基于CoNi-双金属氢氧化物//AC非对称超级电容器的构筑
2016-10-31谢莉婧孙国华谢龙飞苏方远李晓明孔庆强吕春祥李开喜陈成猛
谢莉婧, 孙国华, 谢龙飞, 苏方远, 李晓明,刘 卓, 孔庆强, 吕春祥, 李开喜, 陈成猛
(1.中国科学院山西煤炭化学研究所 炭材料重点实验室,山西 太原030001;2.辽宁工业大学,辽宁 锦州121001)
基于CoNi-双金属氢氧化物//AC非对称超级电容器的构筑
谢莉婧1,孙国华1,谢龙飞2,苏方远1,李晓明1,刘卓1,孔庆强1,吕春祥,李开喜1,陈成猛1
(1.中国科学院山西煤炭化学研究所 炭材料重点实验室,山西 太原030001;2.辽宁工业大学,辽宁 锦州121001)
以高电容特性的CoNi-LDH作正极,活性炭作负极,6 mol/L KOH溶液为电解液构筑CoNi-LDH/AC非对称超级电容器。由于这两种材料在同一种电解液中发生可逆循环时对应的电化学电势范围不同,因此通过组合这两种电极材料可以有效地解决对称电容器工作电压低的问题。用循环伏安、恒电流充放电等测试方法对其电化学性能进行研究。结果表明,所组装非对称电容器在碱性水系电解液中,其工作电压可以达到1.5 V。通过比较它与基于两种电极材料对称电容器的能量密度-功率密度曲线可以看出,非对称电容器的性能有了很大提高,在功率密度为102.3 W·kg-1时,其能量密度可以达到46.3 Wh·kg-1。
钴镍双金属氢氧化物; 纳米复合物; 非对称超级电容器; 能量存储
1 Introduction
Over the past few years, the ever-increasing environmental problems and the up-coming depletion of fossil fuels have stimulated intense research on the development of alternative energy storage/conversion devices with high power and energy densities[1,2]. Electrochemical capacitors often called supercapacitors or ultra-capacitors with attractive characteristics such as high power density, low equivalent series resistance (ESR) and permanent charge-discharge life are considered as promising energy storage devices. They can be applied in a large variety of applications, including consumer electronics, memory back-up systems, industrial power, energy management, public transportation, military devices and electric vehicles[3]. However, the low energy density (normally less than 10 Wh·kg-1) of commercially available supercapacitors has imposed significant challenges for their use as power sources to replace batteries[4]. Therefore, improvement of the energy density of supercapacitors is crucial to meet the future energy storage demands.
Recently, the fabrication of asymmetric supercapacitors using aqueous electrolytes has been regarded as an efficient way to improve their energy density[5, 6]. Such type asymmetric supercapacitors are different from either the electric double layer capacitors (EDLCs) or the conventional batteries,which can make full use of a battery type (faradaic) electrode and a capacitor type (electrochemical double layer) electrode to increase the operation voltage[7]. As a consequence, the high working voltage and high energy density of the faradic reaction electrode can be obtained simultaneously by choosing a proper electrode material, and hence the overall energy density of the asymmetric supercapacitors can be enhanced correspondingly.
Currently, the negative material of an asymmetric supercapacitior is activated carbon (AC)[8-11], and its positive electrode material is mainly transition metal oxides/hydroxide, including V2O5[9], MnO2[10], NiO[11]and Ni(OH)2[12]. Ni(OH)2is one of the very promising materials for energy storage, and has received increasing attention in recent years owing to their relatively high capacitance and fast redox kinetics[13,14]. Actually, the crystal structure of this kind of the materials plays a critical role in the capacitive performance. Nickel hydroxides are well-known to crystallize in two polymorphs,αandβ[15]. Theβ-form is a stoichiometric phase of the composition Ni(OH)2with a brucite-like structure and consists of a hexagonal packing of hydroxyl ions with Ni(II) occupying alternate rows of octahedral sites[16], while theα-hydroxides are reported to be isostructural with hydrotalcite-like compounds that consist of positively charged Ni(OH)2-xlayers and charge balancing anions (e.g., NO3-, CO32-, Cl-, etc.) in the interlayer gallery[17]. Theα-nickel hydroxide is theoretically expected to exhibit superior electrochemical activity as compared to itsβ-counterpart because of its turbostratically crystallized structure. However,α-Ni(OH)2is unstable in alkaline medium and transforms toβ-Ni(OH)2[18]. Numerous efforts have been made to stabilize theα-phase Ni(OH)2by partially substituting Al[19], Co[20], Mn[21]or Zn[22]ions for Ni ions. Recently, we have reported that loose-packed CoNi-layered double hydroxide (LDH) composed of nanoparticles synthesized by a polyvinyl pyrrolidone-assisted chemical co-precipitation method[23], which was shown to be a potential candidate for a supercapacitor electrode material.
In the present work, in order to expand the potential window and enhance the energy density, an asymmetric supercapacitor is assembled in a 6 mol/L aqueous KOH electrolyte using AC as the negative electrode and the CoNi-LDH nanoparticles as the positive electrode. The as-obtained asymmetric supercapacitor showed a high energy density of 46.3 Wh·kg-1at a power density of 102.3 W·kg-1, and a high power density of 1 704.5 W·kg-1at an energy density of ~25.6 Wh·kg-1, all with a 1.5 V cell voltage operating. These features are very attractive for a variety of critical applications in high-performance energy storage systems.
