Genetics of seed flavonoid content and antioxidant activity in cowpea(Vigna unguiculata L.Walp.)
2016-10-24MinAntoineNssourouYnouNiolsNjintngThigmJenBptisteNouissiRihrdMrelNguimouJosephMrtinBell
Min Antoine Nssourou*,Ynou Niols Njintng, Thigm Jen-Bptiste NouissiéRihrd Mrel Nguimou,Joseph Mrtin Bell
aDepartment of Biological Sciences,Faculty of Science,University of Ngaoundéré,P.O.Box 454,Ngaoundéré,Cameroon
bDepartment of Food Science and Nutrition,ENSAI,University of Ngaoundéré,P.O.Box 455,Ngaoundéré,Cameroon
cGenetics and Plant Breeding Unit,Department of Plant Biology,Faculty of Science,University of Yaoundé I,P.O.Box 812,Yaoundé,Cameroon
Genetics of seed flavonoid content and antioxidant activity in cowpea(Vigna unguiculata L.Walp.)
Maina Antoine Nassouroua,*,Yanou Nicolas Njintanga,b, Tchiagam Jean-Baptiste Noubissiéa,Richard Marcel Nguimboub,Joseph Martin Bellc
aDepartment of Biological Sciences,Faculty of Science,University of Ngaoundéré,P.O.Box 454,Ngaoundéré,Cameroon
bDepartment of Food Science and Nutrition,ENSAI,University of Ngaoundéré,P.O.Box 455,Ngaoundéré,Cameroon
cGenetics and Plant Breeding Unit,Department of Plant Biology,Faculty of Science,University of Yaoundé I,P.O.Box 812,Yaoundé,Cameroon
A R T I C L E I N F O
Article history:
Available online 30 June 2016
Cowpea
Genetic improvement
Diallel analysis
Antioxidant properties
Information about the type of gene actiongoverning the inheritance of cowpea seed flavonoid content and antioxidant activity is prerequisite for starting an effective breeding program for developing improved varieties.For this purpose,half-diallel crosses among seven diverse parents were made.The homozygous parents and 21 F1hybrids were evaluated at Maroua in the Sudano-Sahelian zone of Cameroon using a randomizedcomplete block design with three replicates.Flour samples produced from decorticated seeds were used for biochemical analysis.Analysis of variance showed significant differences(P<0.001)among genotypes for the studied traitswith rangesof 363.6-453.9 mg rutin equivalent per 100 g dry weight(DW)for total flavonoids,13.38-30.73 mg ascorbic acid equivalent per 1 g DW for ferric iron reducing activity,70.98-266.93 mg trolox equivalent per 100 g DW for 2,2-diphenyl-1-picrylhydrazyl(DPPH)free radical scavenging activity,and 90.93-370.62 mg trolox equivalent per 100 g DW for 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid)(ABTS)free radical scavenging activity.Both additive and non-additive gene effects were significant in the genetic control of these traits,but dominance variance was greater than additive variance.The traits were mainly controlledbyoverdominance model suggesting a selection inthe delayed generations. Broad-and narrow-sense heritability estimates varied from 0.90 to 0.99 and from 0.12 to 0.45,respectively.The variances due to both general and specific combining ability were highly significant for all studied traits.Recessive alleles had positive effects on DPPH and ABTS scavenging activities,whereas dominant alleles had positive effects on flavonoid content and ferric iron reducing activity.These results could help cowpea breeders to improve the antioxidant potential of cowpea seeds by appropriate selection.
©2016 Crop Science Society of China and Institute of Crop Science,CAAS.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
Cowpea(VignaunguiculataL.Walp.)isastaplefoodthatprovides large amounts of proteins,calories,vitamins,and essential minerals for human nutrition in many countries[1-3].It is consumed in central and western Africa mostly in the form of steamedpastecake(kokiormoinmoin)andfritters(kosaiorakara)[4,5].Cowpeas are also used in the formulation of simple infant weaning foods that are relatively affordable for poor rural populations[6].Cowpea seeds also contain phytochemicals that provide some health benefits to consumers.
Phenolic compounds such as flavonoids are plant secondary metabolites that play an important role in plant protection[7]. Although plant phenolics and specifically flavonoids have been classifiedasantinutrients,theyareusefulasnaturalantioxidants[8].Levels of total flavonoids and antioxidant activity were correlated[9,10].According to Enujiugha et al.[8]and Kumar et al.[11]epidemiological studies have revealed that the consumption of flavonoid-rich foods protects against human diseases associated with oxidative stress.Cowpea seeds are a good source of antioxidants,as reported recently[12-17].Little information about the genetic variation in flavonoid content of cowpea seeds is available,except for the reports of Adeyemi and Olorunsanya[15],Apea-Bahetal.[17],andSalawuetal.[18].Toourknowledge,only Nzaramba et al.[12]and Noubissié et al.[16]have evaluated the genetic variation and inheritance of antioxidant activity by the DPPH method in cowpea.Both studies involved diallel and/or generation mean analysis involving four different pure lines to evaluateinheritanceandothergeneticeffects.Theimportanceof such studies is reinforced by the rejection of the synthetic antioxidants(butylated hydroxyanisole,butylated hydroxytoluene,and tertiary butylhydroxylquinone)by the consumers,in favorofnaturalantioxidantssuchasphenoliccompounds[19,20].
