TLR介导的Nrf2抗氧化通路在对乙酰氨基酚药物性肝损伤中的保护作用
2016-10-26顾佳毅郁丰荣
顾佳毅 郁丰荣
·论著·
TLR介导的Nrf2抗氧化通路在对乙酰氨基酚药物性肝损伤中的保护作用
顾佳毅郁丰荣
目的研究内毒素(LPS)及Toll 样受体(TLR)在对乙酰氨基酚(APAP)药物性肝损伤中的保护作用及其相关机制。方法雄性 C57BL/6 小鼠40只,分为 4 组,每组 10 只。空白对照组腹腔注射0.9%氯化钠溶液,LPS组腹腔注射LPS 10 μg/kg,APAP组腹腔注射APAP 300 mg/kg,LPS+APAP组在APAP造模前16 h给予LPS 10 μg/kg预处理。通过比较各组血清 ALT 和 AST水平,并通过HE染色评价肝组织损伤程度,观察LPS对小鼠肝损伤的保护作用。测定相应时间点的肝脏组织丙二醛(MDA)、还原型谷胱甘肽(GSH)的变化以及肝组织DHE染色,评价小鼠氧化应激水平。应用Western印迹及RT-PCR检测肝脏Nrf2,Gclc及HO-1的表达水平。结果LPS预处理可明显减轻APAP所致的肝脏氧化应激反应及肝损伤程度。LPS预处理组的小鼠血清ALT(518.3±142.3对4542±498.4 U/L)、AST(643.3±105.6对5432.1±569.2 U/L)水平及肝组织MDA(78.0±14.5对141.7±26.4 mmoL/mg)水平与模型组相比明显降低,而GSH (6.2±1.7对3.5±1.0 μmol/g)水平明显升高(P<0.05),肝组织病理损伤明显减轻。同时,LPS预处理可明显促进Nrf2及其下游抗氧化基因的表达。结论LPS在小鼠APAP肝损伤中起到保护作用,作用机制与Nrf2抗氧化通路的激活相关,可能成为药物性肝损伤的新的治疗策略。
对乙酰氨基酚;药物性肝损伤;氧化应激;Nrf2;HO-1
对乙酰氨基酚(acetaminophen,APAP)是一种解热镇痛药,在治疗剂量下,APAP具有较好的疗效及安全性[1]。然而,APAP会导致部分患者肝损伤甚至急性肝功能衰竭。目前APAP肝损伤已取代病毒性肝炎成为欧美等发达国家急性肝衰竭的首要病因[1-3]。很多严重的APAP肝衰竭患者需要肝移植术才能得到救治[4-6]。研究认为,APAP本身并不具有直接杀伤肝细胞的作用,APAP的肝细胞毒性主要是由其活性代谢产物导致的[7]。在常规剂量下,90%的APAP可以通过醛糖酸化和硫酸化作用后经尿液排除[8],10%的APAP经过CYP450代谢转化而产生高活性代谢产物NAPQI[9-13]。在正常情况下,肝细胞通过还原型谷胱甘肽(GSH)结合NAPQI转化为无毒物质[14]。但在GSH耗竭的情况下,如过量饮酒,饥饿,APAP过量或营养不良等,NAPQI无法被有效中和而产生肝毒性[15,16]。NAPQI可造成细胞膜的脂质过氧化, 并影响线粒体、内质网等细胞器的功能, 导致肝细胞氧化应激和线粒体功能失调,从而致肝细胞损伤及坏死[17]。活性氧自由基( reactive oxygen species,ROS)是介导氧化应激的主要物质。研究证明,减少ROS产生是防治APAP肝损伤的关键,增强肝细胞抗氧化应激的能力可有效避免APAP肝损伤[18-22]。
