In vivo anticancer activity of maesopsin 4-O-β-glucoside isolated from leaves of Artocarpus tonkinensis A. Chev. Ex Gagnep
2016-11-14TrinhThiThuyDaoDucThienTranQuangHungNguyenThanhTamNguyenThiHoangAnhNguyenThiNgaNguyenThiCucLePhuongMaiTranVanSungDomenicoDelfinoDoThiThao
Trinh Thi Thuy, Dao Duc Thien, Tran Quang Hung, Nguyen Thanh Tam, Nguyen Thi Hoang Anh, Nguyen Thi Nga, Nguyen Thi Cuc, Le Phuong Mai, Tran Van Sung,Domenico V. Delfino, Do Thi Thao*
1Institute of Chemistry, Vietnam Academy of Science and Technology, 18-Hoang Quoc Viet, Nghia Do, Cau Giay, Hanoi, Vietnam
2Institute of Biotechnology, Vietnam Academy of Science and Technology, 18-Hoang Quoc Viet, Nghia Do, Cau Giay, Hanoi, Vietnam
3National Research Council Canada, Measurement Science and Standards, 1200 Montreal Road, Building M-12, Ottawa, Ontario K1A 0R6, Canada
4Department of Medicine, University of Perugia, Piazzale Gambuli, S.Andrea delle Fratte, 06132 Perugia, Italy
In vivo anticancer activity of maesopsin 4-O-β-glucoside isolated from leaves of Artocarpus tonkinensis A. Chev. Ex Gagnep
Trinh Thi Thuy1*, Dao Duc Thien1, Tran Quang Hung1, Nguyen Thanh Tam1, Nguyen Thi Hoang Anh1, Nguyen Thi Nga2, Nguyen Thi Cuc2, Le Phuong Mai3, Tran Van Sung1,Domenico V. Delfino4, Do Thi Thao2*
1Institute of Chemistry, Vietnam Academy of Science and Technology, 18-Hoang Quoc Viet, Nghia Do, Cau Giay, Hanoi, Vietnam
2Institute of Biotechnology, Vietnam Academy of Science and Technology, 18-Hoang Quoc Viet, Nghia Do, Cau Giay, Hanoi, Vietnam
3National Research Council Canada, Measurement Science and Standards, 1200 Montreal Road, Building M-12, Ottawa, Ontario K1A 0R6, Canada
4Department of Medicine, University of Perugia, Piazzale Gambuli, S.Andrea delle Fratte, 06132 Perugia, Italy
Accepted 15 March 2016
Available online 20 April 2016
Artocarpus tonkinensis
BALB/c
Hematological parameters
Lewis lung carcinoma
Maesopsin 4-O-β-glucoside
Objective: To investigate the antitumor eff ect of maesopsin 4-O-β-glucoside (TAT2) isolated from the leaves of Artocarpus tonkinensis (A. tonkinensis) A. Chev. ex Gagnep. Methods: The antitumor activity of TAT2 was evaluated in Lewis lung carcinoma (LLC) tumor-bearing mice. BALB/c mice had tumors induced by implantation with 2×106LLC cells into the subcutaneous right posterior fl ank. Tumor-bearing mice were treated orally with a range of doses of TAT2 and a standard drug, doxorubicin. Animals were observed for tumor growth and mortality rate. Blood was collected to determine hematological and biochemical parameters. Results: TAT2 was isolated from an ethanolic extract of A. tonkinensis leaves. Its structure was determined by MS and NMR spectroscopy, and identifi ed as TAT2. The compound did not show acute toxicity at the highest dose tested (2 000 mg/kg body weight). TAT2 exhibited antitumor activity by decreasing tumor growth, increasing the survival rate, and ameliorating some hematological and biochemical parameters at doses of 100 and 200 mg/kg body weight (P<0.05). Conclusions: These results indicate that TAT2 possesses clear antitumor activity. Due to its bioavailability and low toxicity,and the fact that it could be isolated in a large scale from A. tonkinensis leaves, the compound shows promise as a potential anticancer drug.