As cowpeas are considered as poor persons'meat and are a principal source of protein for rural populations,it is important to evaluate the inheritance of their health-promoting traits for the development of elite genotypes.Diallel crossing is commonly adopted for evaluating parental lines for performance.It is an appropriate method for rapidly obtaining an overall picture of the genetic control of a trait in a set of inbred lines. This mating design has also been identified by Mather and Jinks[21]as a tool for evaluating genetic components underlying the inheritance of quantitative traits.To our knowledge,little information about the inheritance of antioxidants in various vegetables is available and no studies have evaluated the genetic components of total flavonoid content and antioxidant activity in cowpea in Cameroon's Sudano-Sahelian zone. The aims of this study were to evaluate total flavonoid content and antioxidant activity and elucidate their genetic control and inheritance in order to propose a suitable breeding strategy for improving the antioxidant potential of cowpea seeds.
2.Materials and methods
2.1.Experimental site
Field experiments were conducted from 2011 to 2013 at the IRAD (Institute of Agricultural Research for Development) farm of Giring (09°30′ N, 10°32′ E) in Maroua (Far North Cameroon). Giring is located in the Sudano-Sahelian zone with a ferruginous vertisol soil type. The soil is sandy clay with 8.2 m g k g-1of organic matter and pH of 5.65 [22]. Annual average rainfall ranges between 800 and 900 mm, with a 4-month rainy season from June to September. The mean annual temperature is 28 °C and the mean annual humidity is 40% [22].
2.2.Plant material and experimental design
Cowpea(Vigna unguiculata L.Walp.)seeds of 15 fully homozygous cultivars(two local landraces and 13 improved lines)were obtained from the IRAD in Maroua.Preliminary field screenings were performed during the rainy season in 2011 and 2012 to ensure the purity of the genotypes and evaluate their variation for flavonoid content and antioxidant potential.The experimental design was a randomized complete block design(RCBD)with three replications.Cowpea plants were grown in an experimental area of 384 m2(20.0 m length×19.2 m width).The plot unit consisted of one row of 10 m length with an inter-row spacing of 80 cm.Three seeds were sown with an intra-row spacing of 25 cm and later thinned to one plant per hill.A safety and protection distance of 2 m surrounded the experimental field.At flowering stage,experimental plots were sprayed with a standard insecticide formulation,cypermethrin+dimethoate,at the rate of 30 g+250 g a.i.L-1to control pod borers and other pests.Mature pods were progressively harvested and healthy seeds were carefully selected and kept in tagged envelopes.
2.3.Crossings
Seven genotypes(24-125B,B301,BR1,CRSP,IT97K-573-1-1,Lori,and VYA)were selected as parents for diallel crossing on the basis of their genetic variability for these traits.Seeds of these parents were sown during the 2013 rainy season for crossing.At anthesis,plant-to-plant pollination of all possible crosses except reciprocals was made in 21 cross combinations following the 7×7 diallel crossing pattern.Each cross was tagged for easy identification,and at maturity,the F1seeds were harvested separately.The seven parental lines and the 21 F1hybrids obtained were planted in a RCBD with three replications during the 2014 rainy season.Plot unit size,spacing and treatments were as described above.
2.4.Biochemical analysis
A random sample of 0.25 g of flour prepared from seeds of each genotype following Phillips et al.[23]was used for the methanolic extraction of crude polyphenol compounds.Seeds were extracted with 15 mL of 70%methanol following Abdou Bouba et al.[24],and the extracts were used for all biochemical analyses.
Total flavonoid content was determined following Noudeh et al.[25]based on the flavonoid-aluminum complex with maximum absorption at 430 nm.A calibration curve was prepared with a 1 mg mL-1solution of rutin[26],and results were expressed as mg rutin equivalent on a dry basis.
Ferric iron reducing activity(FIRA)was evaluated by determining the ability of an antioxidant to reduce iron(III)to iron(II)by the method of Oyaizu[27].Absorbance was read at 700 nm and ascorbic acid was used as standard.FIRA was expressed as mg ascorbic acid equivalent per g of flour.
DPPH (2,2-diphenyl-1-picrylhydrazyl)free radical scavenging activity(FRSA)was determined by the capacity of an antioxidant to trap a free radical or to donate a hydrogen atom,following Zhang and Hamauzu[28]with slight modifications. Trolox in varying concentrations was used as standard for the calibration curve,and the absorbance was read at 517 nm. The antioxidant activity of the extracts was expressed as mg trolox equivalent per 100 g dry weight(DW).
ABTS[2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonicacid)]FRSA was determined by ABTS radical cation decoloration following Re et al.[29].Absorbances were read at 734 nm and stable values at room temperature during approximately 1 min were recorded.Trolox(0.625 g L-1)at various concentrations(1.250 mmol,0.833,0.625,and 0.500 mmol L-1)was used for the calibration curve.Results obtained were expressed as mg trolox equivalent per 100 g dry weight.