2 Experimental
2.1Synthesis of electrode materials
Reagents were all of AR grade. Water used in the synthesis and washing was deionized. CoNi-LDH powder was prepared using a chemical co-precipitation method, referencing the previous work[30]. The typical route is as follows. In the first step, CoCl2·6H2O (0.005 mol) and NiCl2·6H2O (0.005 mol) were dissolved in distilled water (50 mL) with vigorous stirring. Then, 5 g polyvinyl pyrrolidone was added to the solution under vigorous stirring for 1 h. Afterward, 5 wt% NH3·H2O was added dropwise into the above solution with continuous stirring until the pH of the solution reached 9. After aging for 12 h, the resulting product was separated by suction filtration and rinsed with distilled water and ethanol several times, and then dried at 60 ℃ for 12 h.
Activated carbon powder with a specific area of 2 384 m2·g-1was prepared from coal powder (Yi chuan, Shanxi, China) via KOH activation at 800 ℃ for 1.5 h with a coal/KOH mass ratio of 1∶6.
2.2Characterization
The obtained products were characterized by field emission scanning electron microscopy (FESEM) (JSM-6701F, Japan), X-ray diffraction (Rigaku, D/Max-2400, Japan) and nitrogen adsorption and desorption (Micromeritics, ASAP 2020M, USA). The specific surface area was obtained using Brunauer-Emmett-Teller (BET) method. The pore size distribution of the CoNi-LDH and activated carbon were calculated using the Barrett-Joyner-Halenda (BJH) model and density functional theory (DFT) method based on the nitrogen adsorption isotherms, respectively. The elemental composition in the as-prepared samples was determined using Inductively Coupled Plasma-Atomic Emission Spectrometry (ICP-AES) (Thermo ICAP6300).
2.3Electrochemical test of the single electrode and asymmetric supercapacitor
The CoNi-LDH used for the positive electrode was prepared by mechanically mixing 75 wt% powered CoNi-LDH, 10 wt% acetylene black, 10 wt% conducting graphite, and 10 wt% polytetrafluoroethylene (PTFE) emulsion with a PTFE content of 50 wt%. Then the mixture was pressed on a Ni-foam grid at a pressure of 10 MPa and dried at room temperature for 24 h. The AC electrodes were prepared by the same method as the positive electrode described above. To construct an asymmetric supercapacitor, the loading mass ratio of active materials (CoNi-LDH/activated carbon) was estimated to be 3∶19 according to their specific capacitances from their galvanostatic charge-discharge curves in a three-electrode cell. The galvanostatic charge-discharge test of the individual electrode was performed in a three-electrode cell, in which a platinum foil and a Hg/HgO were used as counter and reference electrode, respectively, in 6 mol/L KOH aqueous solution. The cyclic voltammetry and galvanostatic charge-discharge tests of the symmetric supercapacitors (AC/AC) and the asymmetric supercapacitors based on CoNi-LDH and activated carbon electrodes separated by a porous nonwoven cloth were performed in a two-electrode cell in 6 mol/L KOH aqueous solution. All of the above electrochemical measurements were carried out on a CHI760D electrochemical working station (CH Instrument).
The specific capacitance of the asymmetric capacitor can be evaluated from the charge/discharge test with the following equation:C=I/[(dE/dt)×m]≈I/[(ΔE/Δt)×m](F/g)
(1)
whereCis the specific capacitance;Iis the constant discharging current;Δt is the time period for the potential changeΔE; andmis the total mass of active material in two electrodes.
The charge on the electrode can be evaluated from the charge/discharge test with the following equation:
C'=IΔt/m(C/g)
(2)whereIis the constant discharging current;Δis the time period for the potential change; and m is the mass of the corresponding electrode materials measured.
The real power densityPrealis determined from the constant current charge/discharge cycles as follows:
Preal=ΔEI/m(W/kg)
(3)
where withEmaxis the potential at the end of the charge andEminat the end of the discharge,Iis the applied current (A), andmis the weight of the active material in the electrode (kg).
The specific energy Erealis defined as:
(4)
whereCis the system capacitance for a cell andUmaxis the maximum cell voltage.
3 Results and discussion
3.1Material characterization
The atomic percentages (at%) of the elements Co and Ni in the CoNi-LDH was obtained by means of Inductively Coupled Plasma-Atomic Emission Spectrometry (ICP-AES). The result shows that the CoNi-LDH material was obtained when the molar ratio of the CoCl2·6H2O∶NiCl2·6H2O in the mixed solution was 1∶1.
The XRD patterns of the Co(OH)2, CoNi-LDH, Ni(OH)2are shown in Fig.1. The patterns in Fig.1 comprise four broad peaks appearing at 2θvalues of 11.3° (0.789 nm), 22.8° (0.391 nm), 34.3° (0.262 nm) and 60.4° (0.153 nm). The XRD patterns correspond to bothα-Co(OH)2andα-Ni(OH)2. It is difficult to differentiate the two phases, since they have very similar structures, and hence their diffraction peaks are very close. Furthermore, theα-phase hydroxides display more disorder and a larger interlayer spacing as the interlamellar space contains water molecules or other anions than theβ-phase[24]. Therefore,α-phase composites are theoretically expected to exhibit superior electrochemical activity as compared with theβ-form[25].
The morphology and structure characters of the CoNi-LDH and the activated carbon are shown in Fig. 2. It can be clearly observed from Fig.2a that the CoNi-LDH is comprised of grain-like particles with diameters in the range from 10 to 30 nm, and these particles arrange randomly and form a relatively loose packed microstructure. This kind of porous structure not only provides a large active area, but also allows a rapid transportation of electrolyte ions, which meet the demand of the appreciable performance at high charge/discharge rate for the asymmetric supercapcitor. The activated carbon sample in Fig. 2b shows mesoporosity, which is essential for the electrochemical activity.
