Molecular survey on zoonotic tick-borne bacteria and chlamydiae in feral pigeons (Columba livia domestica)
2016-11-14ValentinaVirginiaEbaniFabrizioBertelloniPaoloMani
Valentina Virginia Ebani, Fabrizio Bertelloni, Paolo Mani
Department of Veterinary Science, University of Pisa, Viale delle Piagge, 2, 56124 Pisa, Italy
Molecular survey on zoonotic tick-borne bacteria and chlamydiae in feral pigeons (Columba livia domestica)
Valentina Virginia Ebani*, Fabrizio Bertelloni, Paolo Mani
Department of Veterinary Science, University of Pisa, Viale delle Piagge, 2, 56124 Pisa, Italy
Accepted 15 March 2016
Available online 20 April 2016
Pigeon (Columba livia domestica)
Tick-borne bacteria
Chlamydophila psittaci
PCR
Objective: To determine the presence of zoonotic tick-borne bacteria in feral pigeons (Columba livia domestica) from urban areas. Methods: Spleen samples from 84 feral pigeons, found dead with traumatic injuries in urban areas, were examined by PCR to detect DNA of Anaplasma phagocytophilum, Bartonella spp., Borrelia burgdorferi sensu lato, Coxiella burnetii, Rickettsia spp., and Chlamydophila spp. Results: Twenty (23.8%) pigeons were infected by tick-borne agents, in particular 2 (2.38%) animals resulted positive for Bartonella spp., 5 (5.95%) for Coxiella burnetii, 5 (5.95%) for Rickettsia spp., 13 (15.47%) for Borrelia burgdorferi sensu lato. All birds scored negative for Anaplasma phagocytophilum. Moreover, 17 (20.23%) pigeons were positive for Chlamydophila spp. and among them 10 (11.9%) for Chlamydophila psittaci. Mixed infections by two or three agents were detected in 8 (9.52%) animals. Conclusions: Feral pigeons living in urban and periurban areas are a hazard for the human health as source of several pathogens. The obtained results confi rm pigeons as reservoirs of chlamydial agents and suggest that they may be involved in the epidemiology of zoonotic tick-borne infections too.
1. Introduction
Urban and periurban areas are frequently home to wild birds,particularly feral pigeons (Columba livia domestica), which can be present at high density. These animals are known as reservoirs of zoonotic viruses, bacteria, fungi and protozoa[1].
In particular, columbiform birds, including pigeons, have been ranked as the second major reservoir, after psittaciformes, of Chlamydophila psittaci (C. psittaci)[2]. This is a highly infectious,obligate intracellular bacterium which aff ects a wide range of avian hosts inducing asymptomatic forms or pneumonia, poor growth,diarrhoea and central nervous system disorders. C. psittaci is transmissible to humans causing severe zoonotic infections[3].
Wild birds, including feral pigeons, are often carriers of infected ticks. In particular, the most common hard tick species associated with mammals and avian hosts in Europe is Ixodes ricinus, which is known as a competent vector of viral, bacterial and protozoan agents of medical and veterinary importance[4].
Some studies were carried out on vector-borne pathogens in arthropods collected from birds, whereas very little information is available about the presence of tick-borne bacteria in these animals. Previous surveys found Coxiella burnetii (C. burnetii), the causative agent of Q fever, in feral pigeons and other birds[5,6].
Bartonella henselae DNA was detected in two northern mockingbirds (Mimus polyglottus) and one red-winged blackbird(Agelaius phoeniceus), and Bartonella koehlerae in a red-bellied woodpecker (Melanerpes carolinus) and a common loon (Gavia immer) in North Carolina, USA[7].
Rickettsia helvetica (R. helvetica), frequently involved in severe cases of human disease, was recently found in passerine birds,in particular five robins (Erithacus rubecula) and one dunnock(Prunella modularis)[8].
In Europe, wild birds belonging to different species scored positive to Anaplasma phagocytophilum (A. phagocytophilum) by PCR[8,9]. Borrelia burgdorferi (B. burgdorferi) sensu lato (s.l.), agent of the Lyme disease, was frequently detected in bird-associated ticks and in some studies it was directly found in avian specimens worldwide[10].
