胰管支架预防内镜下逆行胰胆管造影术后胰腺炎的临床分析
2016-10-28鲍峻峻梅俏孟翔凌许建明刘付宝李冬苏晓丽
鲍峻峻 ,梅俏,孟翔凌,许建明,刘付宝,李冬 ,苏晓丽
(安徽医科大学第一附属医院,a.腔镜中心,b.消化内科,c.普外科,安徽 合肥 230022)
胰管支架预防内镜下逆行胰胆管造影术后胰腺炎的临床分析
鲍峻峻a,梅俏b,孟翔凌c,许建明b,刘付宝c,李冬a,苏晓丽a
(安徽医科大学第一附属医院,a.腔镜中心,b.消化内科,c.普外科,安徽 合肥230022)
目的回顾性研究内镜下胰管支架置入预防内镜下逆行胰胆管造影术后胰腺炎(PEP)疗效。方法按统一标准入组相关患者,选择接受内镜下逆行胰胆管造影(ERCP)诊治并置入胰管支架的102例患者作为观察组,随机选择同期接受ERCP治疗但未置入胰管支架的患者100例作为对照组,比较两组患者PEP及高淀粉酶血症的发生率。结果(1)观察组与对照组总体术后胰腺炎发生率差异无统计学意义(P=0.113);两组总体高淀粉酶血症发生率相比差异无统计学意义(P=0.491)。(2)两组高危人群术后胰腺炎发生率相比差异有统计学意义(P=0.037);高危人群高淀粉酶血症发生率相比差异无统计学意义(P=0.757)。结论胰管支架置入可降低高危人群ERCP术后胰腺炎的发生率。
胰胆管造影术,内窥镜逆行/方法;支架;胰腺炎
内镜下逆行胰胆管造影(endoscopic retrograde cholangiopancreatography,ERCP) 自1968年问世以来,一直是临床诊断和治疗胆胰系统疾病的重要手段[1];而术后胰腺炎(post-ERCP pancreatitis,PEP)自ERCP技术问世以来一直是其最常见,也可能是最严重的并发症。目前一般认为,PEP的发生率约为1%~10%,而某些高危人群中则可高达30%,甚至40%~60%[2-3]。因此,如何预防PEP一直是临床研究的热点问题,有大量相关的临床研究,但结果不尽相同[4-6]。随着近年来经内镜胰管支架置入用于预防PEP,相关研究显示出十分正面的预防作用[4-5,7-9];为评价胰管支架预防PEP的临床效果,现对国内某三甲医院相关资料进行回顾性研究。
1 资料与方法
1.1一般资料对国内某三甲医院2012年9月至2014年8月间住院行ERCP诊治的患者人群进行回顾性研究,所有病例术前均由患者本人或委托人签署知情同意书。入选标准:年龄>18岁;术前血清淀粉酶在正常范围内。排除标准:妊娠期妇女;严重的心、肺或肾功能不全者;急性胰腺炎或慢性胰腺炎急性发作。
在此基础上,依据是否留置胰管支架分成2组。所有放置胰管支架患者均纳入观察组(支架组)共102例,其中男50例(50/102,49.02%),女52例(52/102,50.98%),年龄21~90岁,平均(56.69±17.45)岁。该组根据术前或术中操作情况均考虑为PEP高危人群,术中均置入胰管支架引流预防术后胰腺炎;对未行胰管支架引流患者随机抽样,设立对照组共100例,其中男42例(42/100,42.00%),女58例(58/100,58%),年龄29~88岁,平均(62.58±14.44)岁,术后未置入胰管支架,按常规给予禁食、抑酶等治疗。
1.2研究方法所有患者均使用美国GE公司9800型C形X光机、日本Olympus公司 TJF-260V型十二指肠镜、德国ERBE公司ICC200型高频电刀及美国COOK公司5F-5 cm型单猪尾胰管支架,余术中所用耗材均为美国波科及美国COOK公司产品。两组患者均于术前30 min肌注地西泮10 mg、哌替啶50 mg、东莨菪碱10 mg(具体用量依据患者年龄、体质、病情及术中情况酌情增减)。所有ERCP均由熟练操作医生按诊疗规范[1]及具体病情完成。支架组术中置入胰管支架,对照组未置入胰管支架。支架组患者胰管支架则根据术中及术后情况择期经十二指肠镜直视下取出。
相关标准:两组患者均于术后3、24 h检测血清淀粉酶水平,并根据目前公认的Cotton[10]标准进行诊断:即ERCP后出现新发的上腹痛或原有腹痛加重,且术后24 h血清淀粉酶升高超过正常上限3倍,超过24 h,即可诊断为PEP;如出现其它脏器功能损害结合影像学等相关表现,即诊断为重型PEP;而仅有血清淀粉酶的升高不伴有上腹痛则为高淀粉酶血症。
高危人群[4]:年轻、女性、术前怀疑Oddi括约肌功能异常、既往有胆源性胰腺炎病史、有PEP史、预切开、胰管内操作(胰管注射,括约肌切开等)、困难插管、球囊扩张等。
1.3统计学方法使用SPSS21.0统计软件进行统计学处理。计数资料的比较采用χ2检验。P<0.05为差异有统计学意义。
2 结果