转录因子Nrf2是体内抗氧化相关基因的直接调控因子,主要包括Gclc、HO-1、G6pdh等,而这些抗氧化基因则是体内清除ROS的重要因子[23]。已报道有多种药物可以通过激活Nrf2抗氧化通路对APAP所致肝损伤起保护作用[24,25]。
研究证明,LPS激活TLR后可促进肝组织内Nrf2及其下游基因的表达[26]。因此,推测LPS对APAP肝损伤的保护作用是通过激活Nrf2抗氧化通路实现的。
资料和方法
一、化学试剂
APAP 与 LPS 购自美国 Sigma 公司;DHE购自上海碧云天公司;MDA和GSH检测试剂盒购自南京建成生物工程研究所;Western 抗体购于美国abcam公司。动物饲料购于上海斯莱克实验动物有限公司。APAP溶于PBS中, 浓度为20 mg/mL, 55 ℃水浴锅加热至完全溶解, 现用现配;LPS溶于0.9%氯化钠溶液,浓度为1 μg/mL。
二、实验动物及分组
清洁级C57BL/6 雄性小鼠40只,8~10周龄, 体质量20~25 g,购自上海斯莱克实验动物有限公司。小鼠均于SPF环境下昼夜规律喂养,自由进食、饮水。所有小鼠在APAP造模前进食过夜,APAP造模均在早上9点进行,LPS预处理在造模前16 h进行。采用随机数表法将小鼠分为4组,每组10只。①空白对照组:腹腔注射0.9%氯化钠溶液(剂量同LPS组),预处理;②APAP组:给予APAP腹腔注射,剂量为300 mg/kg;③LPS组:给予LPS腹腔注射(10 μg/kg)预处理;④LPS+APAP组:LPS处理后禁食16 h,给予APAP造模。所有小鼠在APAP造模后恢复禁食。
三、标本收集
APAP造模后1 h,收集各组5只小鼠标本用于检测GSH和MDA。造模后6 h,收集各组5只小鼠的血清和肝组织标本。用于检测血清ALT, AST,组织病理损伤以及肝组织内Nrf2的表达情况。
四、肝功能及肝组织检测
血清ALT、AST 检测采用全自动生化分析仪(美国德灵公司)。
小鼠肝组织用体积分数为0.04的甲醛固定, 脱水, 石蜡包埋, 切片(厚5 μm), HE染色, 于显微镜下观察肝组织病理改变。
新鲜组织制作冰冻切片后,将二氢乙啶(DHE)终浓度稀释至10 μmoL,将其滴在冰冻组织切片上,37 ℃避光孵育30 min。PBS清洗3遍,用荧光显微镜观察DHE染色情况。红色荧光的强度代表ROS量的多少。
五、RT-PCR
按照Trizol试剂盒操作提取组织总mRNA,并测定浓度。用TAKARA反转录试剂盒进行反转录,20 μL体系。10 μL扩增体系,LightCycler○R480 即时PCR系统进行扩展并测定荧光强度。
六、Western印迹