Further information on the specific surface area and pore structure of the activated carbon (Fig. 3) is obtained from N2adsorption-desorption measurements. A type IV isotherm with a distinct hysteresis loop is observed for the the activated carbon, indicating the presence of a mesoporous structure. The specific surface area and pore volume of the CoNi-LDH are 109.8 m2·g-1and 0.603 6 cm3·g-1, respectively. Its corresponding pore diameter distributions is shown in Fig. 3a (insert), which possesses a narrow mesoporous distribution around 1.5-5 nm. The activated carbon shows a BET surface area of 2 384 m2·g-1and a pore volume of 1.98 cm3·g-1. Moreover, the pores of the activated carbon are mainly mesopores with a narrow size distribution of 3-5 nm (Fig. 3b insert). These intriguing features are of huge benefit for the transport and diffusion of electrolyte ions during the rapid charge-discharge process, hence resulting in an excellent electrochemical performance.
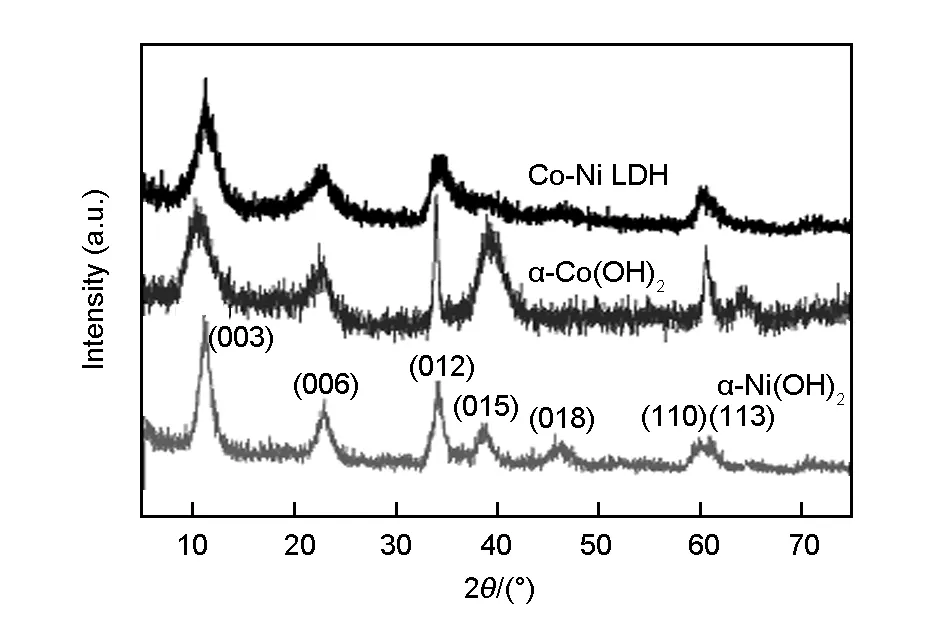
Fig. 1 XRD patterns of Co(OH)2, CoNi-LDH and Ni(OH)2.
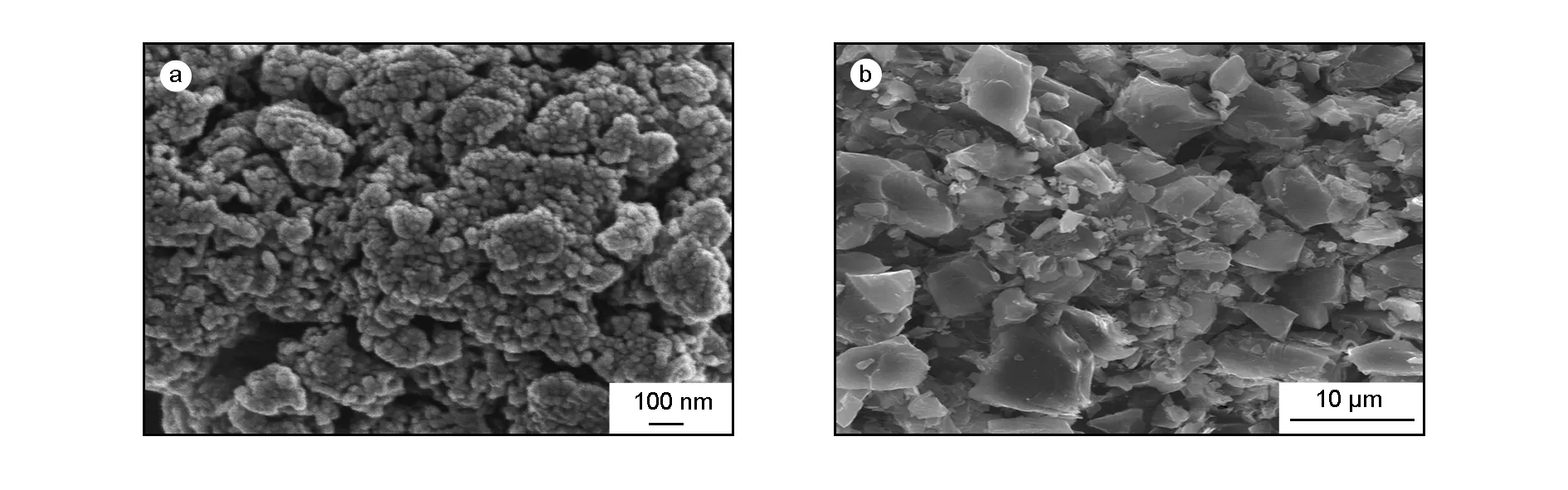
Fig. 2 SEM images of (a) the CoNi-LDH and (b) activated carbon.
