In vitro antiplasmodial and antioxidant activities of bisbenzylisoquinoline alkaloids from Alseodaphne corneri Kosterm
2016-11-14AzeanaZahariAbdulwaliAblatYasodhaSivasothyJamaludinMohamadMuhammadChoudharyKhalijahAwang
Azeana Zahari, Abdulwali Ablat, Yasodha Sivasothy, Jamaludin Mohamad, Muhammad I. Choudhary, Khalijah Awang*
1Department of Chemistry, Faculty of Science, University of Malaya, 50603 Kuala Lumpur, Malaysia
2Institute of Biological Sciences, Faculty of Science, University of Malaya, 50603 Kuala Lumpur, Malaysia
3H.E.J. Research Institute of Chemistry, International Center for Chemical and Biological Sciences, University of Karachi, Karachi - 75270, Pakistan
In vitro antiplasmodial and antioxidant activities of bisbenzylisoquinoline alkaloids from Alseodaphne corneri Kosterm
Azeana Zahari1, Abdulwali Ablat2, Yasodha Sivasothy1, Jamaludin Mohamad2, Muhammad I. Choudhary3, Khalijah Awang1*
1Department of Chemistry, Faculty of Science, University of Malaya, 50603 Kuala Lumpur, Malaysia
2Institute of Biological Sciences, Faculty of Science, University of Malaya, 50603 Kuala Lumpur, Malaysia
3H.E.J. Research Institute of Chemistry, International Center for Chemical and Biological Sciences, University of Karachi, Karachi - 75270, Pakistan
Accepted 15 March 2016
Available online 20 April 2016
Bisbenzylisoquinoline
Laurotetanine
Norstephasubine
Antiplasmodial
Antioxidant
Oxidative stress
Objective: To study antiplasmodial and antioxidant activities of the isolation of alkaloids from the active dichloromethane extract of Alseodaphne corneri. Methods: Phytochemical studies of the crude extract led to the isolation of six alkaloids using recycle high performance liquid chromatography and preparative thin layer chromatography. The antiplasmodial activity of the isolated compounds was evaluated usingthe histidine-rich protein II assay. The isolated alkaloids were also tested for their antioxidant activity using three diff erent assays; DPPH,ferric reducing ability of plasma and metal chelating assays. Results: Malaria infection caused the formation of free radicals which subsequently led to oxidative stress and apoptosis. The antioxidant properties of the alkaloids under investigation revealed that in addition to the antiplasmodial activity, the alkaloids could also prevent oxidative stress. (+)-laurotetanine and (+)-norstephasubine exhibited strong antiplamodial activities with IC50values of 0.189 and 0.116 μM, respectively. Conclusions: Interestingly, the two most potent compounds that exhibit antiplasmodial activity also exhibit good antioxidant activities. The crude dichloromethane extract and the isolated compounds exert substantial antiplasmodial and antioxidative activities which in turn suppress oxidative stress and cause less damage to the host.
1. Introduction
In modern world, vector-borne diseases still pose a great threat to human health. Those from mosquitoes have killed millions of human beings every year. Malaria is one of the serious vectorborne diseases that people can catch after bitten by Anopheles mosquitoes that are infected with parasites called Plasmodium. Four different types of Plasmodium [Plasmodium Falciparum (P. falciparum), Plasmodium vivax, Plasmodium ovale, and Plasmodium malariae] affect humans. According to the World Health Organization[1], malaria is presently considered endemic and has caused approximately 198 million infections each year especially in developing countries, with more than 584 000 deaths in 2013. In Malaysia, 3 850 malaria cases have been reported with 14 deaths in the same year[2]. Plasmodium knowlesi followed by P. falciparum are recognized as the main cause of malaria in Malaysia[3,4]. Although the malaria rate has decreased worldwide, however, the resistance to drug therapy has increased. Chloroquine resistant was first reported in Peninsular Malaysia as early as 1963[2]. Present drugs,artemisinin and chloroquine, have become ineff ective because of the occurrence detected in 4 countries, Cambodia, Myanmar, Thailand and Vietnam that are resistant to P. falciparum[1].
