laboratory tests a clinician must know to achieve a correct diagnosis. Braz J Infect Dis 2004; 8(6): 390-398.
[14] Johnson BW, Russell BJ, Lanciotti RS. Serotype-specific detection of dengue viruses in a fourplex real-time reverse transcriptase PCR assay. J Clin Microbiol 2005; 43(10): 4977-4983.
[15] Humayoun MA, Waseem T, Jawa AA, Hashmi MS, Akram J. Multiple dengue serotypes and high frequency of dengue hemorrhagic fever at two tertiary care hospitals in Lahore during the 2008 dengue virus outbreak in Punjab, Pakistan. Int J Infect Dis 2010; 14(Suppl 3): e54-59.
[16] Chan YC, Salahuddin NI, Khan J, Tan HC, Seah CL, Li J, et al. Dengue haemorrhagic fever outbreak in Karachi, pakistan 1994. Trans R Soc Trop Med Hyg 1995; 89(6): 619-620.
[17] Gupta E, Dar L, Kapoor G, Broor S. The changing epidemiology of dengue in Delhi, India. Virol J 2006; 3:92.
[18] Amjad Ali ZN, Rehman RU, Farzana, Ali S, Zahir F, Iqbal A, et al. Dengue virus serotype 2 and 3 causing high morbidity and mortality in Swat, Pakistan. Biohelikon 2013; 1(2): 1-3.
[19] World Health Organization Department of Communicable Disease Surveillance and Response. WHO report on global surveillance of epidemic-prone infectious diseases. [Online] Available at: http:// www.who.int/csr/resources/publications/surveillance/WHO_Report_ Infectious_Diseases.pdf.
[20] Wasay M, Channa R, Jumani M, Zafar A. Changing patterns and outcome of Dengue infection; report from a tertiary care hospital in Pakistan. J Pak Med Assoc 2008; 58(9): 488-489.
[21] Mourao MP, Lacerda MV, Macedo VO, Santos JB. Thrombocytopenia in patients with dengue virus infection in the Brazilian Amazon. Platelets 2007; 18(8): 605-612.
[22] Martina BE, Koraka P, Osterhaus AD. Dengue virus pathogenesis: an integrated view. Clin Microbiol Rev 2009; 22(4): 564-581.
[23] Ageep AK, Malik AA, Elkarsani MS. Clinical presentations and laboratory fi ndings in suspected cases of dengue virus. Saudi Med J 2006; 27(11): 1711-1713.
[24] Akhtar N, Khan J, Khan A. Dengue Outbreak in Khyber Pakhtoonkhwa,Pakistan 2013. Eur Acad Res 2014; 1(11): 3842-3857.
[25] Pandey BD, Morita K, Khanal SR, Takasaki T, Miyazaki I, Ogawa T, et al. Dengue virus, Nepal. Emerg Infect Dis 2008; 14(3): 514-515.
[26] Halstead SB. Pathogenesis of dengue: challenges to molecular biology. Science 1988; 239(4839): 476-481.
[27] Jaenisch T, Junghanss T, Wills B, Brady OJ, Eckerle I, Farlow A, et al. Dengue expansion in Africa-not recognized or not happening? Emerg Infect Dis 2014; doi: 10.3201/eid2010.140487.
ent heading
10.1016/j.apjtm.2016.03.010
15 January 2016
Syed Sohail Zahoor Zaidi, Department of Virology, National Institute of Health, Park Road, Chak Shahzad, Islamabad-45500, Pakistan.
Tel: +92-51-9255082
Fax: +92-51-9255082
E-mail: sohailz@live.com
in revised form 20 February 2016
ARTICLE INFO
Article history:
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Determination of ligand cluster and binding site within VP40 of Ebola virus: clue for drug development
- Clinacanthus nutans: a review of the medicinal uses, pharmacology and phytochemistry
- Current perspectives on dengue episode in Malaysia
- Etiological agents causing leptospirosis in Sri Lanka: A review
- Phylogeny of Murray Valley encephalitis virus in Australia and Papua New Guinea
- Bioactive extracts of red seaweeds Pterocladiella capillacea and Osmundaria obtusiloba (Floridophyceae: Rhodophyta) with antioxidant and bacterial agglutination potential