取肝脏组织适量,加入蛋白裂解液RIPA进行蛋白提取,用BSA法定量,调整蛋白浓度后加入加样缓冲液100 ℃加热变性,进行聚丙烯酰胺凝胶电泳分离及转膜,5% BSA封闭液常温封闭1 h,一抗4 ℃孵育过夜,用TBST液洗膜3次,每次5 min,荧光二抗37 ℃孵育1 h后洗膜10 min×4次,Odyssey 扫膜仪进行图像扫描分析。
七、统计学处理
结 果
一、LPS预处理对APAP肝损伤的影响
与对照组相比,单纯给予LPS处理对WT小鼠的转氨酶水平无明显影响(P>0.05)。而APAP组小鼠造模后 6 h 血清 ALT和AST较对照组明显升高,差异有统计学意义(P<0.05);与APAP组相比,LPS+APAP组的血清 ALT明显降低,差异有统计学意义(P<0.05),见表1。HE染色显示APAP可以引起小鼠肝组织大片的小叶中央型坏死,而LPS预处理可明显减轻APAP的损伤程度,见图1。

图1 各组小鼠肝组织镜下表现(HE,×200)
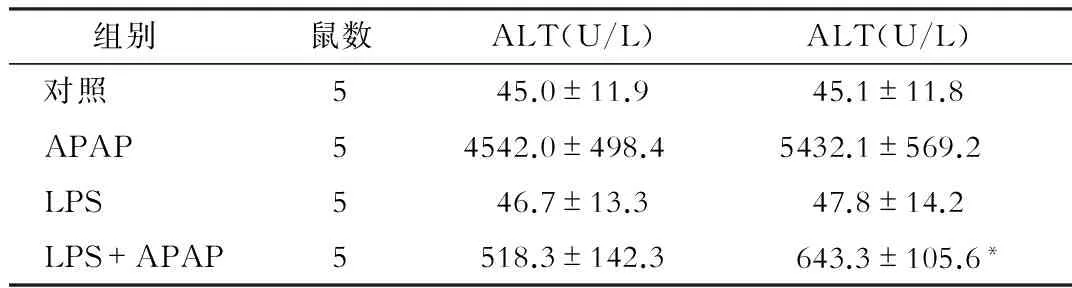
组别鼠数ALT(U/L)ALT(U/L) 对照545.0±11.945.1±11.8 APAP54542.0±498.45432.1±569.2 LPS546.7±13.347.8±14.2 LPS+APAP5518.3±142.3643.3±105.6*
注:*与APAP组相比,P<0.05
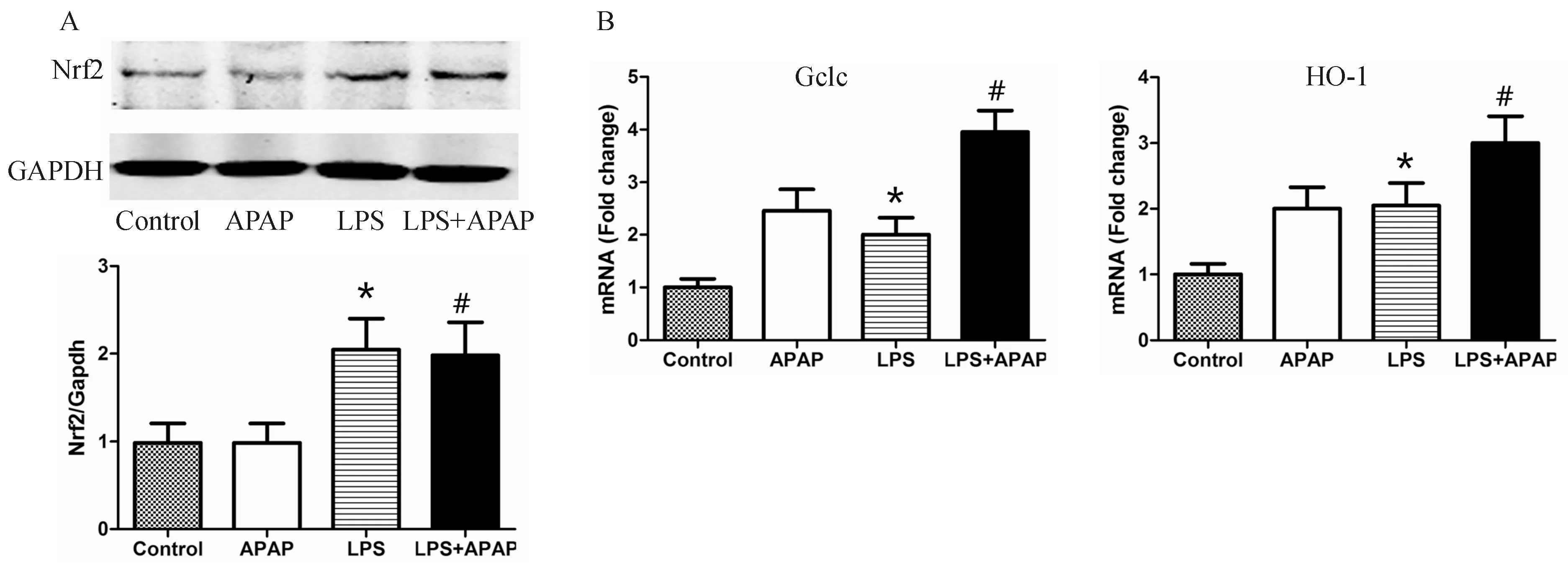
注:与空白对照组相比,*P<0.05;与APAP组相比,#P<0.05图3 LPS预处理可促进Nrf2抗氧化通路的激活
二、LPS对APAP肝毒性过程中氧化应激反应的影响
与对照组相比,APAP处理可引起小鼠肝组织内MDA水平的明显升高(P<0.05),同时造成GSH水平的明显降低(P<0.05)。而LPS预处理可明显减少APAP损伤后的MDA水平(P<0.05),同时减轻APAP对GSH的消耗(P<0.05),见表2。DHE染色也是反映氧化应激的重要指标,如图2所示,APAP组的荧光强度明显高于对照组,而LPS预处理可明显减轻这一趋势。
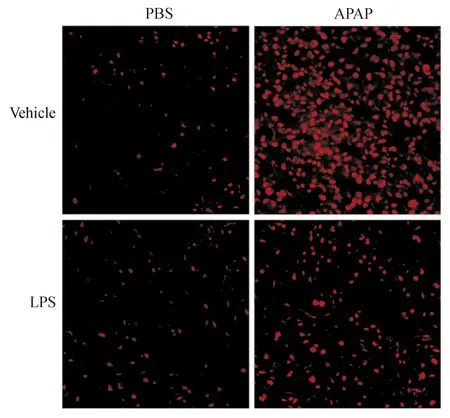
图2 各组小鼠肝组织免疫荧光染色结果
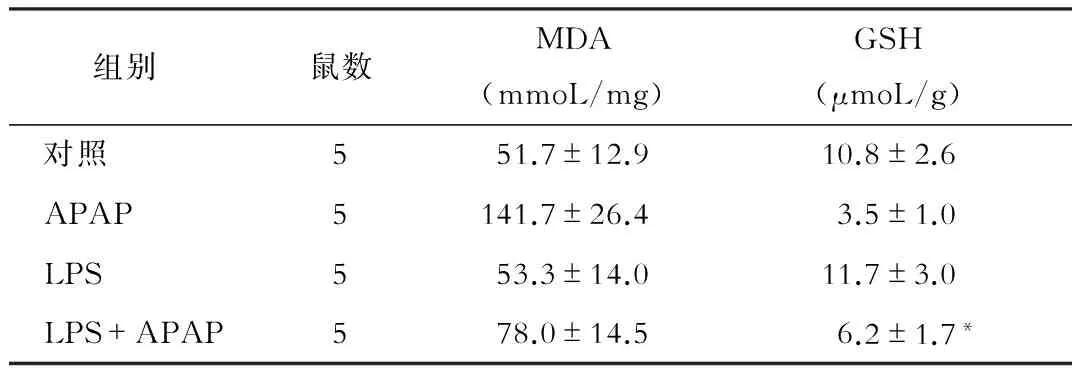
组别鼠数MDA(mmoL/mg)GSH(μmoL/g) 对照551.7±12.910.8±2.6 APAP5141.7±26.43.5±1.0 LPS553.3±14.011.7±3.0 LPS+APAP578.0±14.56.2±1.7*
注:*与APAP组相比,P<0.05
三、LPS对Nrf2抗氧化通路的影响