2.5.Statistical and genetic analyses
All biochemical analyses were performed in triplicate.To estimate genetic variation,data obtained from the 28 genotypes(parents and hybrids)were subjected to an analysis of variance(ANOVA)using STATGRAPHICS Plus 5.0[30].
The genetic analysis was performed for a 7×7 half-diallel mating using the DIAL98 computer program [31].Griffing's[32]method 2(excluding reciprocal F1crosses)and model 1(fixed effects)were used to estimate the general combining ability(GCA)of the lines and the specific combining ability(SCA)of crosses.GCA and SCA estimates of parents and hybrids,respectively,were obtained as
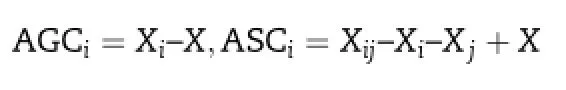
where X is the general mean of the population,Xiis the mean of the hybrids from parent i,Xjis the mean of the hybrids from parent j,and Xijis the value of the hybrid from parents i and j.
Genetic parameters were estimated by Hayman's method[33].Student's t test was used to test the hypotheses that the GCA or SCA effects equal zero.
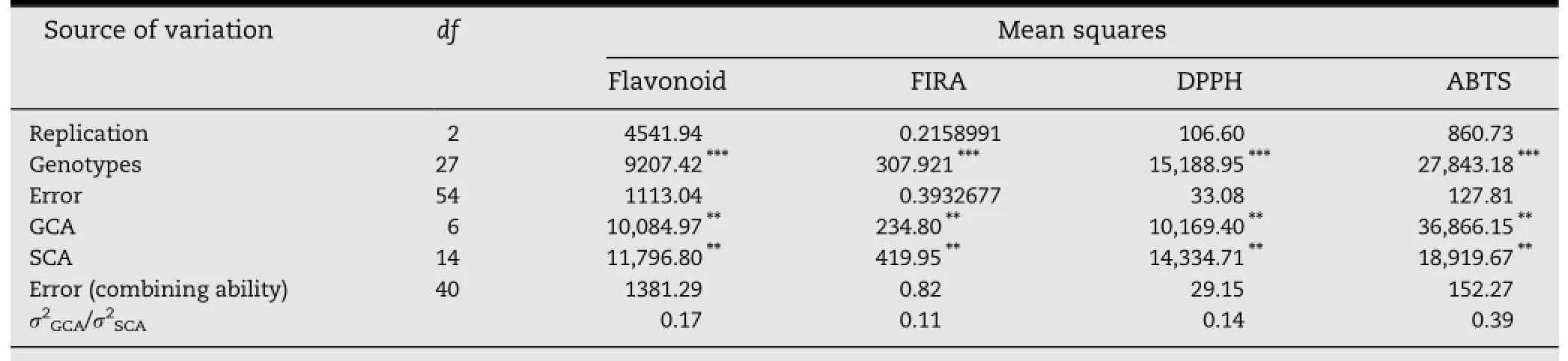
Table 1-Mean squares obtained for total flavonoid and antioxidant activity for genotypes and combining ability in cowpea.
3.Results
3.1.Genotypic variability
The ANOVA for flavonoid content and antioxidant activities showed a significant difference(P<0.001)between the various genotypes and hybrids studied(Table 1).There was also a significant effect(P<0.01)of general and specific combining ability.The ratio σ2GCA/σ2SCAshowed values lower than 1,indicating the prevalence of non-additive gene effects in the genetic control of the studied traits.
3.2.Diallel analysis The average values of parents(per se)and GCA of crosses revealed that the genotypes with highest flavonoids and ABTS-FRSA were BR1and B301,respectively(Table 2),whereas genotype Lori showed the highest FIRA and DPPH-FRSA. However,the genotypes with highest values sometimes showed the lowest GCA.This relationship was observed for BR1(the genotype with highest value),which presented a negative and significant(P<0.05)GCA effect for flavonoid content.In contrast,the genotype 24-125B(with lowest value)presented a positive and significant(P<0.05)GCA effect for flavonoid content(Table 2).Positive and significant combining ability is necessary for improving the antioxidant potential of seeds.The genotypes 24-125B and B301 showed positive and significant(P<0.05)GCA effects for flavonoid content and FRSA(DPPH and ABTS),respectively.For FIRA,there were no positive and significant(P<0.05)GCA effects.
Forthecombinations24-125B×CRSP,B301×IT97K-573-1-1,BR1×IT97K-573-1-1,CRSP×VYA,IT97K-573-1-1×Lori,and Lori×VYA,positive and significant(P<0.05)SCA effects were observed for flavonoid content(Table 3).The combination B301×Lori presented a positive and significant(P<0.05)SCA effect for all antioxidant activities.