Plasmodium must acquire nutrients from the environment (host)and convert it into other molecules or energy. This energy will eventually be used for survival and the reproduction of the parasites. The Plasmodium spp. requires amino acids for the synthesis of their proteins from the host or through the digestion of host hemoglobin. Growing substantial evidences have shown that malaria arises as a result of free radical generation from reactive oxygen species (ROS). Generation of ROS which induces oxidative stress has played an important role in the development of systematic complication in hosts caused by malaria infection[5,6]. Wide varieties of compounds with synthetic antioxidative properties have been used to reduce ROS formation and protect DNA, lipid and protein damage against oxidative stress. The preference of natural products is becoming signifi cant due to the awareness of the public on the negative side effects caused by synthetic drugs[7]. Therefore, natural products could provide a template molecule which in turn leads to producing eff ective antimalarial drugs. The combination of drugs that possess diff erent activities, artemisinin (antimalarial) and metalloporphyrins(oxidizing reagents) acting synergistically on strains of P. falciparum,has been reported[8]. It is known that this synergism improves the survival rates, reduces the development of resistance and might decrease the transmission of drug resistant parasites[9]. Thus, a single drug that can possess both antiplasmodial and antioxidant activities simultaneously suppressing malaria, might be a better choice compared to treatment with multiple drugs.
The genus Alseodaphne belongs to the Lauraceae family which is widely distributed from Yunan to West Malaysia, Sri Lanka and Burma. About 62 species have been identified till present,which include trees or shrubs whereby 20 species are known to occur in Malaysia. Alseodaphne is locally known as ‘Medang’. The plants of this genus have been used as furniture, and to build boats and houses. Alseodaphne corneri (A. corneri) is a small tree,with about 6 m in height. Terminal bud is covered with many glabrous scales. The twigs are stout and grey in colour with prominent leaf scars[10]. This genus is a rich source of isoquinoline alkaloids particularly bisbenzylisoquinoline (BBIQ) alkaloids that exhibit a wide range of pharmacological activities such as antiinfl ammatory, antibacterial and vasorelaxant[11,12]. Most of BBIQ have been isolated from families Menispermaceae, Berberidaceae,Monimaceae and Ranunculaceae plants[13]. Our continuing activities on antiplasmodial screening of Malaysian plants have revealed that dichloromethane (CH2Cl2) bark extracts of A. corneri exhibited promising antiplasmodial activity (IC50= 2.78 μg/mL)[14,15]. Hence,in this study, antiplasmodial and antioxidant activities of the isolation of alkaloids from the active extract was explored.
2. Materials and methods
2.1. Instruments
Spectra were recorded using the following instruments; UV,Shimadzu UV-250, UV-Visible spectrometer; IR, Perkin Elmer 1600; NMR, AVN BRUKER with CDCl3as the solvent to obtain the 400 MHz proton and 100 MHz carbon spectra. Mass spectra were obtained using on Agilent technologies 6530 Accurate-Mass Q-TOF liquid chromatography/mass spectrometry, with ZORBAX Eclipse XDB-C18 Rapid Resolution HT 4.6 mm i.d. × 50 mm × 1.8 μm column. All solvents, except those used for bulk extraction were AR grade. Column chromatography separations were conducted by using Merck silica gel 60 (230-400 mesh) and silica gel 60 F254for thin layer chromatography (TLC) monitoring. Recycle high performance liquid chromatography was performed on LC-908WC60. Chromatographic analysis and separations were performed on a JAIGEL GS320 (21.5 mm internal diameter, 500 mm L, 13 mm)size exclusion column using methanol (MeOH) as the solvent. Glass and aluminium supported silica gel 60 F254plates were used for TLC. TLC spots were visualized under UV light (254 nm and 365 nm) followed by spraying with Dragendroff ’s reagent for alkaloid detection.
2.2. Plant material
The bark of A. corneri Kosterm was collected from Hutan Simpan Kenderong, Gerik, Perak. The plant specimen was identified by Eng and Nor and a voucher specimen (KL5641) of this plant was deposited at the Herbarium of the Department of Chemistry,University of Malaya, Kuala Lumpur, Malaysia.