与对照组相比, APAP处理组小鼠肝组织内Nrf2的蛋白表达量无明显变化,而给予LPS预处理后,无论是否给予APAP造模,肝组织内Nrf2的水平均明显升高(P<0.05)。RT-PCR结果显示,Nrf2的重要的下游抗氧化基因Gclc和HO-1均可被LPS明显激活,见图3。
讨 论
APAP是临床常用药, 过量使用易导致急性药物性肝损伤, 患者预后较差。因此探究APAP的肝毒性机制从而研究防治策略是非常有必要的。已有大量研究证实,氧化应激反应是APAP肝毒性过程中的最重要机制,大量的ROS生成,可损伤线粒体、内质网等重要细胞器的功能,进而导致细胞坏死。本研究也发现,APAP处理的小鼠肝组织内氧化应激反应及ROS水平明显增加。因此,抗氧化应激是防治APAP肝损伤的关键之一。而Nrf2作为一种重要的核转录因子,可以调控一系列抗氧化基因的表达。Nrf2抗氧化通路已成为APAP肝损伤领域的研究热点和重点[27,28]。
TLR是一种病原体识别受体,可以识别病原相关分子模式,通过刺激细胞内炎性信号通路刺激炎性因子的合成和释放,继而促进炎性反应的发生[29]。LPS是革兰阴性杆菌细胞壁的主要组分之一[30]。LPS是TLR的自然配体,该受体的激活可对多种信号通路产生重要影响。TLR在多种肝脏疾病中均扮演重要角色,包括缺血再灌注损伤、非酒精性脂肪性肝病、酒精性肝病、自免肝损伤等[31-34]。研究发现,苯甲醇可以通过激活TLR4对APAP肝损伤起保护作用[35]。尽管LPS预处理对APAP肝损伤的保护作用已有相关报道,但具体机制尚不明确。本研究发现,LPS激活TLRs/Nrf2抗氧化通路,具有减轻氧化应激损伤的作用。
低剂量LPS预处理可明显减轻APAP引起的肝组织坏死。进一步研究发现,LPS可明显增强Nrf2在小鼠肝组织内的表达,同时其代表性的抗氧化基因Gclc及HO-1的mRNA表达水平也明显升高,提示低剂量LPS可以诱导Nrf2抗氧化通路的表达。同时验证了LPS对APAP肝毒性过程中的氧化应激反应的影响。MDA是检测动物体内氧化应激水平和脂质过氧化反应的良好指标,在多种氧化应激相关的肝损伤过程中MDA均会明显升高,而GSH是体内最重要的抗氧化物质,也是中和APAP毒性的主要解毒物质。本研究显示,LPS+APAP组的小鼠肝组织内MDA含量较APAP组减少,而GSH水平明显增多。DHE染色是反映组织内ROS含量的重要指标,DHE结果与MDA结果趋势一致。
综上所述,LPS通过TLR预激活Nrf2抗氧化通路,减轻APAP引起的氧化应激反应,从而明显缓解肝脏损伤。Nrf2抗氧化通路的深入研究可能为药物性肝损伤的临床防治提供一种新的策略。
[1]Larson AM, Polson J, Fontana RJ, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology, 2005, 42: 1364-1372.
[2]Holubek WJ, Kalman S, Hoffman RS. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology, 2006, 43: 880; author reply 882.