At the best of our knowledge, no data were reported about the spreading of arthropod-borne agents among avian populations in Italy. The aim of this study was to evaluate the presence of some zoonotic tick-borne bacteria, in particular A. phagocytophilum,Bartonella spp., B. burgdorferi s.l., C. burnetii, and Rickettsia spp., in feral pigeons found dead in urban areas of Tuscany, Central Italy. The same specimens were also tested for C. psittaci DNA to monitor the spreading of this zoonotic agent among feral pigeons.
2. Material and methods
2.1. Sampling
During 2011-2013, 84 feral pigeons (Columba livia domestica) were examined in the Avian Pathology Division of the Department of Veterinary Science, University of Pisa. All animals had been found dead and collected by private citizens in diff erent urban areas of Tuscany.
The animals were submitted to necropsies, and spleen samples were collected. Animals’ gender and age were also established: 55 pigeons were males and 29 females; 33 were considered young, 51 adult. It was generally accepted that complete development of the left ovary in females and testicles in males was achieved at the end of the fourth month of life. Therefore, pigeons under 4 months of age were considered young and those above 4 months adult[11]. All pigeons showed evidence of traumatic injuries with wing or/and leg trauma probably due to collisions. No ticks were detected during external examinations.
2.2. Molecular analysis
Spleen samples were submitted to DNA extraction using the DNeasy Tissue Kit (Qiagen GmbH, Hilden, Germany) and according to the manufacturer’s instructions. DNA samples were stored at 4 ℃until employment as templates for PCR assays.
2.2.1. A. phagocytophilum
A primary amplification was carried out to amplify a 932 bp fragment of the 16S rRNA gene of A. phagocytophilum, using the primers GE 3a and GE 10r. A nested PCR, with the primers GE 9f and GE 2, amplifi ed a 546 bp fragment of the same gene. Primary and secondary amplifi cations were performed with the same cycling conditions[12].
2.2.2. Bartonella spp.
DNA samples were employed in a PCR assay to identify the Bartonella genus. The primers p24E and p12B were used to amplify a 296 bp fragment of the Bartonella 16S rRNA gene as previously described by Relman et al[13].
2.2.3. B. burgdorferi s.l.
Primers JS1 and JS2 were used to amplify a 261 bp fragment of the 23S rRNA gene of B. burgdorferi s.l.[14].
2.2.4. C. psittaci
A genus-specific amplification was carried out using primers 201CHOMP and CHOMP336s to amplify a 450 bp fragment of the variable domains Ⅲ and Ⅳ of the ompA gene. One μL of the genusspecifi c PCR product was used as template in a second amplifi cation with primers 218PSITT and CHOMP336s specifi c for a 389-404 bp fragment of C. psittaci[15].
2.2.5. C. burnetii
C. burnetii was identifi ed by amplifying a 687 bp fragment of the IS1111a gene using primers Trans-1 and Trans-2, as described by Berri et al[16].
2.2.6. Rickettsia spp.
PCR with the primers Rr190.70p and Rr190.701 were carried out to amplify a 632 bp fragment of the gene encoding the outer surface protein ompA of Rickettsia spp., as described by Roux et al[17]. Since this protocol did not allow to detect R. helvetica, Rickettsia akari,Rickettsia australis, and Rickettsia bellii, a second PCR assay was performed using the primers RpCS.877p and RpCS.1258n which amplifi ed a 381 bp fragment of the gltA gene[18].
HotStarTaq (Qiagen) was used for all PCR assays. Standard precautions were taken to avoid contamination of samples and reaction mixture, including strict separation of the areas for reagent preparation, DNA extraction and amplifi cation. A negative control without template DNA was included to ensure the absence of contamination in the reaction mixture. No DNA positive controls were added to avoid possible cross-contamination.
All the amplifi cation products were analysed by electrophoresis on 1.5% agarose gel at 100 V for 45 min; gel was stained with ethidium bromide and observed. GelPilot 100 bp Plus Ladder (Qiagen) was used as DNA marker.
2.3. Statistical analysis
Statistical evaluation was carried out by the X2test to analyze the results in relationship to age and gender of examined pigeons. Diff erences were considered signifi cant when P<0.05.
3. Results
Tick-borne infections were observed in 20 pigeons, with 23.8% prevalence. More in details, 2 (2.38%) animals showed positive for Bartonella spp., 5 (5.95%) for C. burnetii, 5 (5.95%) for Rickettsia spp., 13 (15.47%) for B. burgdorferi s.l. All birds scored negative for A. phagocytophilum.
