稳定表达Caveolin-3基因突变型P104L和P47L的C2C12骨骼肌细胞株的构建
2016-10-24邓玉风黄亿元尚丽娜杨立会黄勤
邓玉风,黄亿元,尚丽娜,杨立会,黄勤
(1右江民族医学院,广西百色533000;2广西医科大学)
稳定表达Caveolin-3基因突变型P104L和P47L的C2C12骨骼肌细胞株的构建
邓玉风1,黄亿元1,尚丽娜2,杨立会2,黄勤2
(1右江民族医学院,广西百色533000;2广西医科大学)
目的构建稳定表达Caveolin-3(CAV3)基因突变型P104L和P47L的C2C12骨骼肌细胞株。方法 将野生型CAV3(CAV3-WT)、CAV3-P104L、CAV3-P47L装在带有GFP标签的质粒中,构建目的基因与GFP基因融合的表达质粒,以只含GFP的载体为对照。将C2C12细胞随机分为A、B、C、D组,分别转染对照空载体(NC)、CAV3-WT、CAV3-P47L及CAV3-P104L。以遗传霉素(G418)进行阳性克隆筛选,荧光显微镜下挑选稳定转染的细胞系,Western blotting法检测各组细胞中的CAV3-GFP融合蛋白,免疫荧光法检测各组细胞中的CAV3蛋白,以荧光强度代表目的蛋白相对表达量。结果 经酶切和测序验证,证实4种表达质粒构建成功。B、C、D组细胞中测得CAV3-GFP融合蛋白,A组未测得融合蛋白。A、B、C、D组CAV3蛋白相对表达量分别为23.0±2.25、32.3±0.35、25.6±0.92、24.3±1.42,B组CAV3蛋白表达增强,与A组相比,P<0.01;A、C、D组CAV3蛋白表达下调,与B组相比,P均<0.01。结论 成功构建了稳定表达CAV3-P47L和CAV3-P104L的C2C12细胞,两种细胞中CAV3蛋白表达下调。
Caveolin-3;基因突变; 2型糖尿病;肢带型肌营养不良
CAV3蛋白在1996年首次得到克隆和鉴定,它由151个氨基酸组成,是肌肉特有的微囊蛋白,仅在骨骼肌和心肌中大量表达,参与许多细胞生理过程,包括钙离子、钠离子和钾离子通道调节,囊泡转运,胆固醇水平调节及信号转导的调控。CAV3蛋白还可维持体内葡萄糖和脂质平衡,并被认为与胰岛素抵抗有关[1,2],可能在2型糖尿病(T2DM)的发病中发挥一定作用。我们课题组前期从T2DM患者血样中提取DNA,PCR后测序发现有较多CAV3基因突变位点[3],如第47个氨基酸位置由脯氨酸(Pro)突变为亮氨酸(Leu),即P47L。此突变点是否参与T2DM的发病有待研究。CAV3基因突变还与多种肌组织病变有关,其中一种人类常染色体显性遗传的肢带型肌营养不良(LGMD-1C)是由于CAV3基因编码区P104L突变导致[4~8]。目前我国人群LGMD-1C与CAV3基因P104L突变的相关性尚未见报道,其具体机制也无深入研究。为此,我们构建了稳定转染CAV3基因突变型P104L和P47L的C2C12骨骼肌细胞株,为进一步研究打下实验基础。
1 材料与方法