1. Introduction
Artocarpus tonkinensis (A. tonkinensis) A. Chev. ex Gagnep. is usually found in North Vietnam. The decoction of its leaves and roots is used in folk medicine to treat backache, rheumatism and joint disorders[1]. A crude extract from A. tonkinensis leaves has been used successfully in Vietnam as an immunosuppressive agent for skin transplantation in mice[2]. In recent years, our group has focused on the chemical identifi cation of the active ingredients in A. tonkinensis leaves. We demonstrated that fl avonoid glycosides such as maesopsin 4-O-β-glucoside (hovetrichoside C, TAT2),alphitonin-O-β-glucoside, artonkin-4’-O-β-glucopyranosid,and astragalin strongly contributed to its immunosuppressive and anti-inflammatory activities[3,4]. These compounds have potent anti-proliferative and anti-infl ammatory eff ects both in vitro and in vivo[4,5]. The anti-inflammatory effects demonstrated in a rat model of arthritis correlated well with the inhibition of mitogeninduced T-cell proliferation[6]. Furthermore, the main compound,TAT2, inhibited the growth of OCI-AML cells (and additional acute myeloid leukemia cells) but not Jurkat cells, a T lymphoma. Moreover, gene expression profi ling identifi ed 19 genes modulated by TAT2 and, among them, indicated HMOX1 and SRXN1 as consistently and highly upregulated[5]. Although TAT2 is a major component (yield 0.2%) of A. tonkinensis leaves, its antitumor activity has not yet been studied in vivo. Due to its promising pharmacological properties, this study focused on the large-scale isolation of TAT2 and its potential anticancer effect in vivo for possible use in alternative medicine.
2. Materials and methods
2.1. Chemicals
Fractionation was performed over DIAION HP20 resin (Supelco,Bellefonte, PA, USA), Sephadex® LH20 (Sigma, St. Louis, MO,USA); silica gel (Merck). Dulbecco’s modified Eagle’s medium and MEM nonessential amino acid were obtained from Invitrogen(Carlsbad, CA, USA). Fetal bovine serum and antibiotics were purchased from Sigma Chemical Co. (St. Louis, MO., USA).
2.2. Plant material, extraction and isolation
Leaves of A. tonkinensis were collected in Hanoi, Vietnam(December 2013) and identified by the taxonomist -Ngo Van Trai (Institute of Materia Medica, Hanoi, Vietnam). The voucher specimen (Nr. 1482-AT-2013) was deposited in the Institute of Chemistry, Vietnam Academy of Science and Technology.
Dried ground leaves of A. tonkinensis (12 kg) were extracted with 70% EtOH (each 36 litres, overnight, ×3 times) at room temperature. The extract was filtered and concentrated in vacuo(temperature 45-60 ℃) to remove EtOH affording an aqueous solution, and partitioned with n-hexan. The aqueous phase was dissolved in water and loading over Diaion HP20 column, washed with water, 50% aq. MeOH and MeOH to give 4 fractions (Fr1→Fr4). Fr3 (eluting with 50% MeOH, 740 g) was chromatographed over Sephadex LH20 eluted with MeOH to give 4 subfractions (Fr3.1→Fr3.4). Fraction 3 (Fr3.3, 350 g) was further chromatographed over silica gel, eluted with EtOAc-MeOH-H2O(75:25:1→70:30:2 and then 65:35:5) to afford 5 sub-fractions(Fr3.3.1→Fr3.3.5). Sub-fraction 4 (Fr3.3.4, 40 g) was further purified by CC (silica gel, EtOAc-MeOH-H2O, 4:1:0.1) to afford compound TAT2 (24 g, yield 0.2%, compared with dried material).
2.3. Identification of purity and characterization of compound TAT2
The purity of compound TAT2 was determined by HPLCDAD (HPCHEM) using a Synergi Fusion RP80A column(250 mm×2.0 mm i.d.) at 25 ℃. UV spectrum was recorded from 190 nm to 400 nm. The mobile phase consisted of A, water containing 0.1% formic acid, and B, acetonitrile containing 0.1% formic acid. The elution gradient was performed using a pump from 10% to 35% B for 50 min; 35% to 80% B from 50 to 55 min at a fl ow rate of 0.25 mL/min. Detection was carried over 60 min at a fl ow rate of 0.25 mL/min. Detection was carried out at 290 and 294 nm. The injection volume was 5 μL. The HPLC chromatogram of TAT2 showed major peak with retention time (Rt) at 20.39 min,percentage 95.8%.