Thegeneticparametersandtheirratioswereobtainedbythe graphical method of Hayman[33](Table 4).Variances due to additiveandnon-additiveeffectsweresignificantandindicated that all studied traits were under the control of an additivedominance model.The Wr(covariance values between theparents and their offspring in the rth array)on Vr(variance values of the rth array)coefficients of regression were not significant for flavonoids(-0.29),FIRA(-0.11)and DPPH-FRSA(0.49),but ABTS-FRSA showed a positive and significant regression coefficient(0.84).However,it is noteworthy that the additive(D)and environmental(E)variances were lower than the two components of the dominance variance(H1and H2).In addition,the values of average degree of dominance[(H1/D)1/2]above 1 showed overdominance and those of proportion of dominant genes[kd/(kd+kr)]confirmed the prevalence of dominance over additive.The positive sign of the term h indicated that most alleles were dominant for flavonoid levelsand FIRA but not for DPPH-FRSA and ABTS-FRSA.This trend is confirmed by the value of balance of positive and negative alleles(H2/4H1)below 0.25(the theoretical maximum),indicating that dominant and recessive alleles were unevenly distributed among the parents.Overall,the correlation coefficient between the degree of dominance of the parents(Wr+Vr)and the parental value(Pr),was positive and significant for DPPH-FRSA and ABTS-FRSA,but not for FIRA,which showed a positive and nonsignificant coefficient.Recessive alleles thus had a positive effect on DPPH-FRSA and ABTS-FRSA.For flavonoid content,a negative and nonsignificant correlation coefficient was observed;dominant alleles had a positive effect on this trait and a slightly positive effect on FIRA.All the traits studied were highly heritable(h2=0.90-0.99),with the variance due to genetic interactions greater than environmental variance.Low (0.12-0.45)narrow-sense heritability(hn2)values were observed,confirming the superiority of dominance over additivity.
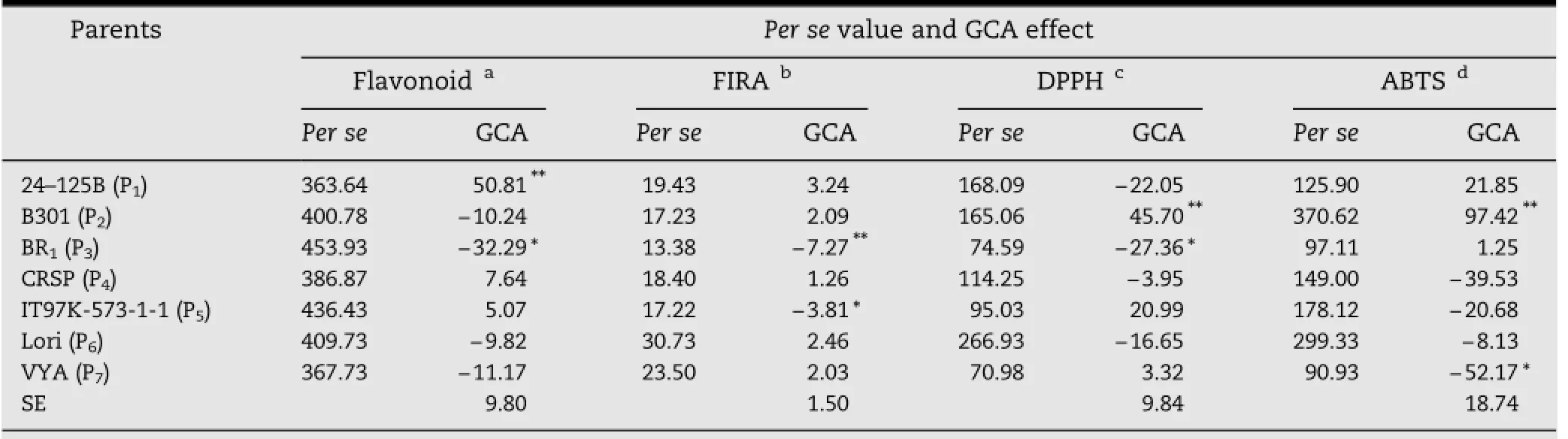
Table 2-Per se performance and general combining ability effects of parents for total flavonoid and antioxidant activity in cowpea.

Table 3-Mean values and estimates of specific combining ability effects of crosses for total flavonoid and antioxidant activity in cowpea.