[3]Norris W, Paredes AH, Lewis JH. Drug-induced liver injury in 2007. Curr Opin Gastroenterol, 2008, 24: 287-297.
[4]Xu X, Liu X, Ling Q, et al. Artificial liver support system combined with liver transplantation in the treatment of patients with acute-on-chronic liver failure. PLoS One, 2013, 8: e58738.
[5]Mendes M, Ferreira AC, Ferreira A, et al. ABO-incompatible liver transplantation in acute liver failure: a single Portuguese center study. Transplant Proc, 2013, 45: 1110-1115.
[6]Akamatsu N, Sugawara Y, Kokudo N. Acute liver failure and liver transplantation. Intractable Rare Dis Res, 2013, 2: 77-87.
[7]Jollow DJ, Mitchell JR, Potter WZ, et al. Acetaminophen-induced hepatic necrosis. II. Role of covalent binding in vivo. J Pharmacol Exp Ther, 1973, 187: 195-202.
[8]James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos, 2003, 31: 1499-1506.
[9]Dahlin DC, Miwa GT, Lu AY, et al. N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen. Proc Natl Acad Sci U S A, 1984, 81: 1327-1331.
[10]Patten CJ, Thomas PE, Guy RL, et al. Cytochrome P450 enzymes involved in acetaminophen activation by rat and human liver microsomes and their kinetics. Chem Res Toxicol, 1993, 6: 511-518.