HR-ESI MS spectrum was obtained on QStar Pulsar (Applied Biosystems).1H NMR (500.13 MHz) and13C NMR (125.77 MHz)spectral data were measured in methanol-d4(CD3OD) on a Bruker Avance 500 NMR spectrometer, at 25 ℃. Chemical shifts were expressed in δ (ppm) downfi eld from TMS as an internal standard,and coupling constants were reported in Hertz.
2.4. Animals
Male and female albino BALB/c mice (8-10 weeks old) were obtained from the Institute of Biotechnology, Vietnam Academy of Science and Technology (Hanoi, Vietnam). All mice were housed in a temperature-controlled room on a 12-h light/12-h dark cycle with food and water provided ad libitum. Experiments were performed in accordance with Vietnamese ethical laws and European Communities Council Directives of November 24, 1986 (86/609/ EEC) guidelines for the care and use of laboratory animals.
2.5. Acute toxicity study
The acute oral toxicity of the compound was tested using the OECD GUIDELINE 420[7]. Mice were administered TAT2 orally by using a stomach tube. Animals received the compound in serial doses of 2 000 mg/kg; 1 500 mg/kg; 1 000 mg/kg; and 500 mg/ kg body weight (bw) after 4 h diet. They were observed for 14 d to monitor survival and toxic symptoms.
2.6. Antitumor study
The tumor cell line Lewis lung carcinoma (LLC) was kindly provided by Dr. Jeanette Maier, University of Milan, Italy. The cells were maintained in 75 cm2tissue culture flasks containing Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA, USA), 1% nonessential amino acids and 1% antibiotics (Sigma, St. Louis, MO,USA) at 37 ℃ in a humidified 5% CO2atmosphere. Cells were subcultured every 2-3 d.
2.7. Treatment schedule
Mice were tumorized by implantation with 2×106LLC cells (in 0.2 mL serum-free DMEM) into the subcutaneous right posterior flank. Drug treatment began when the tumor volume reached approximately 100 mm3. The day the LLC cells were implanted wasday 0 of the experiment. Tumor-bearing mice were divided into six groups of ten: Group Ⅰ received distilled water orally and served as a negative control; Group Ⅱ received a standard drug (doxorubicin)at a dose of 5 mg/kg/day; animals in Group Ⅲ, Ⅳ and Ⅴ were treated with TAT2 at doses of 50 mg/kg, 100 mg/kg and 200 mg/ kg bw, respectively. All compounds were administered orally once daily. The animals were observed every day for tumor growth. Body weight, tumor width and length were assessed every 7 d.
2.8. Determination of tumor volume
Based on tumor size, which was measured by calipers, tumor volume was calculated using the following formula[8,9]: volume(cm3) = W2×L/2, where W is the tumor width and L is the tumor length. The percent of tumor growth inhibition was determined using the following equation: % of tumor inhibition=100%-(100×MT×MC-1), where MC is the mean tumor volume of the control group and MT is the mean tumor volume of the treated group.
2.9. Measurement of hematological parameters
Blood from the control and TAT2-treated groups were collected to determine hematological parameters. Blood was obtained from the tail vein and used for the detection of hemoglobin (Hb), red blood cells (RBC), white blood cells (WBC), monocytes, lymphocytes(LYM), platelets (PLT), mean corpuscular (cell) volume, and mean corpuscular hemoglobin concentration using a blood automatic analyzer (AU680, Beckman Coulter). Levels of other enzymes in the sera such as serum glutamate pyruvate transaminase (SGPT),serum aspartate aminotransferase (SGOT) and creatinin were also measured.
2.10. Statistical analysis
Data are expressed as the mean±SD. Statistical analyses and significance, as measured by one-way analysis of variance(ANOVA), were performed using GraphPad PRISM software version 4.0 (GraphPad Software, USA). In all comparisons, P<0.05 was considered statistically signifi cant.