2.3. Extraction and separation
Plant extraction was carried out by cold percolation. Dried grounded bark of A. corneri (2.0 kg) was fi rst defatted with hexane(17 L) for three days at room temperature. Then, the hexane extract was fi lters and dried on the rotary evaporator. The residues were dried and then moistened with 25% ammonia solution and left for 2 h. The 40.0 g crude CH2Cl2extract of alkaloids was subjected to column chromatography using silica gel (0.040-0.063 mm) as the stationary phase using mixtures of (CH2Cl2: MeOH) as the eluting solvent (100:0, 99:1, 97:3, 96:4, and 90:1) to obtain eight fractions(F1-F8). Alkaloid was purified from fraction F5 by a recycle high performance liquid chromatography over JAIGEL size exclusion column (21.5 mm internal diameter, 500 mm L, 13 mm) using MeOH as solvent at a fl ow rate of 4.0 mL/min. Three recycles in a duration of 40 min aff orded (-)-gyrolidine with retention times of 35 min. Further purifi cation of fraction F6 by a preparative TLC using CH2Cl2: MeOH with 94:6; v/v, saturated with NH4OH; Dragendroff reagent gave alkaloids (+)-O-methyllimacusine, (+)-2-norobaberine,(+)-laurotetanine, (+)-norstephasubine and (+)-stephasubine.
2.4. Antiplasmodial assay
The crude CH2Cl2extract and the isolated compounds were evaluated for their in vitro antiplasmodial activity against P. falciparum strain K1 which was resistant to chloroquine. Chloroquine diphosphate was used as positive controls. The screening was basedon the ability to culture P. falciparum in human erthrocytes in vitro. It was maintained in continuous culture as described by Trager et al[16] with some modifi cation[17]. The synchronization of the malaria culture to one stage was by Lambros et al[18]. Antiplasmodial activity was evaluated using histidine-rich protein II assay by enzyme linked immunosorbent assay[19]. Micro titration techniques were used to measure the activity of samples over a wide range of concentrations. All tests were performed in duplicate. Crude extract was dissolved in DMSO to produce a stock solution of 20 mg/mL. The stock solutions were subsequently diluted with deionized water at 20 concentrations of two-fold dilutions into two 96-well microtiter plates. A total of 10 μL of each concentration was transferred into another 96-well microtiter plates. A total of 200 μL of parasitized red blood cell suspension (1% parasitemia) were added to it. The mixtures were incubated for 24 h at 37 ℃ and were subsequently cooled at -20 ℃ to lyse the red blood cells. The plates were allowed at room temperature, and 20 μL of the blood suspension was dispensed into a new microtiter plate containing 100 μL MALSTAT reagent, 20 μL nitroblue tetrazolium and phenazine ethanosulphate mixture. Absorbance was measured with an ELISA plate reader at 780 nm. The percentage inhibition at each concentration was determined and the mean of IC50values of parasite sustainability was calculated using analysis. The antiplasmodial activity of each compound was expressed as an IC50value defi ned as the concentration of the compounds causing 50% inhibition of parasite growth relative to untreated control[20,21].
2.5. Antioxidant assay
2.5.1. DPPH assay
The DPPH scavenging activity of pure compounds were tested based on the method previously published[22]. Briefly, 40 μL of purifi ed compounds at diff erent concentrations were mixed with 200 μL of 50 μM DPPH solution in MeOH. The mixture was immediately shaken and incubated for 15 min in the dark at room temperature. The decrease in absorbance was measured at 517 nm with a microplate reader (Tecan Sunrise, Austria). Ascorbic acid was used as a standard and the control was MeOH. The percentage of inhibition activity of the compounds was calculated (n= 3) and results were presented in Table 1.
2.5.2. Metal chelating activity assay
The ferum ion chelating activity of the purifi ed compounds was determined according to the previously published[22] by measuring the formation of the Fe2+- ferrozine complex in the reaction mixture. Briefly, 100 μL of purified compounds or standards (6.25-100 μg/mL) were mixed with 120 μL distilled water and 10 μL FeCl2(2 mM) in a 96-well microplate and the absorbance was read as blank. Then, 20 μL of ferrozine (5 mM) was added to the mixture to initiate the reaction. The reaction mixture was incubated at room temperature for 20 min and the absorbance at 562 nm was measured. The results were presented in Table 1 and butylated hydroxyanisole was used as the standard reference.