[11]Lee SS, Buters JT, Pineau T, et al. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J Biol Chem, 1996, 271: 12063-12067.
[12]Cheung C, Yu AM, Ward JM, et al. The cyp2e1-humanized transgenic mouse: role of cyp2e1 in acetaminophen hepatotoxicity. Drug Metab Dispos, 2005, 33: 449-457.
[13]Walubo A, Barr S, Abraham AM, et al. The role of cytochrome-P450 inhibitors in the prevention of hepatotoxicity after paracetamol overdose in rats. Hum Exp Toxicol, 2004, 23: 49-54.
[14]Potter WZ, Thorgeirsson SS, Jollow DJ, et al. Acetaminophen-induced hepatic necrosis. V. Correlation of hepatic necrosis, covalent binding and glutathione depletion in hamsters. Pharmacology, 1974, 12: 129-143.
[15]Saini SP, Zhang B, Niu Y, et al. Activation of liver X receptor increases acetaminophen clearance and prevents its toxicity in mice. Hepatology, 2011, 54: 2208-2217.
[16]Kim YH, Hwang JH, Kim KS, et al. Metformin Ameliorates Acetaminophen Hepatotoxicity via Gadd45beta-Dependent Regulation of JNK Signaling in Mice. J Hepatol, 2015, 63: 75-82.
[17]Moon MS, Richie JP, Isom HC. Iron potentiates acetaminophen-induced oxidative stress and mitochondrial dysfunction in cultured mouse hepatocytes. Toxicol Sci, 2010, 118: 119-127.
[18]Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev, 2012, 44: 88-106.
[19]Song E, Fu J, Xia X, et al. Bazhen decoction protects against acetaminophen induced acute liver injury by inhibiting oxidative stress, inflammation and apoptosis in mice. PLoS One, 2014, 9: e107405.
[20]Michael Brown J, Ball JG, Wright MS, et al. Novel protective mechanisms for S-adenosyl-L-methionine against acetaminophen hepatotoxicity: improvement of key antioxidant enzymatic function. Toxicol Lett, 2012, 212: 320-328.
[21]Liu WX, Jia FL, He YY, et al. Protective effects of 5-methoxypsoralen against acetaminophen-induced hepatotoxicity in mice. World J Gastroenterol, 2012, 18: 2197-2202.
[22]Yuan HD, Jin GZ, Piao GC. Hepatoprotective effects of an active part from Artemisia sacrorum Ledeb. against acetaminophen-induced toxicity in mice. J Ethnopharmacol, 2010, 127: 528-533.
[23]Ma Q, He X. Molecular basis of electrophilic and oxidative defense: promises and perils of Nrf2. Pharmacol Rev, 2012, 64: 1055-1081.
[24]Jiang YM, Wang Y, Tan HS, et al. Schisandrol B protects against acetaminophen-induced acute hepatotoxicity in mice via activation of the NRF2/ARE signaling pathway. Acta Pharmacol Sin, 2016, 37: 382-389.
[25]Guo Q, Shen Z, Yu H, et al. Carnosic acid protects against acetaminophen-induced hepatotoxicity by potentiating Nrf2-mediated antioxidant capacity in mice. Korean J Physiol Pharmacol, 2016, 20: 15-23.
[26]Yin S, Cao W. Toll-Like Receptor Signaling Induces Nrf2 Pathway Activation through p62-Triggered Keap1 Degradation. Mol Cell Biol, 2015, 35: 2673-2683.
[27]Ye D, Wang Y, Li H, et al. Fibroblast growth factor 21 protects against acetaminophen-induced hepatotoxicity by potentiating peroxisome proliferator-activated receptor coactivator protein-1alpha-mediated antioxidant capacity in mice. Hepatology, 2014, 60: 977-989.
[28]Gum SI, Cho MK. Recent updates on acetaminophen hepatotoxicity: the role of nrf2 in hepatoprotection. Toxicol Res, 2013, 29: 165-172.
[29]Sabroe I, Parker LC, Dower SK, et al. The role of TLR activation in inflammation. J Pathol, 2008, 214: 126-135.