3. Results
3.1. Chemical analysis
In extraction experiment, 70% ethanol was used, as TAT2 is soluble in both ethanol and water. TAT2 was isolated as a yellow amorphous powder from the EtOH extract by repeated column chromatography over DIAION HP20, sephadex LH-20 (MeOH) using appropriate solvent systems. An ion exchange resin (Diaion HP20) column and gel-filtrate (Sephadex LH20) chromatography with appropriate solvent systems were eff ective for preparing the compound on a large scale. The purity of TAT2 was determined to be 95.8% by HPLC. HR-ESI-TOF-MS (m/z): 473.1053 [M+Na]+(C21H22O11Na requires 473.1054). ESI-MS (negative ions): 449 [M-H]-.1H and13C NMR spectral data are shown in Table 1. The HPLC chromatogram of TAT2 is shown in Figure 1 and its structure is shown in Figure 2.
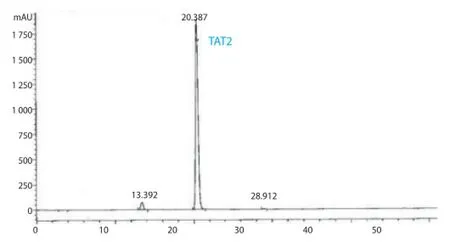
Figure 1. Analytical HPLC chromatograms of compound TAT2 (retention time 20.4 min, purity 95.8%).
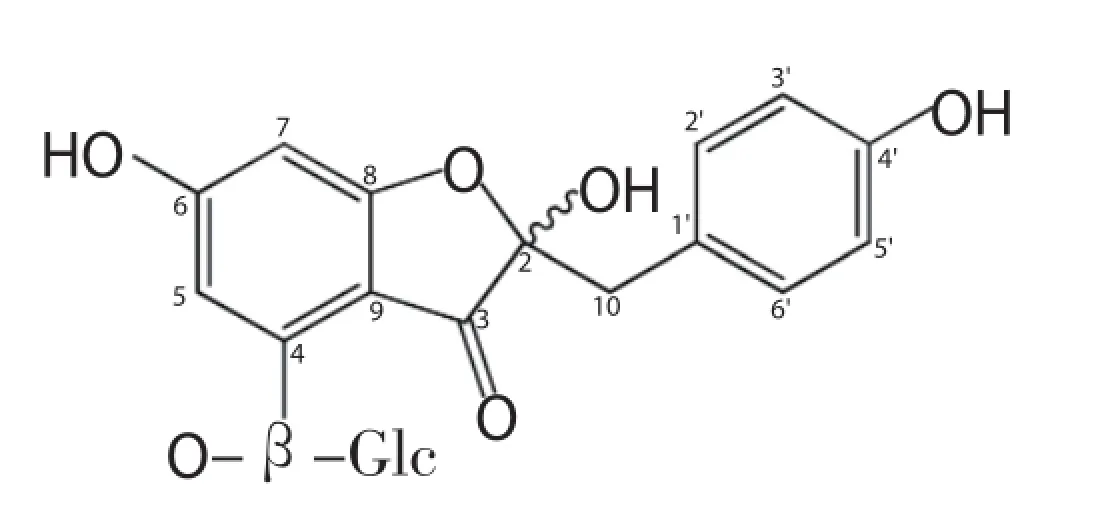
Figure 2. Structure of compound TAT2 isolated from the leaves of A. tonkinensis.