1.1材料C2C12细胞株从中国科学院上海细胞库引进。E.coli DH5α感受态细胞购于TIANGEN公司,表达载体购自美国Gene Copoeia公司,细胞培养试剂购于美国Gibco公司,去内毒素质粒提取试剂盒购于Omega公司,脂质体转染试剂盒LipofectamineTM3000、G418和Alexa Fluor 647荧光二抗购自Invitrogen公司,CAV3抗体购于Santa Cruz公司,GAPDH购于Immunoway公司,远红外IRDye 800二抗购自Li-Cor公司。
1.2CAV3-P104L、CAV3-P47L表达质粒的构建表达质粒由Gene Copoeia公司构建并验证,将目的基因野生型CAV3(CAV3-WT)、CAV3-P104L、CAV3-P47L装在带有GFP标签的表达质粒中,CAV3-WT碱基序列是从NCBI数据库获取的CAV3的CDS序列,两个突变型是在野生型的基础上定点诱变而成。我们把CDS序列的最后3个碱基TAA(终止密码子)去掉,则可构建CAV3与GFP的融合表达载体,以只含GFP的载体为对照。将4种载体转化至E.coli DH5α感受态细胞中,用氨苄青霉素抗性的LB平板培养基筛选阳性克隆,在超净台里用10 μL枪头挑取白色单菌落放至装有LB/Amp(5 mL)培养基的离心管中,混匀后置于280 r/min、37 ℃摇床孵育8 h,取菌液50 μL加入LB/Amp培养基(50 mL)中继续培养12~16 h,用去内毒素质粒提取试剂盒中量提取质粒,送上海生工测序。上游引物序列为5′-GCGGTAGGCGTGTACGGT-3′,下游引物序列为5′-CCGGACACGCTGAACTTGT-3′。
1.3CAV3-P104L、CAV3-P47L转染C2C12细胞取对数生长期的C2C12细胞接种于6孔板,加入含10% FBS无抗生素的DMEM培养基,当细胞达到70%融合时,分为A、B、C、D组,分别转染空载体、CAV3-WT、CAV3-P47L和CAV3-P104L。转染24 h后按1∶10比例传代细胞,加入含G418(400 μg/mL)的培养液进行筛选,设置未转染的细胞作为对照。荧光显微镜下观察,筛选出含绿色荧光的单克隆细胞后行扩大培养。取部分细胞待检。
1.4转染突变型CAV3基因C2C12细胞中CAV3蛋白检测①Western blotting法:取各组细胞用RIPA裂解液裂解,提取细胞总蛋白,用BCA蛋白浓度测定试剂盒定量蛋白浓度后,加入10% SDS-PAGE分离胶分离,用含20%甲醇的转膜液将蛋白转移到PVDF膜;5%脱脂奶粉封闭1 h,4 ℃孵育抗CAV3(1∶100)和抗GAPDH(1∶1 000)过夜;TBST洗3次后,用远红外IRDye 800(1∶10 000)二抗室温孵育2 h;TBST洗3次,以Odyssey双色红外荧光扫描成像系统鉴定反应条带。②免疫荧光法:取四组细胞接种于细胞爬片专用的盖玻片上,每组细胞做3张爬片,待细胞长至60%~70%融合时用4%多聚甲醛固定,5% BSA封闭1 h后滴加抗CAV3抗体4 ℃孵育过夜,避光孵育Alexa Fluor 647(1∶1 000)荧光二抗1 h,用激光扫描共聚焦显微镜拍摄并分析,用Image Pro Plus6.0软件测定荧光强度,作为目的蛋白相对表达量。

2 结果