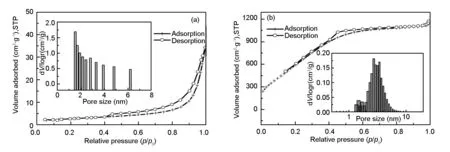
Fig. 3 N2 adsorption-desorption isotherms of (a) the CoNi-LDH and (b) activated carbon (insets show their pore size distributions).
3.2Electrochemical characterization
Fig. 4a shows the CV curves of the CoNi-LDH and the AC as electrode materials, which are measured in a three-electrode system. The AC electrode is measured within a potential window from -1 V to 0 V, and the CoNi-LDH electrode within a potential window from 0 V to 0.5 V at a scan rate of 5 mV·s-1. The CV curve of the AC electrode exhibits a rectangular shape, suggesting a typical characteristic of EDLC behavior. In contrast, the CoNi-LDH electrode exhibits a pair of cathodic and anodic peaks, indicating the existence of fast Faradic redox reactions during the CV tests.
Fig. 4b shows the charge-discharge curves of the individual electrodes vs. the Hg/HgO reference electrode. The AC electrode presents a typical linear relationship with increasing time, being a characteristic of electric double layer capacitance. The CoNi-LDH electrode deviates from the ideal line due to the existence of the faradaic reaction, as indicated by the CV results. According to the Equation (1), the specific capacitances of the AC and CoNi-LDH are 210 and 2 614 F·g-1at a current density of 1 A·g-1, respectively. The stored charges (q) are related with the specific capacitance (C), the potential window (ΔV), and the mass (m) of the electrode according to the Equation (2)[26]. Therefore, on the basis of the specific capacitances and the potential windows of the two materials, the mass ratio between the CoNi-LDH electrode and the AC electrode should be 3∶18 in an asymmetric supercapacitior.
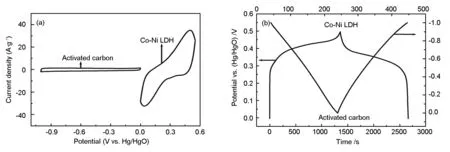
Fig. 4 (a) Cyclic voltammograms of the CoNi-LDH and AC electrodes at a scan rate of 5 mV·s-1in 6 M KOH solution and (b) the charge-discharge curves of the individual electrodes vs. the Hg/HgO reference electrode.
The working principle of the positive and negative electrodes in the asymmetric supercapacitor is illustrated in Fig.5. Based on the physical reasoning, both electrodes of an asymmetric supercapacitor connect in series[27]. In the view of this holistic supercapacitor, the series circuit combines with two parts: one part is the symmetric circuit based on activated carbon and another part is the symmetric circuit based on CoNi-LDH. As these two different materials have different potential ranges operating reversibly in the same aqueous electrolyte, it could effectively increase the operation voltage if these two electrodes are combined. Moreover, in the as-constructed asymmetric supercapacitor system, the positive electrode made from CoNi-LDH to supply pseudo-capacitance and the negative electrodes from activated carbon to obtain double-layer capacitance in their respective potential ranges, which is favorable for its storage charge in the voltage range of 0-1.5 V.
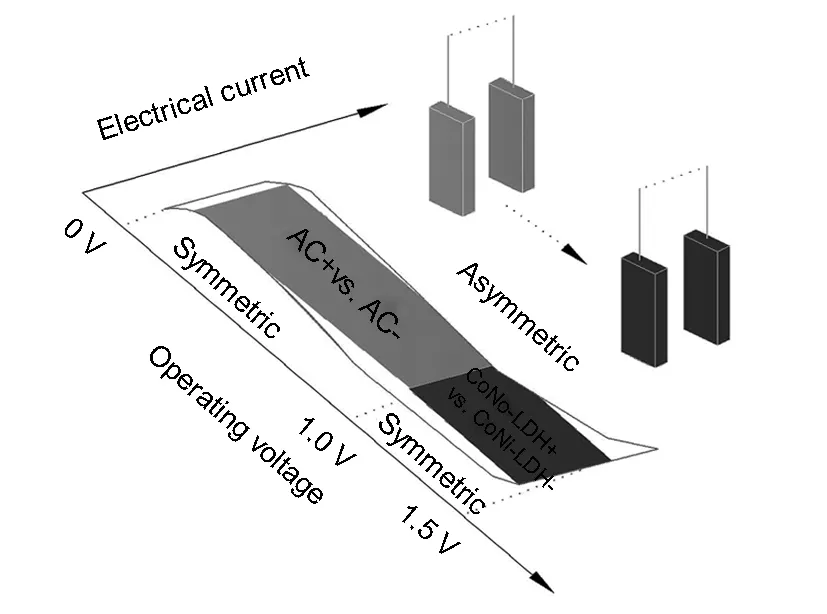
Fig. 5 Schematic diagram of the CoNi-LDH//AC asymmetric supercapacitor.
The CV curves of the CoNi-LDH//activated carbon asymmetric supercapacitor have been tested at various scan rates and are shown in Fig. 6a, which is consistent with our hypothesis, i.e., the voltage of the asymmetric supercapacitor can be extended to be 1.5 V. With the increase of the scan rate from 3 to 30 mV·s-1, the shapes of CV curves of the capacitor do not change, implying its good electrochemical performance. At the same time, no oxygen or hydrogen evolution current is observed in these curves, indicating that no hydrogen or oxygen evolution occurs within the voltage range (0-1.5 V). Fig. 6b shows galvanostatic charge-discharge curves of the CoNi-LDH//activated carbon asymmetric supercapacitor at various current densities. It can be seen that both charge and discharge curves remain a good symmetry at cell voltage as high as 1.5 V, implying that the cell has an excellent electrochemical reversibility and capacitive characteristics. Although an almost linear potential response is observed, the galvanostatic charge-discharge curves are not in an exact triangle shape, which may be due to the pseudocapacitance arising from the redox reaction within this voltage range. The specific capacitance calculated from the galvanostatic charge-discharge curves according to the Equation (1) is 149 F·g-1at a current density of 142.8 mA·g-1and still reaches 112 F·g-1at a high current density of 2 381 mA·g-1, indicating a good rate capability.