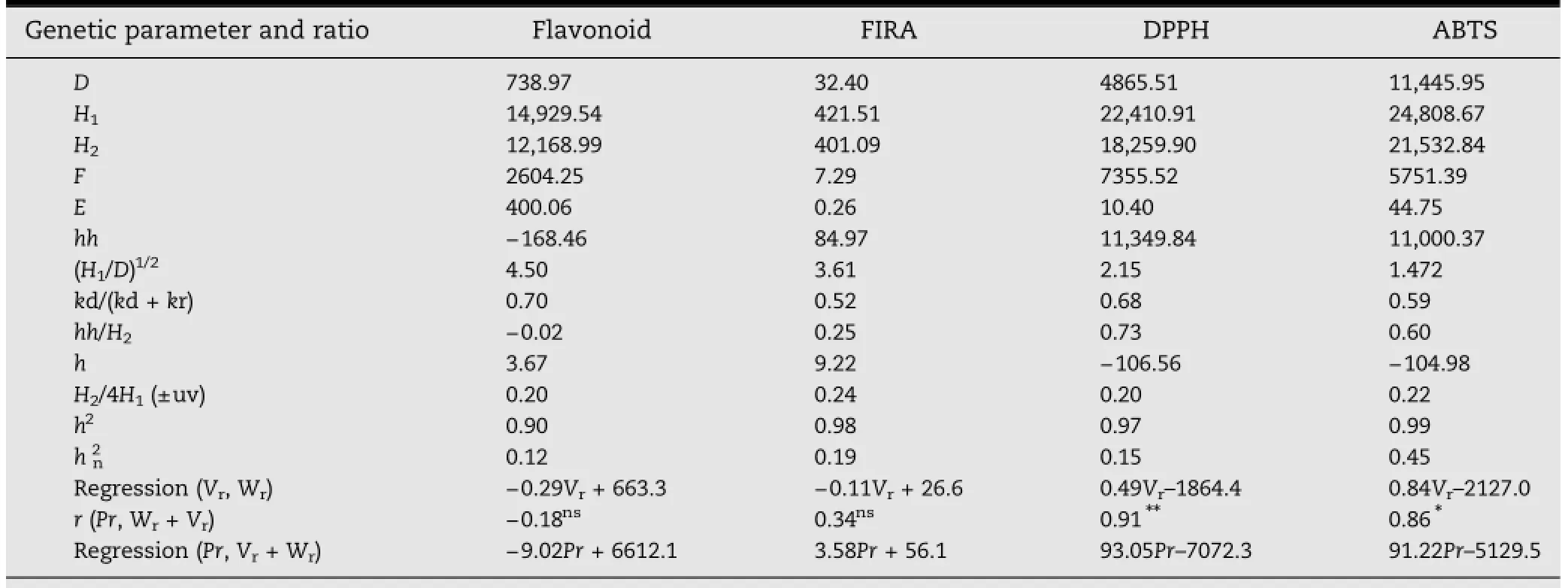
Table 4-Some genetic parameters and ratios obtained from a 7×7 half diallel in cowpea.
4.Discussion
The present results show broad genetic variability for flavonoid content and antioxidant potential in cowpea seeds.This variability can be exploited in selection for the development of new varietieswithhighnutritivevalue.Qualitativetestsperformedby Adeyemi and Olorunsanya[15]showed that three offour cowpea varieties tested contained flavonoids.According to Salawu et al.[18],the total flavonoid content in cowpea ranged from 0.95 to 0.36 mg quercetin equivalents g-1.However,a high flavonoid content(12,226 μg g-1DW)was observed in cowpea flour for which the major flavonoids subclasses were flavonols and flavan-3-ols[17].Recent studies by El-Mergawi and Taie[34]and Fouad and Rehab[35]in faba bean(Vicia faba L.)and lentil(Lens culinarisMedik.)gaveapproximatelysimilarflavonoidcontentsto those obtained in the present study.Several other studies of faba beansaswellasAfricanyambean(SphenostylisstenocarpaHochst. Ex A.Rich),Acacia species,chickpea(Cicer arietinum L.),and lupine(Lupinus albus L.)[8,36-39]revealed low values for total flavonoid content.By contrast,a high flavonoid content(20.9±0.8 mg catechin equivalent in 1 g extract)was obtained for pigeon pea(Cajanus cajan L.)in a methanolic extract of seed coat and whole seed extract[40].Despite using the same standard,like catechin,rutin,quercetin,kaempférol,etc.,we observed a large variation in results.This variation may have been due to the measuring methods used,processing applied,storage conditions and duration,quality of the standard used,and genetic factors[41].
Cultivars with darker seed coat show higher total flavonoid content than white cultivars in two species[18,37].Positive relationships between dark hull color and antioxidant activity in legume seeds have been reported[42,43].Whole seeds of pigeon pea showed higher antioxidant properties than cooked whole pod[40].The antioxidants may be located mainly in the hull or seed coat and be removed by leaching.In general,antioxidant activity of an extract cannot be predicted on the basis only of its total phenolic content[24].
Antiradical activity is determined by two methods,the first generally applied to cereals using the DPPH radical and the second,using the ABTS radical,applied for simple compounds and complex mixtures[44,45].The ABTS radical used is a nonphysiological radical source not found in mammals[46]. Probably for this reason,Ba et al.[47]observed that values obtained by ABTS were greater than those found by DPPH.All cowpea lines used in this study showed high antioxidant activity according to DPPH,ABTS,and ferric reducing power assays.Allgenotypesexcept24-125BandVYAshowedvaluesof ABTS-FRSA greater than those for DPPH-FRSA,in agreementwiththefindingsofBaetal.[47].Highbiologicalandantioxidant activities have been found previously in cowpea seeds and other legume species[8,17,36,39,43].