从扩增的菌液提取质粒并根据载体设计引物测序验证,测序结果与设计序列一致,与CAV3-WT序列比较,P47L、P104L突变都是由C碱基变成T碱基,导致氨基酸由脯氨酸突变为亮氨酸。
CAV3蛋白的分子量为25 kD,GFP分子量为27 kD,所以融合蛋白分子量约为52 kD。A组转染空载体,用抗CAV3抗体未检测到CAV3-GFP融合蛋白表达(52 kD左右无条带),B、C、D组在52 kD左右可检测到条带,说明融合蛋白表达,4组细胞筛选成功(见图1)。共聚焦显微镜拍摄结果显示,转染CAV3-WT、CAV3-P104L、CAV3-P47L的细胞均能表达GFP,且主要定位在胞质和胞膜上,与CAV3蛋白表达情况基本一致(见图2)。A、B、C、D组CAV3蛋白相对表达量分别为23.0±2.25、32.3±0.35、25.6±0.92、24.3±1.42,B组CAV3蛋白表达增强,与A组相比,P<0.01;A、C、D组CAV3蛋白表达下调,与B组相比,P均<0.01。
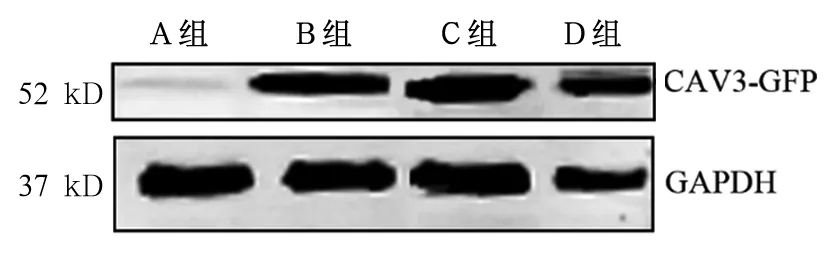
图1 四组CAV3-GFP融合蛋白Western blotting
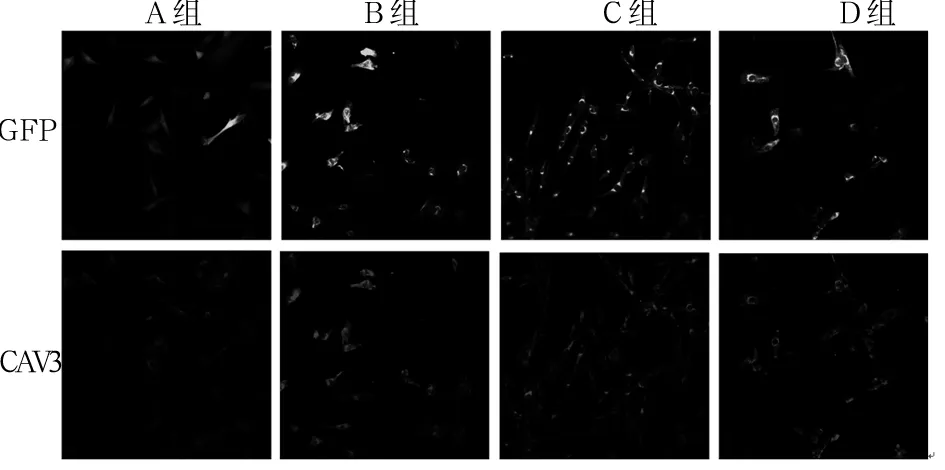
图2 四组CAV3蛋白免疫荧光法检测结果
3 讨论
微囊是细胞囊膜上直径在50~100 nm的特异性囊状小凹结构,主要由胆固醇、鞘磷脂、糖基鞘磷脂及CAV组成。CAV是相对分子质量为21~24 kD的膜蛋白,高度富集于微囊膜,是体内细胞膜微囊的主要和特征性蛋白,在维持微囊的形态、结构和功能中起重要作用。在哺乳动物细胞中,CAV基因家族有三种亚型:CAV1、CAV2和CAV3。CAV1和CAV2共同表达于许多类型细胞中并形成异源寡聚体复合物发挥作用,特别是在脂肪细胞中有高度表达[9]。CAV3仅在骨骼肌和心肌的微囊及肌膜内陷形成的T管中高表达,因其具有肌肉特异性,推测CAV3可能对肌细胞有特殊作用[10,11]。CAV3蛋白在维持微囊正常结构及调节细胞囊泡转运、离子通道开放、细胞内外信号转导功能等方面起着关键作用。CAV3基因位于3p25区,CAV3突变蛋白通过多种可能机制引起骨骼肌、心肌等不同程度的病变,统称微囊蛋白病[12]。目前已发现CAV3基因突变与8种典型的骨骼肌和心肌疾病有关,包括LGMD-1C、波纹肌肉病(RMD)、远端型肌病(DM)、高CK血症(H-CK)、肥厚型心肌病(HCM)、扩张型心肌病(DCM)、长QT综合征(LQTS)、婴儿猝死综合征(SIDS)[12~17]。
在上述疾病中,LGMD-1C是研究热点之一,该病主要特点是局限于肢体肌肉组织的无力和废用,临床表现为轻到中度近端肌无力,Gowers征阳性,小腿肥大,肌痛,血清CK水平是正常值的4~25倍[18]。LGMD-1C患者中最常见的CAV3突变是P104L。文献[19~21]报道P104L突变可引起细胞膜上CAV3含量减少,导致LGMD-1C。P104L转基因小鼠可导致LGMD-1C,伴随着eNOS和肌生成抑制蛋白1型受体活性增加[7]。成熟的CAV3主要定位于肌膜的微囊区。P104L突变使CAV3滞留在高尔基复合体中形成不稳定的高分子聚集团,微囊蛋白生成减少、不能移位到细胞膜[20],严重改变微囊蛋白的功能,导致膜修复缺陷,而膜修复缺陷将加剧病情进展[22]。我们在实验中也发现,稳定转染CAV3-P104L的细胞中CAV3表达量低于稳定转染CAV3-WT的细胞,推测CAV3蛋白表达减少可能通过某些机制影响肌肉功能。