2.1一般情况所有患者均行选择性插管操作,2组患者均未出现死亡病例或需外科、介入科协助处理并发症的病例;支架组中有2例胆道疾病患者未能完成胆管选择性插管,遂放置胰管支架预防术后胰腺炎,操作成功率为98.04%(100/102);本次抽样对照组操作成功率100.00%;对照组中有1例患者出现术后迟发性出血,经内镜止血成功;除部分胰管支架自行脱落外,所有胰管支架均经内镜顺利拔除,且未造成相关并发症。
2.2两组患者总体PEP及高淀粉酶血症发生率比较两组患者中均无重型胰腺炎病例。如表1所示,支架组有4例发生PEP(4/102,3.92%),对照组有7例发生PEP(7/100,7.00%),两者相比差异无统计学意义(P=0.113);支架组有63例发生高淀粉血症(63/102,61.76%),对照组有57例发生高淀粉血症(57/100,57.00%),两者相比差异无统计学意义(P=0.491)。
2.3两组高危人群PEP及高淀粉酶血症发生率比较如表2所示,所有支架组均为PEP高危人群并留置胰管支架,共计102例,而对照组中高危人群数量共计45例。其中支架组有4例发生PEP(4/102,3.92%),对照组高危人群有6例发生PEP(6/45,13.33%),两者相比差异有统计学意义(P=0.037);支架组有63例发生高淀粉血症(63/102,61.76%),对照组高危人群有29例发生高淀粉血症(29/45,64.44%),两者差异无统计学意义(P=0.757)。
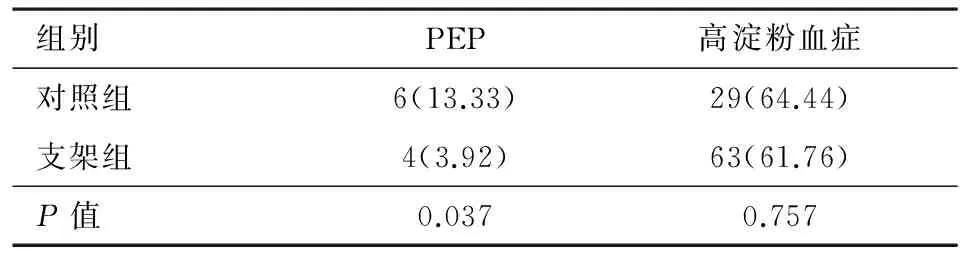
表2 两组高危人群PEP及高淀粉酶血症发生率比较/例(%)
3 讨论
ERCP是胆胰系统疾病诊疗的重要手段,具有疗效确切、创伤小、恢复快等优点,但同时作为一项侵袭性操作,又存在一定并发症甚至致死性并发症发生概率,故而备受临床重视。其中,PEP是ERCP最常见、最严重的并发症之一。研究发现[4,11-12],虽然多数PEP患者为轻中型胰腺炎,但重症胰腺炎的发生率仍可能达到10%。
ERCP术后胰腺炎发生机制目前尚不清楚,目前多认为与胰管引流不畅、机械损伤、化学因素和感染因素等有关[13-16];同时,年轻、女性、Oddi括约肌功能异常、复发性胰腺炎、有PEP史及血清胆红素正常、胰管注射、困难插管、胰括约预切开及球囊扩张[4-5,17]等高危因素均会增加PEP的发生风险。Sherman等[18]研究发现,上述风险因素中,乳头插管致Oddi括约肌痉挛或乳头开口水肿致胰液引流不畅是PEP发生的最主要原因,故通过置入胰管支架可改善胰管引流,从而降低PEP的发生。本组数据也发现,对照组非高危人群及高危人群的PEP发生率分别为1.81%及13.33%,高危人群的PEP发生率为3.92%,由此可见高危人群的PEP术后发生率是非高危人群PEP发生率的7.36倍,通过置入胰管支架可大大降低高危人群的PEP发生率。
目前有大量应用药物预防PEP的临床研究,如奥曲肽、生长抑素、双氯芬酸钠、加贝酯、肝素、硝酸甘油、吲哚美辛栓等,目前认为只有吲哚美辛栓及双氯芬酸是可以有效降低PEP的发生率,特别是近几年术前应用吲哚美辛栓被认为是有效、安全且花费低的预防措施[4,5,19-20],但也有相关认为无效的研究[21,22]。而应用胰管支架预防PEP,目前绝大多数研究均认为有效或可能有效,且效果优于现有的药物,并写入相关临床指南[1,4,23]。
Cha SW等[7]研究表明,对困难插管且应用针状刀预切开的此类PEP高危患者置入胰管支架,可显著减少PEP及重症胰腺炎的发生(P<0.05);Takero Mazaki等[9]在一项包括1 541例患者(其中760例患者置入胰管支架)的荟萃分析发现,置入胰管支架可降低PEP(RR 0.39;95% CI 0.29~0.53;P<0.001)及重症胰腺炎(RR 0.26;95% CI 0.09~0.76;P=0.01)的发生率。而Tsuchiya等[24]研究发现,ERCP术中留置5F胰管支架,术后胰腺炎发生率无明显差异,支架组平均淀粉酶水平显著低于对照组(257.9 vs 456.2 IU·L-1,P=0.035),认为胰管支架可能有助于PEP的预防;Aizawa等[25]研究发现在胆总管结石取石后置入胰管支架并不能显著减少PEP的发生,但术后高淀粉酶血症发生率较对照组显著减少,支架组PEP发生率有下降趋势,认为可能降低PEP的发生率。具体原因可能与该研究样本量不大,或研究针对行EST取石患者,而目前认为行EST患者往往PEP的发生率较未行EST患者发生率为低,且该研究未进一步区分高危人群的PEP发生率有关。陈龙艳[26]对国内相关研究进行荟萃分析发现,胰管支架置入可降低PEP和重度PEP的发生率,可能是预防PEP的有效措施。本研究中,如果单纯考量2组患者总体的PEP发生率,胰管支架置入同样无法降低PEP的发生率,与近年来由于培训、技术的发展使得ERCP操作从总体技术、安全及规范层面均较前进一步提升有关;但针对高危人群的PEP发生率,则胰管支架置入则可以降低PEP的发生率。另外,无论是总体还是高危人群,2组的高淀粉酶血症发生率均无差异,可能与高淀粉酶血症选择的诊断标准,即血淀粉酶高于正常上限不伴有腹痛即诊断为高淀粉酶血症,未强调升高的程度有关,因此后续需进一步探讨胰管支架对高淀粉酶血症发生率的影响。