2.5.3. FRAP
The reducing power was determined using the method of Oyaizu[23]. The tested compounds (0.5 mL) dissolved in ethanol at different doses (0, 50, 100, 150, 200 μg/mL) were mixed with phosphate buff er (0.5 mL, 0.2 M, pH 6.6) and potassium ferricyanide[K3Fe(CN)6] (0.5 mL, 1%). The mixture was then incubated at 50 ℃ for 20 min. A total of 0.5 mL trichloroacetic acid (10%) was added to the mixture, which was then centrifuged for 10 min at 3 000 rpm (1 000 g). The upper layer of the solution (0.5 mL) was mixed with distilled water (0.5 mL) and FeCl3(0.1 mL, 0.1%) for 10 min, and then the absorbance was measured at 700 nm using a spectrophotometer. Ethylenediaminetetraacetic acid was used as the standard reference (Table 1).
3. Results
This report communicated the isolation and characterization of fi ve BBIQ alkaloids (Figure 1), (-)-gyrolidine, (+)-O-methyllimacusine,(+)-2-norobaberine, (+)-norstephasubine, (+)-stephasubine, and oneaporphine alkaloid (+)-laurotetanine from the CH2Cl2bark extract of A. corneri.
Structural elucidation was performed with the aid of spectroscopic methods notably; UV, IR, liquid chromatography/Mass spectrometry,1D and 2D NMR (COSY, HMBC, HMQC, NOESY). The ESIMS showed a pseudomolecular ion peak m/z at 623.30, 623.30,609.29, 328.16, 576.23, and 591.13 corresponding to alkaloids(-)-gyrolidine, (+)-O-methyllimacusine, (+)-2-norobaberine,(+)-laurotetanine, (+)-norstephasubine and (+)-stephasubine respectively. All the BBIQ alkaloids belonged to type VI that had two diphenyl ether linkages between (C7-O-C8’) and (C11-OC12’). Generally, this could be deduced from presence of seven aromatic proton signals in the form of ABX and AA’BB’ systems,representing H-10, H-13, H-14 (ring C) and H-10’, H-11’, H-13’,H-14’ (ring C’) respectively. As for the aporphine alkaloid,(+)-laurotetanine, only three aromatic proton signals appeared at δH6.58, δH6.78, δδH8.06 corresponding to H-3, H-8 and H-11. The13C NMR spectrum of BBIQ showed between 34-36 carbon signals depending on the number of substituents; whereas, aporphine only gave 20 carbon signals. In addition, this was the fi rst occurrence of both antiplasmodial and antioxidant activities of (-)-gyrolidine,(+)-O-methyllimacusine, (+)-norstephasubine and (+)-stephasubine. Among the six compounds evaluated for their antiplasmodial activity, (+)-norstephasubine clearly showed the most potent in vitro antiplasmodial activity with an IC50value of 0.116 μM that was slightly better than the standard, chloroquine (IC50= 0.090 μM). In addition, (+)-laurotetanine also displayed a strong inhibition capacity with an IC50value of 0.189 μM, followed by; (-)-gyrolidine,(+)-2-norobaberine , (+)-O-methyllimacusine , and (+)-stephasubine(Table 1).