研究[23]认为,T2DM是由多种因素联合作用引起,并不是由单一的病理生理机制导致,胰岛素抵抗是T2DM的病理基础之一。CAV3蛋白是肌肉特有的微囊蛋白,在维持体内葡萄糖和脂质平衡中发挥重要作用,与胰岛素抵抗的发生也有关。有研究[24]表明,CAV3与糖尿病有密切联系,糖尿病大鼠CAV3表达水平减少,而CAV3转基因可恢复骨骼肌的胰岛素通路[25]。CAV3可调节GLUT4易位到细胞膜表面的过程[26],胰岛素通过其受体发挥作用使胞内GLUT4转移到细胞膜,并引导GLUT4到达特定的微囊区域,从而提高肌肉等组织对葡萄糖的摄取能力[27]。在本实验中,我们发现转染CAV3-P47L的细胞中CAV3蛋白表达量低于转染CAV3-WT的细胞,提示P47L突变可能会下调CAV3的表达水平而参与T2DM的发病。
总之,CAV3基因与骨骼肌疾病、心肌疾病及T2DM的发病均有关,但CAV3在这些疾病中的发病机制尚未明确。通过CAV3敲除动物模型或体外细胞转染实验可进一步研究CAV3在骨骼肌和心肌中的作用,探究CAV3基因突变所致相关疾病的发病机制。我们通过稳定转染技术,将CAV3-P104L、CAV3-P47L转入C2C12细胞并稳定表达。在转染的过程中,我们除了使用较好的转染试剂,还对转染使用的质粒进行了去内毒素处理,防止内毒素对细胞的伤害。另外,我们对细胞状态和密度也作了调整,提高了转染效率,有利于更准确地获取稳定转染细胞株。在研究中我们发现,稳定转染CAV3-P47L和CAV3-P104L的C2C12细胞株CAV3蛋白表达量低于转染CAV3-WT的细胞,这为进一步研究CAV3基因突变在T2DM和LGMD-1C发病中的作用机制奠定了实验基础。
[1] Marx J. Caveolin-3 helps build muscles[J].Science, 2001,294(5548):1864.
[2] Murfitt L, Whiteley G, Iqbal MM, et al. Targeting caveolin-3 for the treatment of diabetic cardiomyopathy[J]. Pharmacol Ther, 2015,151:50-71.
[3] 黄勤,黄亿元,邓玉风,等.Caveolin-3基因多态性的筛查及其在中国汉族糖尿病患者中的特点[J].实用医学杂志,2014,30(11):1757-1759.
[4] Sotgia F, Woodman SE, Bonuccelli G, et al. Phenotypic behavior of caveolin-3 R26Q, a mutant associated with hyperCKemia, distal myopathy, and rippling muscle disease[J]. Am J Physiol Cell Physiol, 2003,285(5):C1150-1160.