In the present study,the traits studied were controlled by both additive and non-additive gene effects with a preponderance of non-additive gene effects.The traits studied were thus controlled mainly by dominant genes and were not strongly affectedbyenvironment.Indeed,highdominancevariance,low narrow-sense heritability,and an average degree of dominance greater than unity were estimated.The same conclusion was reported by Karmakar et al.[48]for antioxidants in fresh fruits of ridge gourd(Luffa acutangula Roxb.).Hence,attention must be focused on hybrid breeding to produce flavonoid-and antioxidant-rich genotypes of cowpea seeds for flavonoids and FIRA,but not for DPPH-FRSA and ABTS-FRSA,where judicious selectionofsuperior parents would be effective.The superiority of hybrids over parents may have been due to the presence of heterozygouslociinthe hybrids,leading toheterosis[48].These traits seemed to be controlled by partial dominance.In this case,theselection of elite parents would be anefficient method for breeding varieties rich in these two antioxidant activities. The negative values for DPPH-FRSA and ABTS-FRSA were in agreement with the findings of Karmakar et al.[48]for antioxidant potential,indicating a prevalence of recessive alleles for these traits in parents.The average degree of dominance greater than unity for all of the studied traits revealed the presence of overdominance in the genetic control of traits.Karmakar et al.[48]identified 2-3 groups of genes that controlled the traits and exhibited dominance for DPPH-and ABTSradical scavenging activity.Ourstudyindicated aboutone group of genes,perhaps because of the prevalence of epistasis for these traits.Moreover,the additive-dominant model was not established in this study,given the nonsignificant regression coefficients(Vr,Wr)for flavonoids(-0.29),FIRA(-0.11),and DPPH-FRSA(0.49).Epistasis could be involved in the genetic control of these traits.Only ABTS-FRSA obeyed the additivedominant model without epistasis.Additivity,dominance,and epistasis have been reported to be involved in the genetic control of phenolics and antioxidant activity[10,14].All traits studied were highly heritable,but narrow-sense heritability values lower than 50%showed once again that non-additive gene action played a major role in the inheritance of flavonoids and antioxidant activities.
From the present study,the prevalence of non-additive gene action suggests the adoption of a hybrid breeding strategy.According to Nzaramba et al.[12],breeding for high antioxidant activity in cowpea is achievable by use of dark-colored genotypes.In fact,these authors established a positive correlation between testa color and antioxidant activity.
5.Conclusions
Wide genetic variability was found for total flavonoid content and antioxidant activity in cowpea seeds.These traits were highly heritable and controlled mainly by non-additive gene effects.Recessivealleles exerted a positiveeffectonDPPH-FRSA and ABTS-FRSA and a negative effect on flavonoids and FIRA. Thus,recurrent selection schemes will be a relevant breeding strategy for the improvement of the antioxidant potential of cowpea seed in the Sudano-Sahelian zone.
Acknowledgments
This work was supported in part by funds from the International Foundation for Science(IFS)(agreement number C/5262-1).The authors are grateful to Ukai Yasuo of the University of Tokyo(Japan)forprovidingthecomputerprogramDIAL98.Theyarealso thankful to the Institute of Agricultural Research for Development(IRAD)of Maroua-Cameroon for kindly providing the seed samples and the field for experiments.
R E F E R E N C E S
[1]E.A.Hall,N.Cissé,S.Thiaw,H.O.A.Elawad,J.D.Ehlers,A.M. Ismail,R.L.Fery,P.A.Roberts,L.W.Kitch,L.L.Murdock,O. Boukar, R.D. Phillips, K.H. Mc Watters, Development of cowpea cultivars and germplasm by the bean/cowpea CRSP,Field Crops Res. 82 (2003) 103-134.
[2]I.M.Vasconcelos,F.M.M.Maia,D.F.Farias,C.C.Campello,A.F.O.Carvalho,R.A.Moreira,J.T.Abreu de Oliveira,Protein fractions,amino acid composition and antinutritional constituents of high-yielding cowpea cultivars,J.Food Compos.Anal.23(2010)54-60.
[3]Y.N.Sreerama,V.B.Sashikala,V.M.Pratape,V.Singh,Nutrients and antinutrients in cowpea and horse gram flours in comparison to chickpea flour:evaluation of their flour functionality,Food Chem.131(2012)462-468.
[4]C.M.F.Mbofung,Y.N.Njintang,W.K.Waldrom,Functional properties of cowpea-soy-dry red beans composite flour paste and sensorial characteristics of akara(deep fat fried food):effect of whipping conditions,pH,temperature and salt concentration,J.Food Eng.54(2002)207-214.
[5]K.G.Kaptso,N.Y.Njintang,J.D.Hounhouigan,J.Scher,C.M.F. Mbofung,Production of Bambara groundnut(Voandzeia subterranea)flour for use in the preparation of koki(a steamed cooked paste):effect of pH and salt concentration on the physicochemical properties of flour,Int.J.Food Eng.3(2007),Article 5.
[6]Y.Mensa-Wilmot,R.D.Phillips,J.L.Hargrove,Protein quality evaluation of cowpea-based extrusion cooked cereal/legume weaning mixtures,Nutr.Res.21(2001)849-857.
[7]M.H.Dicko,H.Gruppen,C.Barro,W.J.H.van Berkel,A.G.J. Voragen,Impact of phenolics and related enzymes in sorghum varieties for the resistance and susceptibility to biotic and abiotic stresses,J.Chem.Ecol.31(2005)2671-2688.
[8]V.N.Enujiugha,J.Y.Talabi,S.A.Malomo,A.I.Olagunju,DPPH radical scavenging capacity of phenolic extracts from African yam bean(Sphenostylis stenocarpa),Food Nutr.Sci.3(2012)7-13.