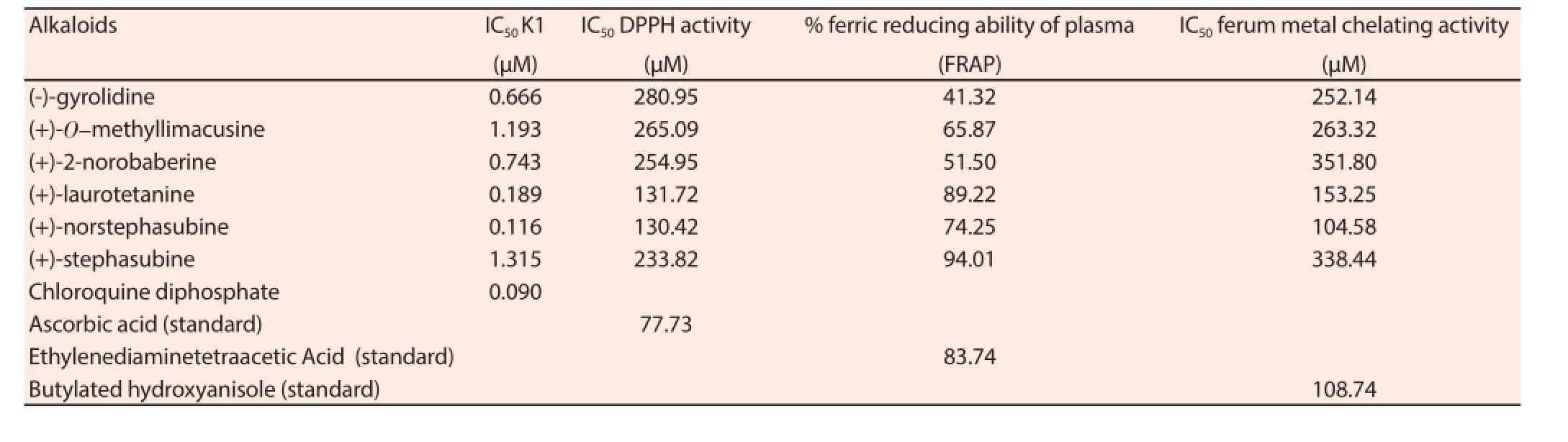
Table 1Antiplasmodial and antioxidant activities of isolated compounds from A. corneri.
Furthermore, in order to evaluate the relationship between suppression of oxidative stress and the cause of malaria, various antioxidant assays (DPPH, FRAP, ferum metal chelating) were performed. Interestingly, the two most potent compounds that revealed antiplasmodial activity also exhibited good antioxidant activities. Both (+)-laurotetanine and (+)-norstephasubine showed high DPPH free radical scavenging activity and ferum metal chelating activities which indicated that they were good reductants and they also possessed the ability to chelate ferum and prevent prooxidant activity (Table 1).

Figure 1. Structures of isolated compounds from A. corneri.1: (-)-gyrolidine; 2: (+)-O-methyllimacusine; 3: (+)-2-norobaberine; 4:(+)-laurotetanine; 5: (+)-norstephasubine; 6: (+)-stephasubine.
4. Discussion
The importance of antioxidant activity was to reverse or minimize the oxidative damage to the hosts caused by Plasmodium parasite from malaria infection. The iron bound in hemoglobin is in Fe2+form, and upon destruction of the hemoglobin, oxidation of Fe2+to Fe3+occurs, followed by the release of a free electron that eventually generate ROS. The generation of ROS such as hydrogen peroxide,hydroxyl radical and superoxide anion radical will induce oxidative damage[24,25]. Malaria infection induces the generation of the hydroxyl radical in the liver, which will most probably lead to the induction of oxidative stress and apoptosis[8].
It is worthy to note that, Plasmodium parasites from malaria infection invade the host hemoglobin to make their own protein. Upon destruction, free heme (ferum atom) will be released and converted to hemazoin which is important for the survival of Plasmodium parasites. Therefore by having strong ferum chelation,as shown by (+)-laurotetanine and (+)-norstephasubine, the killing of Plasmodium by binding to toxic free heme could prevent the formation of hemazoin[8,26].
The high antioxidant activities of those alkaloids may be due to the hydroxyl group that could donate electron to the free radicals which showed high radical scavenging activities that could suppress the oxidative stress on the host. The hydroxyl group that could donate electron to the notorious free radical could be the reason why it possesses potent radical scavenging activities (DPPH)[27,28].