综上所述,针对ERCP高危人群行胰管支架置入是有效、安全的预防术后胰腺炎的措施,可在日常工作常规使用。
[1]中华医学会消化内镜分会ERCP学组.ERCP诊治指南[M].上海:上海科学技术出版社,2010:2.
[2]Hauser G,Milosevic M,Stimac D,et al.Preventing post-endoscopic retrograde cholangiopancreatography pancreatitis:What can be done?[J].World J Gastroenterol,2015,21(4):1069-1080.
[3]DiMagno MJ,Spaete JP,Darren D,et al.Risk models for post-ERCP pancreatitis(PEP) smoking and chronic liver disease are predictors of protection against PEP[J].Pancreas,2013,42(6):996-1003.
[4]Dumonceau JM,Andriulii A,Elmunzer BJ,et al.Prophylaxis of post-ERCP pancreatitis:European Society of Gastrointestinal Endoscopy(ESGE) Guideline-Updated June 2014[J].Endoscopy,2014,46:799-815.
[5]Freeman ML.Preventing post-ERCP pancreatitis:Update 2016[J].Curr Treat Options Gastro,2016,27[Epub ahead of print]
[6]Levenick JM,Gordon SR,Fadden LL,et al.Rectal indomethacin does not prevent post-ERCP pancreatitis in consecutive patients,a randomized trial[J].Gastroenterology,2016,150:911-917.
[7]Cha SW,Leung WD,Lehman GA,et al.Does leaving a main pancreatic duct stent in place reduce the incidence of precut biliary sphincterotomy-associated pancreatitis? A randomized,prospective study[J].Gastrointest Endosc,2013,77:209-16.
[8]Arain MA,Freeman ML.Pharmacologic prophylaxis alone is not adequate to prevent post-ERCP pancreatitis[J].Am J Gastroenterol,2014,109:910-912.
[9]Mazaki T,Mado K,Masuda H,et al.Prophylactic pancreatic stent placement and post-ERCP pancreatitis:an updated meta-analysis[J].J Gastroenterol,2014,49:343-355.
[10] Cotton PB,Lehman G,Vennes J,et al.Endoscopic sphincterotomy complications and their management:an attempt at consensus[J].Gastrointest Endosc,1991,37:383-393.
[11] Ding X,Chen M,Huang S,et al.Nonsteroidal anti-inflammatory drugs for prevention of post-ERCP pancreatitis:a meta-analysis[J].Gastrointest Endosc,2012,76(6):1152-1159.