[5] Weiss N, Couchoux H, Legrand C, et al. Expression of the muscular dystrophy-associated caveolin-3(P104L) mutant in adult mouse skeletal muscle specifically alters the Ca(2+) channel function of the dihydropyridine receptor[J]. Pflugers Arch, 2008,457(2):361-375.
[6] Couchoux H, Allard B, Legrand C, et al. Loss of caveolin-3 induced by the dystrophy-associated P104L mutation impairs L-type calcium channel function in mouse skeletal muscle cells[J]. J Physiol, 2007,580(Pt.3):745-754.
[7] Ohsawa Y, Hagiwara H, Nakatani M, et al. Muscular atrophy of caveolin-3-deficient mice is rescued by myostatin inhibition[J]. J Clin Invest, 2006,116(11):2924-2934.
[8] Ohsawa Y, Toko H, Katsura M, et al. Overexpression of P104L mutant caveolin-3 in mice develops hypertrophic cardiomyopathy with enhanced contractility in association with increased endothelial nitric oxide synthase activity[J]. Hum Mol Genet, 2004,13(2):151-157.
[9] Scherer PE, Lewis RY, Volonte D, et al. Cell-type and tissue-specific expression of caveolin-2. Caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo[J]. J Biol Chem, 1997,272(46):29337-29346.
[10] Parton RG, Way M, Zorzi N, et al. Caveolin-3 associates with developing T-tubules during muscle differentiation[J]. J Cell Biol, 1997,136(1):137-154.
[11] Minetti C, Bado M, Broda P, et al. Impairment of caveolae formation and T-system disorganization in human muscular dystrophy with caveolin-3 deficiency[J]. Am J Pathol, 2002,160(1):265-270.
[12] Gazzerro E, Sotgia F, Bruno C, et al. Caveolinopathies: from the biology of caveolin-3 to human diseases[J]. Eur J Hum Genet, 2010,18(2):137-145.
[13] Woodman SE, Sotgia F, Galbiati F, et al. Caveolinopathies: mutations in caveolin-3 cause four distinct autosomal dominant muscle diseases[J]. Neurology, 2004,62(4):538-543.
[14] Hayashi T, Arimura T, Ueda K, et al. Identification and functional analysis of a caveolin-3 mutation associated with familial hypertrophic cardiomyopathy[J]. Biochem Biophys Res Commun, 2004,313(1):178-184.
[15] Cronk LB, Ye B, Kaku T, et al. Novel mechanism for sudden infant death syndrome: persistent late sodium current secondary to mutations in caveolin-3[J].Heart Rhythm, 2007,4(2):161-166.
[16] Catteruccia M, Sanna T, Santorelli FM, et al. Rippling muscle disease and cardiomyopathy associated with a mutation in the CAV3 gene[J]. Neuromuscular disorders: NMD, 2009,19(11):779-783.
[17] Traverso M, Gazzerro E, Assereto S, et al. Caveolin-3 T78M and T78K missense mutations lead to different phenotypes in vivo and in vitro[J]. Lab Invest, 2008,88(3):275-283.
[18] Gazzerro E, Bonetto A, Minetti C. Caveolinopathies: translational implications of caveolin-3 in skeletal and cardiac muscle disorders[J]. Handbook Clin Neur, 2011,101:135-142.
[19] Couchoux H, Bichraoui H, Chouabe C, et al. Caveolin-3 is a direct molecular partner of the Cav1.1 subunit of the skeletal muscle L-type calcium channel[J]. Int J Biochem Cell Biol, 2011,43(5):713-720.
[20] Galbiati F, Volonte D, Minetti C, et al. Limb-girdle muscular dystrophy (LGMD-1C) mutants of caveolin-3 undergo ubiquitination and proteasomal degradation. Treatment with proteasomal inhibitors blocks the dominant negative effect of LGMD-1C mutanta and rescues wild-type caveolin-3[J]. J Biol Chem, 2000,275(48):37702-37711.