Malaria infection causes the formation of free radicals which subsequently leads to oxidative stress and apoptosis. The crude CH2Cl2extract and the isolated compounds from the bark of A. corneri exert substantial antiplasmodial and antioxidative effects through the DPPH free radical scavenging FRAP and metal chelating activities which could suppress oxidative stress that cause less damage to the host. The two most potent antiplasmodial alkaloids,(+)-laurotetanine and (+)-norstephasubine, also exhibited potent FRAP and metal chelating antioxidant activities. Therefore, these alkaloids may be good candidates for drug development of potential antimalarial properties possessing antioxidant capability.
Conflicts of Interest Statement
The authors declare no confl ict of interest.
Acknowledgments
This work was supported by University of Malaya Research Grant(RP001/2012A), (RP001/2012B) and Postgraduate Research Fundsof University of Malaya (PV050/2012A). The authors would like to thank Mr. Teo Leong Eng, Mr Din Mat Nor and Mr Rafly Syamsir from Herbarium Group of Chemistry Department, University of Malaya, Kuala Lumpur, Malaysia. Special thanks to the Institute of Medical Research, Kuala Lumpur, Malaysia for conducting of antiplasmodial activities.
[1] WHO. World malaria report 2014. Geneva: World Health Organization;2014. p. 242.
[2] Malaysia MoH. Guidelines of malaria in Malaysia. Division DC ed. Malaysia: Ministry of Health Malaysia; 2014.
[3] Rajahram G, Barber B, William T, Menon J, Anstey N, Yeo T. Deaths due to Plasmodium knowlesi malaria in Sabah, Malaysia: association with reporting as Plasmodium malariae and delayed parenteral artesunate. Malar J 2012; 11: 284.
[4] William T, Rahman H, Jelip J, Ibrahim M, Menon J, Grigg M, et al. Increasing incidence of Plasmodium knowlesi malaria following control of P. falciparum and P. vivax Malaria in Sabah, Malaysia. PLoS Negl Trop Dis 2013; 7(1): e2026.
[5] Nethengwe MF, Opoku AR, Dludla PV, Madida KT, Shonhai A, Smith P, et al. Larvicidal, antipyretic and antiplasmodial activity of some Zulu medicinal plants. J Med Plants Res 2012; 6(7): 1255-1262.
[6] Tchinda AT, Fuendjiep V, Sajjad A, Matchawe C, Wafo P, Khan S,et al. Bioactive compounds from the fruits of Zanthoxylum leprieurii. Pharmacologyonline 2009; 1: 406-415.
[7] Umemura T, Kodama Y, Hioki K, Nomura T, Nishikawa A, Hirose M, et al. The mouse rasH2/BHT model as an in vivo rapid assay for lung carcinogens. Cancer Sci 2002; 93(8): 861-866.
[8] Percario S, Moreira DR, Gomes BAQ, Ferreira MES, Goncalves ACM,Laurindo PSOC, et al. Oxidative stress in malaria. Int J Mol Sci 2012; 13(12): 16346-16372.
[9] Nosten F, White NJ. Artemisinin-based combination treatment of falciparum malaria. Am J Trop Med Hyg 2007; 77(Suppl 6): 181-192.
[10]Whitmore TC, Ng FSP. Tree flora of malaya: a manual for foresters. Forest Research Institute Malaysia: Longman; 1989.
[11]K Thakur B, Anthwal A, Singh Rawat D, Rawat B, Rashmi, Rawat MSM. A review on genus alseodaphne: phytochemistry and pharmacology. Mini-Rev Org Chem 2012; 9(4): 433-445.
[12]Mukhtar MR, Zahari A, Nafiah MA, Hadi AHA, Thomas NF, Arai H, et al. 3',4'-dihydronorstephasubine, a new bisbenzylisoquinoline from the bark of Alseodaphne corneri. Heterocycles 2009; 78(10): 2571-2578.
[13]Phillipson JD, Roberts MF, Zenk M. The chemistry and biology of isoquinoline alkaloids. 1st ed. Berlin Heidelberg: Springer-Verlag; 1985.
[14]Zahari A, Cheah F, Mohamad J, Sulaiman S, Litaudon M, Leong K, et al. Antiplasmodial and antioxidant isoquinoline alkaloids from Dehaasia longipedicellata. Planta Medica 2014; 80(7): 599-603.