[9]J.M.Awika,L.W.Rooney,X.Wu,R.L.Prior,L.Cisneros-Zevalos,Screeningmethodstomeasureantioxidantactivityofsorghum(Sorghum bicolor)and sorghum products,J.Agric.FoodChem.50(2003)6657-6662.
[10]D.Prochazkova,I.Bouova,N.Wilhelmova,Antioxidant and prooxidant properties of flavonoids,Fitoterapia 82(2011)513-523.
[11]P.Kumar,S.Kumar,M.K.Tripathi,N.Mehta,R.Ranjan,Z.F. Bhat,P.K.Singh,Flavonoids in the development of functional meat products:a review,Vet.World 6(2013)573-578.
[12]M.N.Nzaramba,A.L.Hale,D.C.Schewing,J.C.Miller,Inheritance of antioxidant activity and its association withseed coat colour in cowpea,J.Am.Soc.Hortic.Sci.130(2005)386-391.
[13]R.Doblado,J.Frias,C.Vidal-Valverde,Changes in vitamin C content and antioxidant capacity of raw and germinated cowpea(Vigna sinensis var.carilla)seeds induced by high pressure treatment,Food Chem.101(2007)918-923.
[14]E.N.Herken,S.Ibanoglu,M.D.Oner,N.Bilgicli,S.Guzel,Effect of storage on the phytic acid content,total antioxidant capacity and organoleptic properties of macaroni enriched with cowpea flour,J.Food Eng.78(2007)366-372.
[15]K.D.Adeyemi,A.O.Olorunsanya,Comparative analysis of phenolic composition and antioxidant effect of seed coat extracts of four cowpea(Vigna unguiculata)varieties on broiler meat,2(2012)343-349.
[16]J.B.T.Noubissié,E.Youmbi,N.Y.Njintang,M.Aladji Abatchoua,R.M.Nguimbou,J.M.Bell,Inheritance of phenolic contents and antioxidant capacity of dehulled seeds in cowpea(Vigna unguiculata L.Walp.),Int.J.Agron.Agric.Res.2(2012)7-18.
[17]F.Apea-Bah,A.Minnaar,M.J.Bester,K.G.Duodu,Does a sorghum-cowpea composite porridge hold promise for contributing to alleviating oxidative stress?Food Chem.157(2014)157-166.
[18]O.S.Salawu,I.E.Oluwafemi,D.Oladipupo,B.O.S.Bukola,Effect of Callosobruchus maculatus infestation on the nutrient-antinutrient composition,phenolic composition and antioxidant activities of some varieties of cowpeas(Vigna unguiculata),Adv.J.Food Sci.Technol.6(2014)322-332.
[19]R.Kahl,H.Kappus,Toxicology of synthetic antioxidants BHA and BHT in comparison with natural antioxidant vitamin C,Z.Lebensm.-Unters.Forsch.196(1993)329-338(in German with English abstract).
[20]S.S.Gray,E.A.Gomnae,D.J.Buckley,Oxidative quality and shelf life of meat,Meat Sci.42(1996)127-132.
[21]K.Mather,J.L.Jinks,Biometrical Genetics:The Study of Continuous Variation,third ed.Chapman and Hall,London,New York,1982.
[22]J.B.T.Noubissié,J.M.Bell,S.G.Birwe,S.Gonne,E.Youmbi,Varietal response of cowpea(Vigna unguiculata(L.)Walp.)to Striga gesnerioides(Willd.)Vatke race SG5 infestation,Not.Bot. Hort.Agrobot.Cluj 38(2010)33-41.
[23]R.D.Phillips,M.S.Chinnan,A.L.Branch,J.Miler,K.H. McWatters,Effects of treatment on functional and nutritional properties of cowpea meal,J.Food Sci.53(1988)805-809.
[24]A.Abdou Bouba,Y.N.Njintang,J.Scher,C.M.F.Mbofung,Phenolic compounds and radical scavenging potential of twenty Cameroonian spices,Agric.Biol.J.N.Am.1(2010)213-224.
[25]G.D.Noudeh,F.Sharififar,M.Khatib,E.Behravan,M.A. Afzadi,Study of aqueous extract of three medicinal plants on cell membrane-permeabilizing and their surface properties,Afr.J.Biotechnol.9(2010)110-116.
[26]T.Miyase,H.Kohsaka,A.Ueno,Tragopogonosids A-I,oleanane saponins from Tragopogon pratensis,Phytochemistry 31(1992)2087-2091.
[27]M.Oyaizu,Studies on products of browning reaction: antioxidative activity of products of browning reaction,Jpn.J. Nutr.40(1986)307-315.
[28]D.Zhang,Y.Hamauzu,Phenolics,ascorbic acid,carotenoids and antioxidant activity of broccoli and their changes during conventional and microwave cooking,Food Chem.88(2004)503-509.
[29]R.Re,N.Pellegrini,A.Proteggente,A.Pannala,M.Yang,C.A. Rice-Evans,Antioxidant activity applying an improved ABTS radical cation decolorization assay,Free Radic.Biol.Med.26(1999)1231-1237.
[30]Statgraphics,Statgraphics plus for Windows 3.0,MD,Manugistics Inc.,Rockville,1997.