A total of 17 (20.23%) pigeons were positive for Chlamydophila spp. and among them 10 (11.9%) were positive for C. psittaci.
Statistical analysis did not fi nd signifi cant diff erences between the age and gender groups (Table 1).
Mixed infections were detected in 8 (9.52%) pigeons: 3 animals were positive for Rickettsia spp. and B. burgdorferi s.l., 2 for C. psittaci and B. burgdorferi s.l., 1 for C. psittaci and C. burnetii, 2 for C. psittaci, Rickettsia spp. and B. burgdorferi s.l..
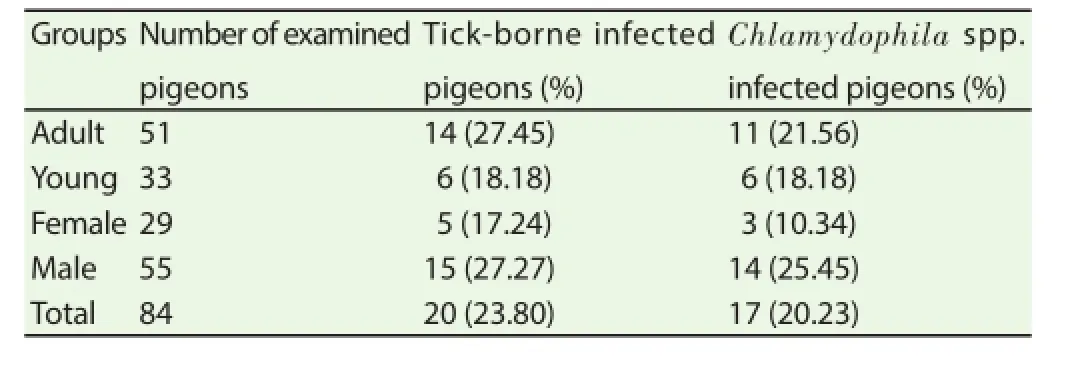
Table 1Number of examined pigeons PCR-positive for tick-borne agents and Chlamydophila spp. according to age and gender.
4. Discussion
Contamination of urban environments caused by feral pigeons and the resultant health risks for humans have been known for a long time. Direct and indirect contact between feral pigeons and humans commonly occurs in squares, public gardens, parks, markets, and railway stations. In addition, the behavioral habit of pigeons in assembling and resting on roofs, balconies, window sills and shutters brings them even closer to humans. Epidemiological studies in feral pigeon populations have detected at least 110 organisms that are pathogenic to humans, including 8 viruses, 41 bacteria, 55 fungi and 6 protozoa. Among them, the most important pathogen transmissible from feral pigeons to humans is considered C. psittaci[19].
Our results, with 11.9% of C. psittaci-positive feral pigeons,confi rm these animals as reservoirs of these bacteria. C. psittaci is usually excreted in feces and ocular and respiratory secretions of infected birds; infection in other animals and humans occurs through fecal dust, feather particles and dried excreta from infected birds that contaminate water, food and aerosols. Moreover, C. psittaci could be considered an arthropod-borne agent, because it may be transmitted by red mites Dermanyssus gallinae[20]. About 8% of the tested pigeons showed positive to Chlamydophila genus. In the literature,studies about avian infections by chlamydiae have usually been confi ned to the search of C. psittaci, thus data about the presence of other chlamydiae are scant. A previous survey detected Chlamydia trachomatis, Chlamydophila abortus and Chlamydophila pecorum in urban pigeons; moreover 19.5% of all Chlamydiaceae-positive cases turned out to be infected by non-classifi ed organisms[21].
The 23.8% of the pigeons examined during the present investigation showed infected by tick-borne bacterial pathogens. All pigeons had no ectoparasites at the moment of examination and sampling. This finding was probably due to the fact that ectoparasites, in particular ticks, quite promptly leave dead animals; thus it cannot be excluded that the examined pigeons had been previously infested by hematophagous arthropods.