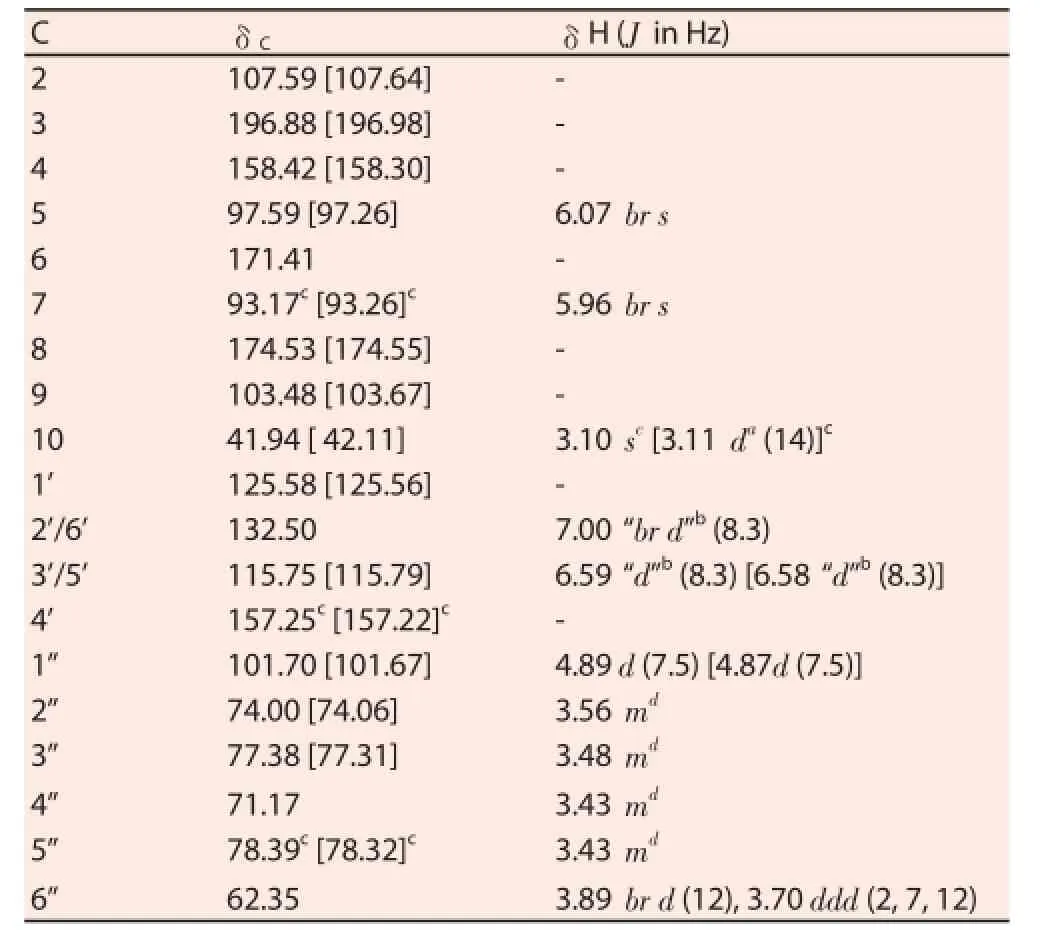
Table 113C- and1H-NMR data of compound TAT2 [CD3OD, 125/500 MHz, δ(ppm)] (shifts of the minor compound in brackets, if diff erent).

Table 2Eff ect of TAT2 on tumor growth in LLC tumor-bearing mice.
3.2. In vivo antitumor activity of TAT2
3.2.1. Acute toxicity studies
Results from the acute toxicity study showed that TAT2 did not cause toxicity in mice even at the highest dose of 2 000 mg/kg body weight. All mice treated with TAT2 survived and did not show any toxic symptoms or abnormal behavior at the tested doses over the 14 d of observation (data not shown). Therefore, TAT2 was considered safe to use to determine the antitumor activity at lower doses (200 mg/kg, 100 mg/kg and 50 mg/kg bw).
3.2.2. Tumor growth and survival indices
In the in vivo antitumor experiment, TAT2 suppressed solid tumor growth in a dose-dependent manner (Table 2). Although the tumor volumes were the same for the fi rst 14 d between the control group and the TAT2-treated groups, TAT2 increased at a much slower speed than the negative control (vehicle-treated group) over the same time period. The data collected at the end of the study demonstrated that TAT2 showed signifi cant activity at doses of 100 mg/kg and 200 mg/kg body weight, with tumor inhibition being 35.47% and 51.24%, respectively (Figure 3).

Figure 3. Percentage of tumor growth inhibition by TAT2.