[30]Zamoshnikova A, Gross CJ, Schuster S, et al. NLRP12 is a neutrophil-specific, negative regulator of in vitro cell migration but does not modulate LPS- or infection-induced NF-kappaB or ERK signalling. Immunobiology, 2016, 221: 341-346.
[31]Rao J, Yue S, Fu Y, et al. ATF6 mediates a pro-inflammatory synergy between ER stress and TLR activation in the pathogenesis of liver ischemia-reperfusion injury. Am J Transplant, 2014, 14: 1552-1561.
[32]Hoque R, Farooq A, Ghani A, et al. Lactate reduces liver and pancreatic injury in Toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology, 2014, 146: 1763-1774.
[33]Byun JS, Suh YG, Yi HS, et al. Activation of toll-like receptor 3 attenuates alcoholic liver injury by stimulating Kupffer cells and stellate cells to produce interleukin-10 in mice. J Hepatol, 2013, 58: 342-349.
[34]Tu CT, Han B, Yao QY, et al. Curcumin attenuates Concanavalin A-induced liver injury in mice by inhibition of Toll-like receptor (TLR) 2, TLR4 and TLR9 expression. Int Immunopharmacol, 2012, 12: 151-157.
[35]Cai C, Huang H, Whelan S, et al. Benzyl alcohol attenuates acetaminophen-induced acute liver injury in a Toll-like receptor-4-dependent pattern in mice. Hepatology, 2014, 60: 990-1002.
(本文编辑:钱燕)
TLR protects liver against acetaminophen-induced Hepatotoxicitin via activating the Nrf2 antioxidant signaling pathway
GUJia-yi,YUFeng-rong.
DepartmentofGastroenterologicalSurgery,RenJiHospital,SchoolofMedcine,ShanghaiJiaotongUniversity,Shanghai200127,ChinaCorrespondingauthor:YUFengrong,Email:rry@126.com
ObjectiveTo investigate the protective effect of Lipopolysaccharide (LPS) and toll-like receptors (TLRs) on acetaminophen (APAP)-induced liver injury and its potential mechanism. MethodsForty male mice were randomly divided into 4 groups. Mice in control group were intraperitneally (i.p.) injected with saline, in LPS group were i.p. given with 10 μg/kg LPS, and in APAP group were i.p. administrated with APAP (300 mg/kg). LPS+APAP group were i.p. pretreated with LPS (10 μg/kg) 16 h before APAP (300 mg/kg) injection. Serum and liver tissue among 4 groups were collected for further analysis. Liver injury was assessed by detection of serum ALT and AST levels and HE staining of liver tissue. The oxidative stress was evaluated by measuring hepatic glutathione (GSH) and malondialdehyde (MDA) levels, and Dihydroethidium (DHE) staining. Expression of Nrf2, Gclc and HO-1 were measured by western blot and real time-polymerase chain reaction. ResultsCompared with APAP groups, LPS+APAP group showed lower serum levels of ALT (518.3±142.3 vs. 4542±498.4 U/L,P<0.05) and AST (643.3±105.6 vs. 5432.1±569.2 U/L,P<0.05), milder liver tissue damage, lower MDA levels (78.0±14.5 vs.141.7±26.4 mmol/mg tissue,P<0.05) and higher GSH levels (6.2±1.7 vs. 3.5±1.0 μmol/g tissue,P<0.05), which revealed that APAP-induced liver injury and oxidative stress response were significantly attenuated by LPS pretreatment. Moreover, LPS pretreatment could enhance the expression of Nrf2 and other antioxidant genes significantly. ConclusionLPS plays an important role in protection against APAP-induced hepatotoxicity in mice via activating antioxidant signaling pathway of Nrf2, which might become a new strategy for APAP-induced liver injury therapy.
Acetaminophen;Drug induced liver injury; Oxidative stress;Nrf2;HO-1
200127上海交通大学医学院附属仁济医院胃肠外科
郁丰荣,Email:rry@126.com
2016-07-26)