[31]Y.Ukai,A microcomputer program DIALL for diallel analysis of quantitative characters,Jpn.J.Breed.39(1989)107-109.
[32]B.Griffing,A generalized treatment of the use of diallel crosses in quantitative inheritance,Heredity 30(1956)31-51.
[33]B.I.Hayman,The theory and analysis of diallel crosses,Genetics 39(1954)789-809.
[34]R.El-Mergawi,H.A.A.Taie,Phenolic composition and antioxidant activity of raw seeds,green seeds and sprouts of ten faba bean(Vicia faba)cultivars consumed in Egypt,Int J Pharm.Bio.Sci 5(B)(2014)609-617.
[35]A.A.Fouad,F.M.A.Rehab,Effect of germination time on proximate analysis,bioactive compounds and antioxidant activity of lentil(Lens culinaris Medik.)sprouts,Acta Sci.Pol. Technol.Aliment.14(2015)233-246.
[36]H.Hannachi,W.Elfalleh,I.Ennajeh,M.Laajel,M.L.Khouja,A. Ferchichi,N.Nasri,Chemicals profiling and antioxidants activities of Acacia seeds,J.Med.Plants Res.5(2011)6869-6875.
[37]S.Shruti,Y.Neelam,S.Alka,K.Rajendra,Antioxidant activity,nuetraceutical profile and health relevant functionality of nine newly developed chickpea cultivars(Cicer arietinum L.),Int.J.Nat.Prod.Res.3(2013)44-53.
[38]W.I.M.Aniess,A.F.Khalil,Z.M.Mosa,Phenolic compounds and antioxidants capacity of sweet lupine derivatives-wheat flour mixtures and the effects on diabetic rats,IOSR J.Environ Sci.Toxicol.Food Technol.9(2015)61-69.
[39]M.A.Khan,M.H.Ammar,H.M.Migdadi,E.H.El-Harty,M.A. Osman,M.Farooq,S.S.Alghamdi,Comparative nutritional profiles of various faba bean and chickpea genotypes,Int.J. Agric.Biol.17(2015)449-457.
[40]S.Rani,G.Poswal,R.Yadav,M.K.Deen,Screening of pigeonpea(Cajanus cajan L.)seeds for study of their flavonoids,total phenolic content and antioxidant properties,Int.J.Pharm.Sci.Rev.Res.28(2)(2014)90-94.
[41]S.Fiorucci,Activités biologiques de composés de la famille de flavonoïdes:approches par des méthodes de chimie quantique et de dynamique moléculaire,Thèse de doctorat,Nice 2006(in French).
[42]B.D.Oomah,F.Caspar,L.J.Malcolmson,A.S.Bellido,Phenolics and antioxidant activity of lentil and pea hulls,Food Res.Int.44(2011)436-441.
[43]P.Polthum,A.Ahromrit,GABA content and antioxidant activity of Thai waxy corn seeds germinated by hypoxia method,Songklanakarin J.Sci.Technol.36(2014)309-316.
[44]L.L.Yu,K.Q.Zhou,Antioxidant properties of bran extracts from‘Platte'wheat grown at different location,Food Chem. 90(2004)311-316.
[45]K.Q.Zhou,J.J.Laux,L.L.Yu,Comparison of Swiss red wheat grain and fractions of their antioxidant properties,J.Agric. Food Chem.52(2004)1118-1123.
[46]R.L.Prior,X.L.Wu,K.Schaich,Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements,J.Agric.Food Chem.53(2005)4290-4302.
[47]K.Ba,E.Tine,J.Destain,N.Cissé,P.Thonart,Étude comparative des composés phénoliques,du pouvoir antioxydant de différentes variétés de sorgho sénégalais et des enzymes amylolytiques de leur malt,Biotechnol.Agron. Soc.Environ.14(2010)131-139(in French with English abstract).
[48]P.Karmakar,A.D.Munshi,T.K.Behera,R.Kumar,A.K.Sureja,C.Kaur,B.K.Singh,Quantification and inheritance of antioxidant properties and mineral content in ridge gourd(Luffa acutangula),Agric.Res.2(2013)222-228.
2 December 2015
in revised form 15 May 2016 Accepted 20 June 2016
.Tel.:+237 674 142 748.
E-mail address:mainantoine@gmail.com(M.A.Nassourou).
Peer review under responsibility of Crop Science Society of China and Institute of Crop Science,CAAS.
http://dx.doi.org/10.1016/j.cj.2016.05.011
2214-5141/©2016 Crop Science Society of China and Institute of Crop Science,CAAS.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
杂志排行
The Crop Journal的其它文章
- Editorial:Food Legume Diversity and Legume Research Policies
- QTL and candidate genes associated with common bacterial blight resistance in the common bean cultivar Longyundou 5 from China
- Two major er1 alleles confer powdery mildew resistance in three pea cultivars bred in Yunnan Province,China
- Construction of an integrated map and location of a bruchid resistance gene in mung bean
- Evaluation of common bean(Phaseolus vulgaris L.)genotypes for drought stress adaptation in Ethiopia
- Large-scale evaluation of pea(Pisum sativum L.)germplasm for cold tolerance in the field during winter in Qingdao