Tumor weights are shown in Table 3, which indicated that growth was clearly reduced in the TAT2-treated groups at doses of 100 mg/ kg and 200 mg/kg body weight compared with the negative control(P<0.05). The mean tumor weight of the negative control group was(11.95±0.72) g, while the means of the TAT2 -administered groups at doses of 100 mg/kg and 200 mg/kg body weight decreasedto (8.38±1.05) g and (6.36±1.08) g, respectively. There was no significant difference between the body weights of the tumorbearing mice treated with TAT2 and the control group at the end of the experiment (P>0.05). The antitumor activity of the compound was also measured through survival; the survival rate improved considerably in the TAT2-treated groups.

Table 4Eff ect of TAT2 on hematological and biochemical parameters of tumor-bearing mice.
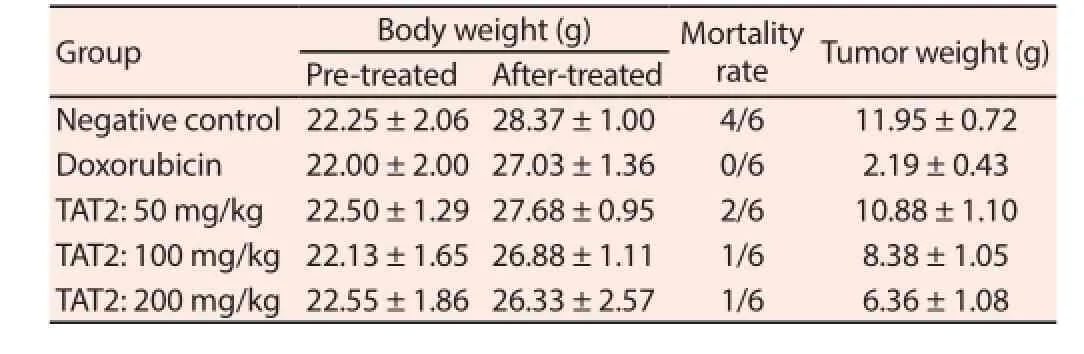
Table 3Eff ect of TAT2 on body weight and tumor weight in LLC-bearing mice.
3.2.3. Hematological parameters
Hematological parameters are presented in Table 4. The WBC and LYM counts were higher in the LLC tumor-bearing mice and lower in the doxorubicin-treated group. TAT2 administration (200 mg/kg body weight) also signifi cantly reduced the number of WBC and LYM compared with the negative control group (P<0.05). In contrast to WBC, the number of RBC and PLT and Hb content were improved in tumor-bearing mice treated with TAT2, but only at doses of 100 and 200 mg/kg body weight.
During the evaluation, the activities of some functional enzymes of the liver and kidney were also measured (Table 4). Although there were no diff erences in SGOT or creatinin levels between groups,the mean SGPT level was (43.05±4.45) UI/L in the group treated with the highest dose of TAT2 and was signifi cantly decreased in comparison with the negative control group (51.73±1.40 UI/L)(P<0.05). These results indicate that TAT2 could ameliorate several hematological and biochemical parameters in LLC-induced tumorbearing mice.
4. Discussion
In traditional medicine, A. tonkinensis leaves are used as immunosuppressive drugs for the treatment of arthritis. To the best of our knowledge, our study is the first to report phytochemical investigations of the leaves of this plant. Compound TAT2 (yield 0.2%, purity 95.8%) was isolated from a 70% EtOH extract of A. tonkinensis leaves by repeated column chromatography. The molecular formula of TAT2 was determined to be C21H22O11by HR ESI MS (m/z 473.1053 [M+Na]+, C21H22O11Na requires 473.1054). The13C NMR spectrum revealed 21 carbons as two sets of signals due to an isomeric hemiketal at C-2. On the basis of the MS results, NMR spectroscopic data and comparison with previously reported data[4,10], its structure was confirmed as TAT2, the rare auronol glycoside in nature[11].