The effect of positive electrode/negative electrode mass ratio on specific capacitance of the assembled asymmetric supercapacitors was investigated by chronopotentiometry and the corresponding results are shown in Fig.7. The assembled asymmetric supercapacitors have the same active-material loading on positive electrode but varied loadings on negative electrode from 12 to 27 mg. As seen in Fig.7, the specific capacitances of the CoNi-LDH//activated carbon asymmetric supercapacitors firstly increase markedly, and then decrease when the mass ratio is in the range between 1∶7 and 1∶9. The maximal specific capacitance at different current densities is obtained when the cathode/anode mass ratio is 1∶6. These results show that the CoNi-LDH/activated carbon mass ratio with 1∶6 is ideal for the CoNi-LDH//activated carbon asymmetric supercapacitor. The experiment result is consistent with theoretical calculations above.
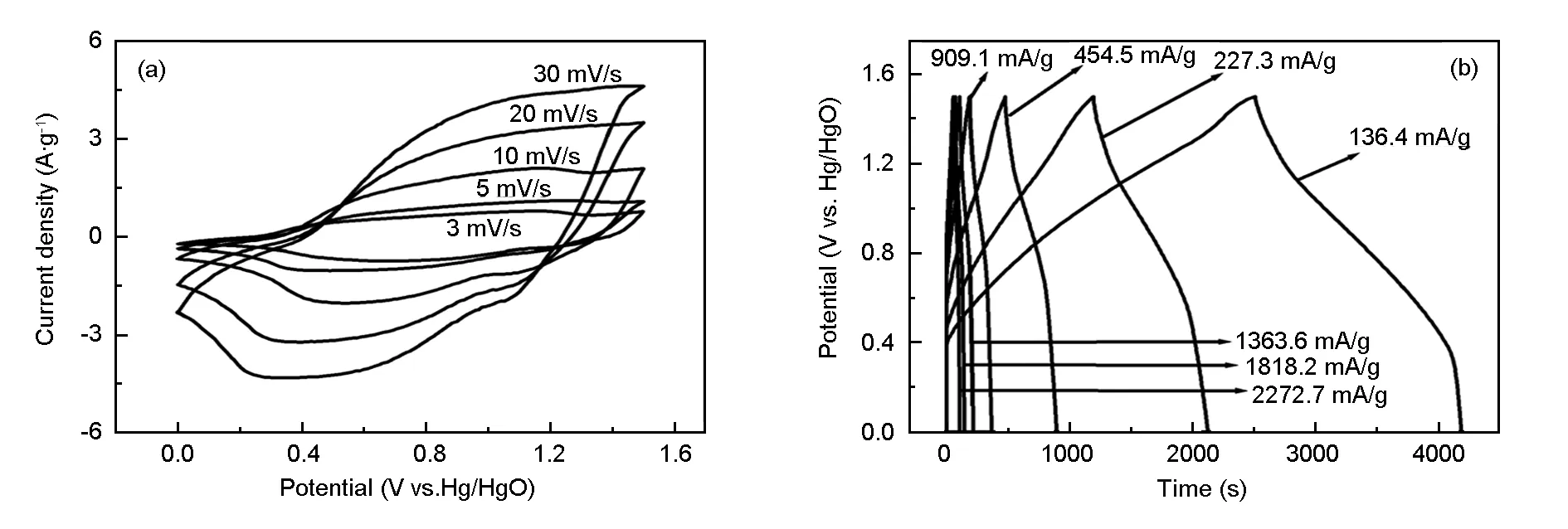
Fig. 6 (a) Cyclic voltammograms at different scan rates and (b) galvanostatic charge-discharge curves at different current densities of the asymmetric supercapacitor.
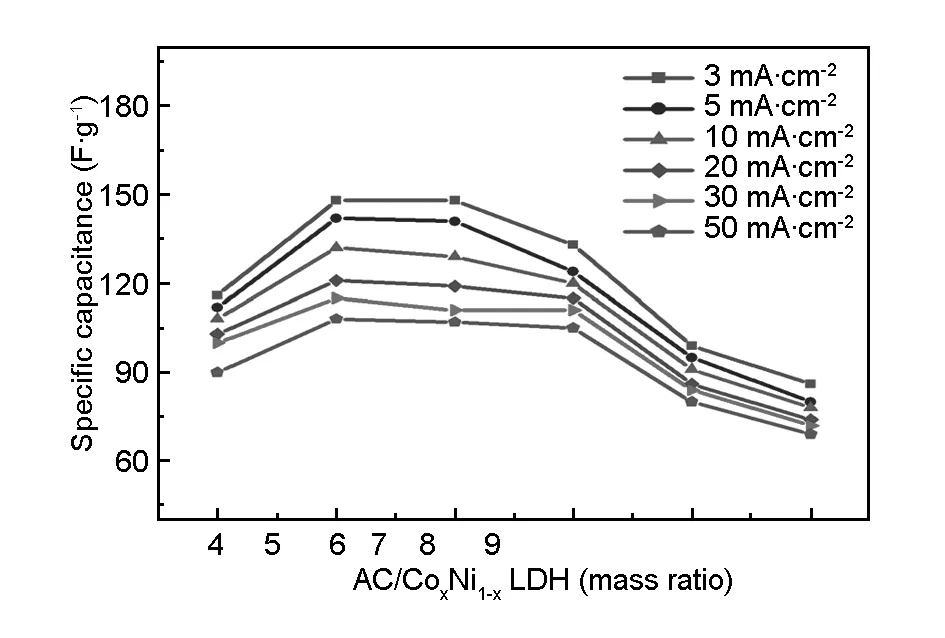
Fig. 7 Specific capacitances of the CoNi-LDH//AC asymmetric supercapacitors having different positive-to-negative electrode mass ratios at different current densities.