[21] Galbiati F, Razani B, Lisanti MP. Caveolae and caveolin-3 in muscular dystrophy[J]. Trends Mol Med, 2001,7(10):435-441.
[22] Cai C, Weisleder N, Ko JK, et al. Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin[J]. J Biol Chem, 2009,284(23):15894-15902.
[23] 潘长玉,尹士男.第一讲:胰岛素抵抗——2型糖尿病发病机制的重要因素[J].中华内分泌代谢杂志,2000,16(1):59-60.
[24] Lei S, Li H, Xu J, et al. Hyperglycemia-induced protein kinase C beta2 activation induces diastolic cardiac dysfunction in diabetic rats by impairing caveolin-3 expression and Akt/eNOS signaling[J]. Diabetes, 2013,62(7):2318-2328.
[25] Liewluck T, Goodman BP, Milone M. Electrically active immune-mediated rippling muscle disease preceding breast cancer[J]. Neurologist, 2012,18(3):155-158.
[26] Tan Z, Zhou LJ, Mu PW, et al. Caveolin-3 is involved in the protection of resveratrol against high-fat-diet-induced insulin resistance by promoting GLUT4 translocation to the plasma membrane in skeletal muscle of ovariectomized rats[J]. J Nutr Biochem, 2012,23(12):1716-1724.
[27] Gustavsson J, Parpal S, Stralfors P. Insulin-stimulated glucose uptake involves the transition of glucose transporters to a caveolae-rich fraction within the plasma membrane: implications for type Ⅱ diabetes[J]. Mol Med, 1996,2(3):367-372.
Establishment of C2C12 skeletal muscle cell lines stably overexpressing Caveolin-3 gene P104L and P47L mutations
DENGYufeng1,HUANGYiyuan,SHANGLina,YANGLihui,HUANGQin
(1YoujiangMedicalUniversityforNationalities,Baise533000,China)
ObjectiveTo construct C2C12 skeletal muscle cell lines stably expressing Caveolin-3 (CAV3) gene P104L and P47L mutations. MethodsThe wild type CAV3 (CAV3-WT), CAV3-P104L, CAV3-P47L were installed in the plasmid with GFP tag, and then we constructed the target gene and GFP gene fusion expression plasmid, with only GFP carrier as the control. C2C12 cells were randomly divided into four groups: groups A, B, C and D which were transfected with empty vector (NC), CAV3-WT, CAV3-P47L and CAV3-P104L. After treated with geneticin (G418), we selected the stable transfected cell lines under the fluorescence microscope. The expression of CAV3 and GFP fusion protein was detected by Western blotting, and the expression of CAV3 protein was detected by immunofluorescence. The relative expression of the target protein was expressed by the fluorescence intensity. Results After enzyme digestion and sequencing, the four expression vectors proved to be successfully constructed. Cell lines of groups B, C and D contained the fusion protein expression of CAV3 and GFP, but fusion protein was not detected in group A. The relative expression of CAV3 in groups A, B, C and D was 23.0±2.25, 32.3±0.35, 25.6±0.92 and 24.3±1.42. The expression of CAV3 in group B was increased compared with that of group A,P<0.01, and the expression of CAV3 in groups A, C and D was significantly decreased compared with that of B group, allP<0.01. ConclusionWe successfully constructed the C2C12 cells stably expressing CAV3-P104L and CAV3-P47L, and the expression of CAV3 protein was down-regulated in these two cells.
Caveolin-3; gene mutation; type 2 diabetes mellitus; limb girdle muscular dystrophy
邓玉风(1989-),女,助教,主要研究方向为2型糖尿病和肌病。E-mail: dyfgxmu@163.com
简介:黄勤(1969-),女,副教授,主要研究方向为2型糖尿病和肌病。E-mail: hqgxmu@163.com
10.3969/j.issn.1002-266X.2016.31.004
R34
A
1002-266X(2016)31-0012-04
2016-02-26)