In recent years, the cancer rate has increased, especially in less developed countries. Identification and development of new chemotherapy drugs has been critical for cancer treatment. Plants have been a rich source of natural drugs[12,13]. In addition,compounds derived from plants are diverse in structure and bioactivity and exhibit low toxicity, therefore they play an important role in pharmaceutical research[14]. In cancer therapy, many plantderived drugs such as vincristine, paclitaxel and taxol have been identified and developed. As reported, TAT2 isolated from A. tonkinensis showed promising anticancer properties in vitro[5]. Therefore, we assessed the antitumor activity of this compound in vivo. Because of its high tumorigenic properties, the LLC cell line was selected to create a tumor model for this study. TAT2 caused a signifi cant decrease in tumor growth at various doses, being the most eff ective at a dose of 200 mg/kg body weight.
Inflammatory responses play critical roles in the evolution of cancer from initiation to metastasis. It has become evident that the infl ammatory microenvironment is an essential component of all tumors[15]. Solid tumor growth induces a region of hypoxia,stimulating necrosis in the tumor’s core. In addition, malignant cells also produce many cytokines and chemokines that attract leukocytes[15,16]. Today, inflammation has become a target for cancer prevention and therapy[17]. In this study, the decreased WBC count including LYM indicated that infl ammation was reduced in mice administrated TAT2. This result was in accordance with other reported effects that A. tonkinensis exerted immunomodulatory activity against disorders of chronic inflammation such as rheumatoid arthritis[3,6]. Some compounds isolated from this plant,including TAT2, were found to cause an anti-infl ammatory eff ect due to the inhibition of T-cell proliferation at high concentrations[3]. This explains why the number of LYM was reduced at the highest dose of TAT2 but not at the lowest dose (50 mg/kg body weight) compared to the negative control.
In addition to infl ammation, anemia is a common phenomenon in patients with cancer and impacts survival and quality of life[18]. Anemia causes multiple function disturbances of various organs and tissues[19]. The management of anemia in cancer patient has to improve treatment effi cacy and increase survival[20]. In cancer,anemia occurs due to the reduction of RBC count and Hb levels. Increasing the RBC count and Hb content in TAT2-administrated mice indicated that this compound may improve the hematopoietic system and survival rate. Further, TAT2 had low toxicity when studied in mice. According to these results, TAT2 has potential as anantitumor agent.
In conclusion, the results of the present study indicate that TAT2 can be isolated from A. tonkinensis leaves in large quantities (0.2%). Furthermore, in vivo tests demonstrated a signifi cant inhibition of tumor growth at 100 and 200 mg/kg body weight. These results indicate that TAT2, a potential antitumor agent from A. tonkinensis,may be valuable for preparing functional foods and pharmaceutical medicines.
Conflict of interest statement
We declare that we have no confl ict of interest.
Acknowledgements
The authors thank Vietnam MOST via project 1/39/2013/NDT and the Italian Ministerodegli Esteri e della Cooperazione Internazionale(MAECI) for fi nancial support.
[1] Ma JP, Qiao X, Pan S, Shen H, Zhu GF, Hou AJ. New isoprenylated flavonoids and cytotoxic constituents from Artocarpus tonkinensis. J Asian Nat Prod Res 2010; 12(7): 586-592.
[2] Ha NTV, Anh PT, Chinh TT, Phi PTP, Lam PC. Anapathological changes in spleen, thymus and bone-marrow of mice treated by products of traditional medicine plant (DY1). Revue Pharmaceutique 1994; 2: 51-55.
[3] Dang DT, Eriste E, Liepinsh E, Trinh TT, Erlandsson-Harris H, Sillard R, et al. A novel anti-infl ammatory compound, artonkin-4’-O-glucoside,from the leaves of Artocarpus tonkinensis suppresses experimentally induced arthritis. Scand J Immunol 2009; 69(2): 110-118.
[4] Thuy TT, Kamperdick C, Ninh PT, Lien TP, Thao TT, Sung TV. Immunosuppressive auronol glycosides from Artocarpus tonkinensis. Pharmazie 2004; 59(4): 297-300.
[5] Pozzesi N, Pierangeli S, Vacca C, Falchi L, Pettorossi V, Martelli MP,et al. Maesopsin 4-O-beta-D-glucoside, a natural compound isolated from the leaves of Artocarpus tonkinensis, inhibits proliferation and upregulates HMOX1, SRXN1 and BCAS3 in acute myeloid leukemia. J Chemother 2011; 23(3): 150-157.