Electrochemical impedance spectroscopy (EIS) is a very powerful tool to characterize the electrochemical process of an electrochemical capacitor. To better evaluate the performance of the asymmetric supercapacitor, the electrochemical impedance measurements were carried out at applied potentials of 0.5, 0.8, 1.0 and 1.3 V and the frequency range of 105-10-2Hz and typical plots are shown in Fig. 8. Three distinct regions, which are dependent on the frequency, are shown in Fig. 8a-d. From the points intersecting the real axis in high frequency, it can be seen that the values of the internal resistance (Ri) of the hybrid electrochemical capacitor at all the applied potentials are nearly the same, which are estimated as little as 0.88 Ω. In the high-medium frequency region, from the semi-circles observed for all the plots, the charge-transfer resistances (Rct) related to charge-transfer process on the surfaces of the electroactive materials are estimated the same as 0.35 Ω at various applied potentials. And it is clear that, in the low frequency region, the slope of all the impedance plots increases and almost tends to a vertical asymptote, indicative of the good electrochemical capacitance performance of the asymmetric supercapacitor within the applied voltage range from 0 V to 1.5 V, which is well consistent with the analysis of the CV tests in Fig. 5b.
Ragone plots relating the energy density to the power density are an efficient way to evaluate the capacitive performance of a supercapacitor. Fig. 9(a) shows the Ragone plot of the as-prepared the CoNi-LDH//activated carbon asymmetric supercapacitor. The energy and power densities were derived from the discharge curves at different current densities according to the Equation (3) and (4). For comparison, the symmetric supercapacitor based activated carbon and activated carbon, has also been constructed and evaluated in 6 M electrolyte (Fig. 9 (b)). It is worth noting that the maximum energy density for the asymmetric supercapacitor with a cell voltage of 1.5 V is 46.59 Wh·kg-1, which is much higher than the symmetric activated carbon/activated carbon supercapacitor (6.8 Wh·kg-1). Moreover, the asymmetrical supercapacitor still shows a high energy density of 35.3 Wh·kg-1even at a power density of 1 785 W·kg-1. Such a high energy density is comparable to or even higher than other reported asymmetric capacitors[9,10,13,28,29]. The enhancement of the energy density here is attributed to the enlarged potential window and the high specific capacitance, which considerably boosts its potential application in the replacement of traditional symmetric supercapacitor in advanced energy storage devices.
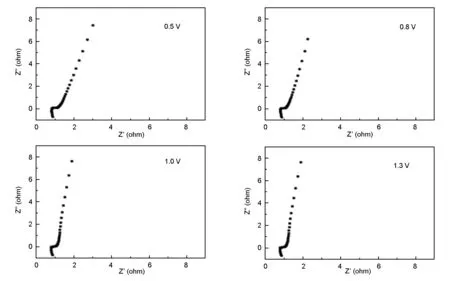
Fig. 8 Nyquist plots of the CoNi-LDH//AC asymmetric supercapacitor at different applied potentials.
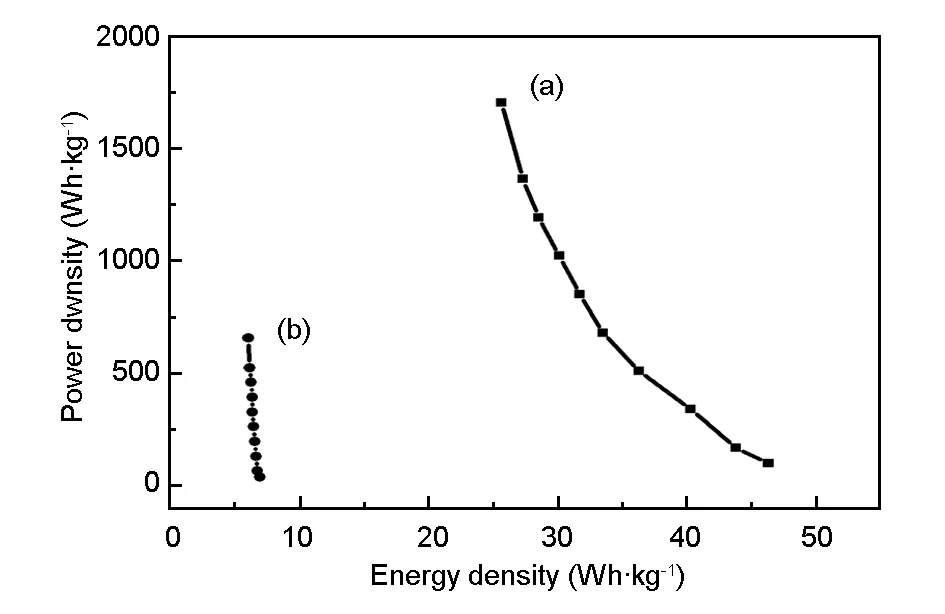
Fig. 9 Ragone plot relating power density and energy density of (a) the CoNi-LDH/AC asymmetric and(b) the AC/AC symmetric supercapacitor.