The 15.47% prevalence found for B. burgdorferi s.l. shows that,among birds, also C. livia is susceptible to this pathogen. Previous investigation found that the bird host competency for maintaining and transmitting Borrelia spirochetes varies in diff erent bird species. Pheasants in the United Kingdom[22,23], blackbirds and song thrushes in Central Europe have been shown to be important reservoirs of Borrelia garinii and Borrelia valaisiana[24-26]. Borrelia turdi DNA was recently detected in Turdus merula and their ticks in Portugal[27]. Two pigeons resulted infected by Bartonella spp. Various Bartonella spp. have been associated to human infections causing mild or severe diseases; these agents have been identifi ed in domestic and wild animals, including canids, deer, cattle, rodents and marine mammals. Bartonella henselae and Bartonella koehlerae have been found in a few birds in USA[7], and Bartonella grahamii DNA has been amplifi ed from a bird tick in Korea[28]. However, attention to the spreading of these bacteria in avian population is very scant, thus data about the prevalence and the pathogenic role in birds are not available.
No pigeons showed positive to A. phagocytophilum. Information about infection by this pathogen in feral pigeons are not available,but studies carried out in Europe indicate that migrating birds may be important in the dispersal of A. phagocytophilum infected Ixodes ricinus[29,30]. However, A. phagocytophilum DNA has been sometimes detected in ticks collected from birds at low prevalence, and it was questioned by some authors whether birds may really be involved in the spreading of the pathogen whereas other authors discussed their possible involvement[31].The involvement of birds and their ticks in the lifecycle of A. phagocytophilum has been also tested in a transmission study in the US. For the two bird species involved,Turdus migratorius and Dumetella carolinensis, no signifi cant role in the lifecycle was found[32].
C. burnetii DNA was amplifi ed from spleen samples of fi ve pigeons. C. burnetii is considered a tick-borne bacterium, even if infection is usually acquired by humans and animals through inhalation of contaminated aerosol or ingestion of contaminated food, mainly raw milk and dairy products[16]. Moreover, infected mammals shed the organism in placentas, and feces. It is not possible to determine the source of infection for the fi ve positive pigeons; in fact they could have contracted coxiellae from arthropods, but also through direct or indirect contact with infected animals living in the neighboring rural areas.
The implication of birds in C. burnetii infection was described in Italy by Babudieri and Moscovici[5], who found that C. livia and Anser anser domesticus may be naturally infected with C. burnetii. Successively, it has been shown that infected domestic poultry can transmit coxiellosis to humans who consume raw eggs from infected hens or through aerosolized fomites. It has been also observed that experimentally infected hens shed C. burnetii in their feces for 7-14 d. The excretion of coxiellae in feces of infected birds has been demonstrated by Stein and Raoult[6] too, when they described a human Q fever outbreak resulted from exposure to contaminated pigeon feces and ticks.
Rickettsia spp. DNA was found in spleen samples collected from fi ve pigeons. Infections by rickettsiae belonging to the Spotted Fever Group have been frequently reported in Europe, including Italy. However, literature data concern the detection of these pathogens in ticks and in wild and domestic animals; moreover cases of human rickettsiosis have been often described. Previous studies have found Rickettsia spp. DNA in ticks collected from birds, and R. helvetica bacteraemia has been recently demonstrated in passerine birds. However, it has been supposed that the transmission of rickettsiae from birds to tick vectors may occur with low effi cacy[8].
Signifi cant diff erences were not detected between the gender and age groups; however, the higher values of prevalence observed in adult birds could be related to the time of exposure to the pathogens,which was longer in this group.
In conclusion, feral pigeons, which live in urban and periurban areas, are traditionally considered a hazard for the human health, as source of pathogens excreted with feces, such as salmonellae and chlamydiae. The results of the present survey, even if carried out on a small number of animals, suggest that these birds may be source of chlamydial agents, other than C. psittaci. Moreover, pigeons seem to be involved in the epidemiology of tick-borne zoonotic pathogens too.
Although it is well known that wild birds are vectors of infected ticks, further studies should be performed to better understand the role of pigeons as reservoirs of the tick-borne agents.
Conflict of interest statement
We declare that we have no confl ict of interest.
Acknowledgments
The present research was carried out with funds from the University of Pisa.
[1] Haag-Wackernagel D, Moch H. Health hazards posed by feral pigeons. J Infect 2004; 48(4): 307-313.
[2] Harkinezhard T, Geens T, Vanrompay D. Chlamydophila psittaci infections in birds: a review with emphasis on zoonotic consequences. Vet Microbiol 2009; 135(1-2): 68-77.