[6] Ngoc DD, Catrina AI, Lundberg K, Harris HE, Ha NT, Anh PT, et al. Inhibition by Artocarpus tonkinensis of the development of collageninduced arthritis in rats. Scand J Immunol 2005; 61(3): 234-241.
[7] OECD. Test No. 420: Acute Oral Toxicity - Fixed Dose Procedure.[Online] Available at: https://ntp.niehs.nih.gov/iccvam/suppdocs/ feddocs/oecd/oecd_gl420.pdf.
[8] Zhou X, Shi H, Jiang G, Zhou Y, Xu J. Antitumor activities of ginseng polysaccharide in C57BL/6 mice with Lewis lung carcinoma. Tumour Biol 2014; 35(12): 12561-12566.
[9] Chen KJ, Tang L, Garcia MA, Wang H, Lu H, Lin WY, et al. The therapeutic efficacy of camptothecin-encapsulated supramolecular nanoparticles. Biomaterials 2012; 33(4): 1162-1169.
[10] Wu SB, Wen Y, Li XW, Zhao Y, Zhao Z, Hu JF. Chemical constituents from the fruits of Sonneratia caseolaris and Sonneratia ovata(Sonneratiaceae). Biochem Syst Ecol 2009; 37(1): 1-5.
[11] Yoshikawa K, Eiko K, Mimura N, Kondo Y, Arihara S. Hovetrichosides C-G, five new glycosides of two auronols, two neolignans, and a phenylpropanoid from the bark of Hovenia trichocarea. J Nat Prod 1998;61(6): 786-790.
[12] Cragg GM, Newman DJ. Natural products: a continuing source of novel drug leads. Biochim Biophys Acta 2013; 1830(6): 3670-3695.
[13] Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod 2012; 75(3): 311-335.
[14] Gali-Muhtasib H, Hmadi R, Kareh M, Tohme R, Darwiche N. Cell death mechanisms of plant-derived anticancer drugs: beyond apoptosis. Apoptosis 2015; 20(12): 1531-1562.
[15] Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010; 140(6): 883-899.
[16] Balkwill FR. The chemokine system and cancer. J Pathol 2012; 226(2): 148-157.
[17] Rayburn ER, Ezell SJ, Zhang R. Anti-Inflammatory Agents for Cancer Therapy. Mol Cell Pharmacol 2009; 1(1): 29-43.
[18] Schrijvers D. Management of anemia in cancer patients: transfusions. Oncologist 2011; 16(suppl 3): 12-18.
[19] Dicato M. Anemia in cancer: some pathophysiological aspects. Oncologist 2003; 1: 19-21.
[20] Calabrich A, Katz A. Management of anemia in cancer patients. Future Oncol 2011; 7(4): 507-517.
ent heading
10.1016/j.apjtm.2016.03.012
15 January 2016
Do Thi Thao, Dr., Institute of Biotechnology, Vietnam Academy of Science and Technology, 18-Hoang Quoc Viet, Nghia Do, CauGiay,Hanoi, Vietnam.
Tel: +84438361774
Phone: 84(0)904588486
E-mail: thaodo74@yahoo.com; thaodo@ibt.ac.vn
Prof. Trinh Thi Thuy, Dr., Institute of Chemistry, Vietnam Academy of Science and Technology, 18-Hoang Quoc Viet, Nghia Do, CauGiay, Hanoi, Vietnam.
Tel: +84432121149
Phone: 84(0)978987562
Fax: +84438361283
E-mail: thuy@ich.vast.vn; drthuy2001@yahoo.de
in revised form 20 February 2016
ARTICLE INFO
Article history:
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Determination of ligand cluster and binding site within VP40 of Ebola virus: clue for drug development
- Clinacanthus nutans: a review of the medicinal uses, pharmacology and phytochemistry
- Current perspectives on dengue episode in Malaysia
- Etiological agents causing leptospirosis in Sri Lanka: A review
- Phylogeny of Murray Valley encephalitis virus in Australia and Papua New Guinea
- Dengue outbreak in Swat and Mansehra, Pakistan 2013; an epidemiological and diagnostic perspective