The cycling stability of the asymmetric superca pacitor was further investigated by the galvanostatic charge-discharge between 0 and 1.5 V at a current density of 476 mA·g-1(Fig. 10a). Results have shown that the asymmetric supercapacitor retains 84.5% of initial capacitance even after 1 000 consecutive cycles of charge-discharge testing, displaying a long cycling ability of the CoNi-LDH/activated carbon asymmetric supercapacitor. In order to understand the excellent electrochemical performance, SEM imaging of the CoNi-LDH electrode after 1 000 cycling test was carried out. The morphology as well as particle size of the CoNi-LDH electrode are almost the same as in the initial state (Fig. 2a), as shown in the Fig. 10b. The results display that the CoNi-LDH composite can effectively accommodate the strain and stress of volume change during cycling, which consequently enhances the cyclic stability of the CoNi-LDH/activated carbon asymmetric supercapacitor.
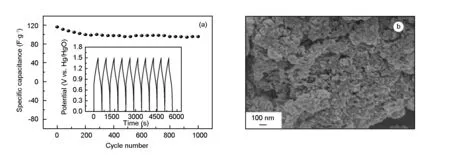
Fig. 10 (a) Long-term cycling stability of the CoNi-LDH//AC asymmetric supercapacitor at a constant current density of 476 mA·g-1 over 1 000 cycles and (b) the morphology of CoNi-LDH after the long cycling test.
4 Conclusions
An asymmetric supercapacitor based on CoNi-LDH as the positive electrode and activated carbon as the negative was successfully assembled in a 6 mol/L aqueous KOH electrolyte, which can be cycled reversibly in the high-voltage region of 0-1.5 V, delivers a high energy density of 46.59 Wh·kg-1at a power density of 107 W·kg-1, and can still operate at a high power density of 1 785 W·kg-1with an energy density of 35.3 Wh·kg-1. Furthermore, the asymmetric supercapacitor also exhibits a long cycle life along with a 84.5% capacitance retention after 1 000 cycles. Such impressive electrochemical performance of the CoNi-LDH//activated carbon capacitor opens new opportunities for its application in high power and energy storage devices.
[1]Wei T Y, Chen C H, Chien H C, et al. A cost-effective supercapacitor material of ultrahigh specific capacitances: Spinel nickel cobaltite aerogels from an epoxide-driven sol-gel process[J]. Adv Mater, 2010, 22: 347-351.
[2]Wang H, Casalongue H S, Liang Y, et al. Ni(OH)2nanoplates grown on graphene as advanced electrochemical pseudo-capacitor materials[J]. J Am Chem Soc, 2010, 132: 7472-7477.
[3]Hu Z A, Xie Y L, Wang Y X, et al. Synthesis ofα-cobalt hydroxides with different intercalated anions and effects of intercalated anions on their morphology, basal plane spacing and capacitive property[J]. J Phys Chem C, 2009, 113: 12502-12508.
[4]Frackowiak E. Carbon materials for supercapacitor application[J]. Phys Chem Chem Phys, 2007, 9: 1774-1785.
[5]Khomenko V, Raymundo-Piňero E, Frackowiak E, et al. High-voltage asymmetric supercapacitors operating in aqueous electrolyte[J]. Appl Phys A: Mater Sci Process, 2006, 82: 567-573.
[6]Park J H, Park O O. Hybrid electrochemical capacitors based on polyaniline and activated carbon electrodes[J]. J Power Sources, 2002, 111: 185-190.
[7]Yan J, Fan Z, Sun W, et al. Advanced asymmetric supercapacitors based on Ni(OH)2/graphene and porous graphene electrodes with high energy density[J]. Adv Funct Mater, 2012, 22: 2632-2641.
[8]Frackowiak E, Béguin F. Carbon materials for the electrochemical storage of energy in capacitors[J]. Carbon, 2001, 39: 937-950.
[9]Qu Q T, Shi Y, Li L L, et al. V2O5·0.6H2O nanoribbons as cathode material for asymmetric supercapacitor in K2SO4solution[J]. Electrochem Commun, 2009, 11: 1325-1328.
[10]Khomenko V, Raymundo-Pińero E, Béguin F. Optimisation of an asymmetric manganese oxide/activated carbon capacitor working at 2 V in aqueous medium[J]. J Power Sources, 2006, 153: 183-190.
[11]Wang D W, Li F, Cheng H M. Hierarchical porous nickel oxide and carbon as electrode materials for asymmetric supercapacitor[J]. J Power Sources, 2008, 185: 1563-1568.
[12]Wang Y G, Yu L, Xia Y Y. Electrochemical capacitance performance of hybrid supercapacitors based on Ni(OH)2/carbon nanotube composites and activated carbon[J]. J Electrochem Soc, 2006, 153: A743-A748.
[13]Song Q S, Li Y Y, Lchan S L. Physical and electrochemical characteristics of nanostructured nickel hydroxide powder[J]. J Appl Electrochem, 2005 35: 157-162.
[14]Cheng J, Cao G P, Yang Y S. Characterization of sol-gel-derived NiOx xerogels as supercapacitors[J]. J Power Sources, 2006, 159: 734-731.
[15]Oliva P, Leonardi J, Laurent J F, et al. Review of the structure and the electrochemistry of nickel hydroxides and oxy-hydroxides[J]. J Power Sources, 1982, 8: 229-255.
[16]Mockenhaupt C, Zeiske T, Lutz H D. Crystal structure of brucite-type cobalt hydroxideβ-Co{O(H,D)}2-neutron diffraction, IR and Raman spectroscopy[J]. J Mol Struct, 1998, 443: 191-203.