[15]Nasrullah AA, Zahari A, Mohamad J, Awang K. Antiplasmodial alkaloids from the bark of Cryptocarya nigra (Lauraceae). Molecules 2013; 18: 8009-8017.
[16]Trager W, Jensen JB. Human malaria parasites in continuous culture. Science 1976; 193(4254): 673-675.
[17]Makler MT, Hinrichs DJ. Measurement of the lactate dehydrogenase activity of Plasmodium falciparum as an assessment of parasitemia. Am J Trop Med Hyg 1993; 48(2): 205-210.
[18]Lambros C, Vanderberg JP. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol 1979; 65(3): 418-420.
[19]Noedl H, Bronnert J, Yingyuen K, Attlmayr B, Kollaritsch H, Fukuda M. Simple histidine-rich protein 2 double-site sandwich enzyme-linked immunosorbent assay for use in malaria drug sensitivity testing. Antimicrob Agents Chemother 2005; 49(8): 3575-3577.
[20]Chan KL, Choo CY, Abdullah NR, Ismail Z. Antiplasmodial studies of Eurycoma longifolia Jack using the lactate dehydrogenase assay of Plasmodium falciparum. J Ethnopharmacol 2004; 92(2-3): 223-227.
[21]Adjalley SH, Lee MCS, Fidock DA. A method for rapid genetic integration into Plasmodium falciparum utilizing mycobacteriophage Bxb1 integrase. Methods Mol Biol 2010; 634: 87-100.
[22]Ablat A, Mohamad J, Awang K, Shilpi JA, Arya A. Evaluation of antidiabetic and antioxidant properties of Brucea javanica seed. Sci World J 2014; 2014: 8.
[23]Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr 1986; 44: 307-315.
[24]Greve B, Lehman LG, Lell B, Luckner D, Schmidt-Ott R, Kremsner PG. High oxygen radical production is associated with fast parasite clearance in children with Plasmodium falciparum malaria. J Infect Dis 1999; 179: 1584-1586.
[25]Yang MH, Yoon KD, Chin YW, Park JH, Kim J. Phenolic compounds with radical scavenging and cyclooxygenase-2 (COX-2) inhibitory activities from Dioscorea opposita. Bioorg Med Chem 2009; 17(7): 2689-2694.
[26]Warhurst D, Craig J, Adagu I, Meyer D, Lee S. The relationship of physico-chemical properties and structure to the diff erential antiplasmodial activity of the cinchona alkaloids. Malaria J 2003; 2: 26.
[27]Jang M, Kim H, Kang K, Yokozawa T, Park J. Hydroxyl radical scavenging activities of isoquinoline alkaloids isolated from Coptis chinensis. Arch Pharm Res 2009; 32(3): 341-345.
[28]Pradines B, Rolain JM, Ramiandrasoa F, Fusai T, Mosnier J, Rogier C,et al. Iron chelators as antimalarial agents: in vitro activity of dicatecholate against Plasmodium falciparum. J Antimicrob Chemoth 2002; 50: 177-187.
ent heading
10.1016/j.apjtm.2016.03.008
15 January 2016
Khalijah Awang, Ph.D, Professor, Department of Chemistry,Faculty of Science, University of Malaya, 50603 Kuala Lumpur, Malaysia.
E-mail: khalijah@um.edu.my
Tel: +6 037967 4064
Fax: +6 037967 419
Foundation project: This work was supported by University of Malaya Research Grant (RP001/2012A), (RP001/2012B) and Postgraduate Research Funds of University of Malaya (PV050/2012A).
in revised form 20 February 2016
ARTICLE INFO
Article history:
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Determination of ligand cluster and binding site within VP40 of Ebola virus: clue for drug development
- Clinacanthus nutans: a review of the medicinal uses, pharmacology and phytochemistry
- Current perspectives on dengue episode in Malaysia
- Etiological agents causing leptospirosis in Sri Lanka: A review
- Phylogeny of Murray Valley encephalitis virus in Australia and Papua New Guinea
- Dengue outbreak in Swat and Mansehra, Pakistan 2013; an epidemiological and diagnostic perspective