[3] Ling Y, Chen H, Chen X, Yang X, Yang J, Bavoli BM, et al. Epidemiology of Chlamydia psittaci infection in racing pigeons and pigeon fanciers in Beijing, China. Zoonoses Public Health 2015; 62(5): 401-406.
[4] Rizzoli A, Silaghi C, Obiegala A, Rudolf I, Hubálek Z, Földvári G, et al. Ixodes ricinus and its transmitted pathogens in urban and peri-urban areas in Europe: new hazards and relevance for public health. Front Public Health 2014; 2: 251. doi:10.3389/fpubh.2014.00251.
[5] Babudieri B, Moscovici L. Experimental and natural infection of birds by Coxiella burnetii. Lancet 1952; 169: 195-196.
[6] Stein A, Raoult D. Pigeon pneumonia in Provence: a bird-borne Q fever outbreak. Clin Infect Dis 1999; 29(3): 617-620.
[7] Mascarelli PE, McQuillan M, Harms CA, Harms RV, Breitschwerdt EB. Bartonella henselae and B. koehlerae DNA in birds. Emerg Infect Dis 2014;20(3): 490-492.
[8] Hornok S, Kováts D, Csörgö T, Meli ML, Gönczi E, Hadnagy Z, et al. Birds as potential reservoirs of tick-borne pathogens: fi rst evidence of bacteraemia with Rickettsia helvetica. Parasit Vectors 2014; 7(1): 128.
[9] De La Fuente J, Naranjo V, Ruiz-Fons F, Hofle U, Fernandez De Mera IG, Villanua D, et al. Potential vertebrate reservoir hosts and invertebrate vectors of Anaplasma marginale and A. phagocytophilum in central Spain. Vector-Borne Zoon Dis 2005; 5: 390-401.
[10] Rudenko N, Golovchenko M, Grubhoffer L, Oliver Jr JH. Updates on Borrelia burgdorferi sensu lato complex with respect to public health. Ticks Tick-Borne Dis 2011; 2(3): 123-128.
[11] Senlik B, Gulegen E, Akyol V. Effect of age, sex and season on the prevalence and intensity of helminth infections in domestic pigeons(Columba livia) from Bursa province, Turkey. Acta Vet Hung 2005; 53(4): 449-456.
[12] Massung RF, Slater K, Owens JH, Nicholson WL, Mather TN, Solberg VB, et al. Nested PCR assay for detection of granulocytic Ehrlichiae. J Clin Microbiol 1998; 36(4): 1090-1095.
[13] Relman DA, Loutit SJ, Schmidt TM, Falkow S, Tompkins S. The agent of bacillary angiomatosis: an approach to the identifi cation of uncultured pathogens. N Engl J Med 1990; 323: 1573-1580.
[14] Chang YF, Novosol V, McDonough SP, Chang CF, Jacobson RH, Divers T, et al. Experimental infection of ponies with Borrelia burgdorferi by exposure to Ixodid ticks. Vet Pathol 2000; 37(1): 68-76.
[15] Sprague LD, Schubert E, Hotzel H, Scharf S, Sachse K. The detection of Chlamydophila psittaci genotype C infection in dogs. Vet J 2009; 181(3): 272-279.
[16] Berri M, Rekiki A, Boumedine A, Rodolakis A. Simultaneous diff erential detection of Chlamydophila abortus, Chlamydophila pecorum, and Coxiella burnetii from aborted ruminant’s clinical samples using multiplex PCR. BMC Microbiol 2009; 9: 130. doi:10.1186/1471-2180-9-130.
[17] Roux V, Fournier PD, Raoult D. Diff erentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplifi ed DNA of the gene encoding the protein rOmpA. J Clin Microbiol 1996; 34(9): 2058-2065.
[18] Regnery RL, Spruill CL, Plikaytis BD. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J Bacteriol 1991; 173(5): 1576-1589.
[19] Magnino S, Haag-Wackernagel D, Geigenfeind I, Helmecke S, Dovc A, Prukner-Radovcic’E, et al. Chlamydial infections in feral pigeons in Europe: review of data and focus on public health implications. Vet Microbiol 2009; 135(1-2): 54-67.