[17]El-Batlouni H, El-Rassy H, Al-Ghoul M. Cosynthesis, coexistence, andself-organization ofα-andβ-cobalt hydroxide based on diffusion and reaction in organic gels[J]. J Phys Chem A, 2008, 112: 7755-7757.
[18]Jayashree R S, Kamath P V. Suppression of theα→β-nickel hydroxide transformation in concentrated alkali: Role of dissolved cations[J]. J Appl Electrochem, 2001, 31: 1315-1320.
[19]Zhao M Q, Zhang Q, Huang J Q, et al. Hierarchical nanocomposites derived from nanocarbons and layered double hydroxides-properties, synthesis, and applications[J]. Adv Funct Mater, 2012, 22: 675-694.
[20]Yang J, Yu C, Fan X M, et al. 3D architecture materials made of NiCoAl-LDH nanoplates coupled with Ni Co-carbonate hydroxide nanowires grown on flexible graphite paper for asymmetric supercapacitors[J]. Adv Energy Mater, 2014, DOI:10.1002/aenm.201400761.
[21]Zhao J W, Chen J L, Xu S M, et al. Hierarchical NiMn layered double hydroxide/carbon nanotubes architecture with superb energy density for flexible supercapacitors[J]. Adv Funct Mater, 2014, 24: 2938-2946.
[22]Guerlou-Demourgues L, Tessier C, Bernard P, et al. Influence of substituted zinc on stacking faults in nickel hydroxide[J]. J Mater Chem, 2004, 14: 2649-2654.
[23]Xie L J, Hu Z A, Lv C X, et al. CoxNi1-x double hydroxide nanoparticles with ultrahigh specific capacitances as supercapacitor electrode materials[J]. Electrochim Acta, 2012, 78: 205-211.
[24]Coudun C, Hochepied J F. Nickel hydroxide “stacks of pancakes” obtained by the coupled effect of ammonia and template agent[J]. J Phys Chem B, 2005, 109: 6069-6074.
[25]Liu Z, Ma R, Osada M, et al. Selective and controlled synthesis ofα- andβ-cobalt hydroxides in highly developed hexagonal platelets[J]. J Am Chem Soc, 2005, 127: 13869-13874.
[26]Fan Z J, Yan J, Wei T, et al. Asymmetric supercapacitors based on graphene/MnO2and activated carbon nanofiber electrodes with high power and energy density[J]. Adv Funct Mater, 2011, 21: 2366-2375.
[27]Qi X C. Study of asymmetric hybrid supercapacitor's modeling. Proceedings of ISES solar world congress 2007: Solar energy and human settlement[C]. September 18-21, 2007, Beijing, 2811-2814.
[28]Wang D W, Fang H T, Li F, et al. Aligned titania nanotubes as an intercalation anode material for hybrid electrochemical energy storage[J]. Adv Funct Mater, 2008, 18: 3787-3793.
[29]Wang Q, Wen Z, Li J. A hybrid supercapacitor fabricated with a carbon nanotube cathode and a TiO2-B nanowire anode[J]. Adv Funct Mater, 2006, 16: 2141-2146.
A high energy density asymmetric supercapacitor based on a CoNi-layered double hydroxide and activated carbon
XIE Li-jing1,SUN Guo-hua1,XIE Long-fei2,SU Fang-yuan1,LI Xiao-ming1,LIU Zhuo1,KONG Qing-qiang1,LU Chun-xiang1,LI Kai-xi1,CHEN Cheng-meng1
(1.KeyLaboratoryofCarbonMaterials,InstituteofCoalChemistry,ChineseAcademyofSciences,Taiyuan030001,China;2.LiaoningUniversityofTechnology,Jinzhou121001,China)
A high performance asymmetric supercapacitor was fabricated using a CoNi-layered double hydroxide (CoNi-LDH) as the positive electrode and activated carbon as the negative electrode in an electrolyte of 6 M aqueous KOH. The supercapacitor can be cycled reversibly in a wide potential window of 0-1.5 V and exhibits a remarkable energy density of 46.3 Wh·kg-1at a power density of 102.3 W·kg-1, a high energy density of 25.6 Wh·kg-1even at a high power density of 1 704.5 W·kg-1These values are much higher than those of a symmetric supercapacitor using only activated carbon (6.9 Wh·kg-1). The results can be ascribed to the high specific capacitance and rate capability of the CoNi-LDH, and the complementary potential windows of the two electrodes that increase the working voltage of the supercapacitor. The supercapacitor also exhibits stable cycling with a 84.5% capacitance retention after 1 000 cycles.
Cobalt nickel double hydroxides; Nanocomposite; Asymmetric supercapacitor; Energy storage
date: 2015-12-12;Reviseddate: 2016-01-11
National Natural Science Foundation of China (51402324,51402325,51302281).
LI Kai-xi, Ph. D, Professor. E-mail: likx99@yahoo.com;
introduction: XIE Li-jing, Ph. D, Assistant Professor. E-mail:xielijing@sxicc.ac.cn
1007-8827(2016)01-0037-09
TB333
A
国家自然科学基金(51402324,51402325,51302281).
李开喜,博士,研究员. E-mail: likx99@yahoo.com; 陈成猛,博士,副研究员. E-mail: ccm@sxicc.ac.cn
谢莉婧,博士,助理研究员. E-mail:xielijing@sxicc.ac.cn
CHEN Cheng-meng, Ph. D, Associate Professor. E-mail: ccm@sxicc.ac.cn
10.1016/S1872-5805(16)60003-3
English edition available online ScienceDirect ( http:www.sciencedirect.comsciencejournal18725805 ).