[12] Andriulli A,Loperfido S,Napolitano G,et al.Incidence rates of post-ERCP complications:a systematic survey of prospective studies[J].Am J Gastroenterol,2007,102:1781-1788.
[13] Sherman S.ERCP and endoscopic sphincterotomy-induced pancreatitis[J].Am J Gastroenterol,1994,89:303-305.
[14] Johnson GK,Geenen JE,Johanson JF,et al.Evaluation of post-ERCP pancreatitis:potential causes noted during controlled study of differing contrast media[J].Gastrointest Endosc,1997,46:217-222.
[15] Sherman S,Hawes RH,Troiano FP,et al.Pancreatitis following bile duct sphincter of Oddi manometry:utility of the aspirating catheter[J].Gastrointest Endosc,1992,38:347-350.
[16] Ratani RS,Mills TN,Ainley CC,et al.Electrophysical factors influencing endoscopic sphincterotomy[J].Gastrointest Endosc,1999,49:43-52.
[17] ASGE Standards of Practice Committee.Complications of ERCP[J].Gastrointest Endosc,2012,75(3):467-473.
[18] Sherman S,Lehman GA.ERCP and endoscopic sphincterotomy-induced pancreatitis [J].Pancreas,1991,6(3):350-367.
[19] Zhao XW,Bao JJ,Hu C,et al.Effect of diclofenac on the levels of lipoxin A4 and Resolvin D1 E1 in the post-ERCP pancreatitis[J].Dig Dis Sci,2014,59(12):2992-2996.
[20] BJ Elmunzer,JM Scheiman,GA Lehman,et al.A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis[J].N Engl J Med,2012,366(15):1414-1422.
[21] Luo H,Zhao L,Leung J,et al.Routine pre-procedural rectal indometacin versus selective post-procedural rectal indometacin to prevent pancreatitis in patients undergoing endoscopic retrograde cholangiopancreatography:a multicentre single-blinded,randomised controlled trial[J].Lancet,2016,387(10035):2293-2301.
[22] John M,Stuart R,Linda L,et al.Rectal indomethacin does not prevent post-ERCP pancreatitis in consecutive patients[J].Gastroenterology,2016,150:911-917.
[23] Li GD,Jia XY,Dong HY,et al.Pancreatic stent or rectal indomethacin——which better prevents post-ERCP pancreatitis?:a propensity score matching analysis[J].Medicine(Baltimore),2016,95:e2994.
[24] Tsuchiya T,Itoi T,Sofuni A,et al.Temporary pancreatic stent to prevent post endoscopic retrograde cholangiopancreatography pancreatitis:a preliminary,single-center,randomized controlled trial[J].J Hepatobiliary Pancreat Surg,2007,14(3):302-307.
[25] Aizawa T,Ueno N.Stent placement in the pancreatic duct prevents pancreatitis after endoscopic sphincter dilation for removal of bile duct stones[J].Gastrointest Endosc,2001,54(2):209-213.
[26] 陈龙艳,于红刚.国内胰管支架置入预防内镜逆行胰胆管造影术后胰腺炎的Meta分析[J].疑难病杂志,2016,15(1):65-69.
Pancreatic duct stent in prevention of post-ERCP pancreatitis
BAO Junjun1,MEI Qiao2,MENG Xiangling3,et al
(1.EndoscopyCenter;2.DepartmentofGastroenterology;3.DepartmentofGeneralSurgery,TheFirstAffiliatedHospitalofAnhuiMedicalUniversity,Hefei,Anhui230022,China)
ObjectiveTo retrospectively study the effect of the placement of endoscopic pancreatic duct stent in prevention of post-ERCP pancreatitis(PEP).MethodsAccording to the unified standards,102 patients with pancreatic duct stent and 100 patients without pancreatic duct stent during ERCP treatment were assigned into treatment group and control group,and the incidences of PEP and hyperamylasemia were compared between the two groups.ResultsThere was no significant difference in the incidence of PEP(P=0.113) and hyperamylasemia(P=0.491) between the two groups.There was significant difference in the incidence of PEP between the high risk population of two groups(P=0.037),but there was no significant difference in the incidence of hyperamylasemia between the high risk population of two groups(P=0.757).ConclusionsPancreatic duct stent can reduce the incidence of PEP in high risk population.
Cholangiopancreatography,endoscopic retrograde/methods;Stents;Pancreatitis
安徽省科技厅重点项目(12070403071)
梅俏,男,主任医师,硕士生导师,研究方向:消化内镜诊治、炎症性肠病,E-mail:meiqiao@hotmail.com
10.3969/j.issn.1009-6469.2016.09.028
2016-04-09,
2016-07-01)