[20] Circella E, Pugliese N, Todisco G, Cafiero MA, Sparagano OA,Camarda A. Chlamydia psittaci infection in canaries heavily infested by Dermanyssus gallinae. Exp Appl Acarol 2011; 55(4): 329-338.
[21] Sachse K, Kuehlewind S, Ruettger A, Schubert E, Rohde G. More than classical Chlamydia psittaci in urban pigeons. Vet Microbiol 2012;157(3-4): 476-480.
[22] Kurtenbach K, Carey D, Hoodless AN, Nuttall PA, Randolph SE. Competence of pheasants as reservoirs for Lyme disease spirochetes. J Med Entomol 1998; 35(1): 77-78.
[23] Kurtenbach K, Peacey M, Rijpkema SG, Hoodless AN, Nuttall PA,Randolph SE. Diff erential transmission of the genospecies of Borrelia burgdorferi sensu lato by game birds and small rodents in England. Appl Environ Microbiol 1998; 64(4): 1169-1174.
[24] Kipp S, Goedecke A, Dorn W, Wilske B, Fingerle V. Role of birds in Thuringia, Germany, in the natural cycle of Borrelia burgdorferi sensu lato, the Lyme disease spirochaete. Int J Med Microbiol 2006; 296(Suppl 1): 125-128.
[25] Taragel’ova V, Koci J, Hanincova K, Kurtenbach K, Derdakova M,Ogden NH, et al. Blackbirds and song thrushes constitute a key reservoir of Borrelia garinii, the causative agent of borreliosis in Central Europe. Appl Environ Microbiol 2008; 74(4): 1289-1293.
[26] Dubska L, Literak I, Kocianova E, Taragelova V, Sychra O. Differential role of passerine birds in distribution of Borrelia spirochetes, based on data from ticks collected from birds during the postbreeding migration period in Central Europe. Appl Environ Microbiol 2009; 75(3): 596-602.
[27] Norte AC, Ramos JA, Gern L, Núncio MS, Lopes de Carvalho I. Birds as reservoirs for Borrelia burgdorferi s.l. in Western Europe: circulation of B. turdi and other genospecies in bird-tick cycles in Portugal. Environ Microbiol 2013, 15(2): 386-397.
[28] Kang JG, Kim HC, Choi CY, Nam HY, Chae HY, Chong ST, et al. Molecular detection of Anaplasma, Bartonella, and Borrelia species in ticks collected from migratory birds from Hong-do Island, Republic of Korea. Vector Borne Zoonotic Dis 2013; 13(4): 215-225.
[29] Alekseev AN, Dubinina HV, Semenov AV, Bolshakov CV. Evidence of ehrlichiosis agents found in ticks (Acari: Ixodidae) collected from migratory birds. J Med Entomol 2001; 38(4): 471-474.
[30] Bjöersdorff A, Bergström S, Massung RF, Haeming PD, Olsen B. Ehrlichia-infected ticks on migrating birds. Emerg Infect Dis 2001; 7(5): 877-879.
[31] Stuen S, Granquist EG, Silaghi C. Anaplasma phagocytophilum - a widespread multi-host pathogen with highly adaptive strategies. Front Cell Infect Microbiol 2013, 3: 31. doi: 10.3389/fcimb.
[32] Johnston E, Tsao JI, Muñoz JD, Owen J. Anaplasma phagocytophilum infection in American robin sand gray catbirds: an assessment of reservoir competence and disease in captive wildlife. J Med Entomol 2013; 50(1): 163-170.
ent heading
10.1016/j.apjtm.2016.03.005
15 January 2016
Valentina Virginia Ebani, DVM, PhD, Department of Veterinary Science, University of Pisa, Viale delle Piagge, 2, 56124 Pisa, Italy.
Tel: +39 050 2216968
Fax: +39 050 2210655
E-mail: valentina.virginia.ebani@unipi.it
in revised form 20 February 2016
ARTICLE INFO
Article history:
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Determination of ligand cluster and binding site within VP40 of Ebola virus: clue for drug development
- Clinacanthus nutans: a review of the medicinal uses, pharmacology and phytochemistry
- Current perspectives on dengue episode in Malaysia
- Etiological agents causing leptospirosis in Sri Lanka: A review
- Phylogeny of Murray Valley encephalitis virus in Australia and Papua New Guinea
- Dengue outbreak in Swat and Mansehra, Pakistan 2013; an epidemiological and diagnostic perspective
