Large-scale evaluation of pea(Pisum sativum L.)germplasm for cold tolerance in the field during winter in Qingdao
2016-10-24XioynZhngShuweiWnJunjieHoJinguoHuToYngXuxioZong
Xioyn Zhng,Shuwei Wn,Junjie Ho,Jinguo Hu,To Yng,Xuxio Zong,*
aQingdao Academy of Agricultural Sciences,Qingdao 266100,China
bNational Key Facility for Crop Gene Resources and Genetic Improvement/Institute of Crop Science,Chinese Academy of Agricultural Sciences,China
cUSDA,Agricultural Research Service,Western Regional Plant Introduction Station,Washington State University,Pullman,WA 99164,USA
Large-scale evaluation of pea(Pisum sativum L.)germplasm for cold tolerance in the field during winter in Qingdao
Xiaoyan Zhanga,1,Shuwei Wana,1,Junjie Haoa,Jinguo Huc,Tao Yangb,Xuxiao Zongb,*
aQingdao Academy of Agricultural Sciences,Qingdao 266100,China
bNational Key Facility for Crop Gene Resources and Genetic Improvement/Institute of Crop Science,Chinese Academy of Agricultural Sciences,China
cUSDA,Agricultural Research Service,Western Regional Plant Introduction Station,Washington State University,Pullman,WA 99164,USA
A R T I C L E I N F O
Article history:
Available online 28 July 2016
Pisum sativum L.
Cold tolerance
Germplasm evaluation
Open-field experiment
As a cool-season crop,pea(Pisum sativum L.)can tolerate frost at the vegetative stage but experiences yield loss when freezing stress occurs at the reproductive stage.Cold-tolerance improvement of pea varieties is important for stable yield and expansion of the winter pea planting area.Under natural low-temperature conditions during winter in Qingdao,Shandong,China,we evaluated the cold tolerance of 3672 pea germplasm accessions in the field and categorized them as displaying high resistance(214),moderate resistance(835),or susceptibility(2623).The highly and moderately resistant genotypes were validated in the following year.We found that genotypes from the winter production region showed higher cold tolerance than genotypes from the spring production region.The accessions identified as having high levels of cold tolerance are recommended as potential genetic resources in cold-tolerance breeding of pea.
©2016 Crop Science Society of China and Institute of Crop Science,CAAS.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
Pea(Pisum sativum L.)is one of the most important legume crops in temperate climates and is classified by end use into field and green pea.Field pea is grown for harvest of dry seeds as food or as forage and garden pea is grown for its immature seeds or tender pods as vegetables.In some countries,such as China,young shoots of pea seedlings are also cooked by several methods for popular consumption.
According to statistics of the Food and Agriculture Organization(FAO)[1],97 and 85 countries produced dry and green pea,respectively,in 2012 and the annual production exceeds 28 million tons.The Russian Federation(1,160,200 ha),Canada(1,475,000 ha),China(905,000 ha),India(735,000 ha),and Australia(248,900 ha)are the largest dry pea-producing countries.Total pea production worldwide has steadily increased since 1993,owing to increasing green pea volume,and the top fiveproducingcountriesareChina(1,300,915 ha),India(380,000 ha),the United States(77,090 ha),the United Kingdom(34,553 ha),andAlgeria(34,110 ha).Asthelargestgreen pea and second largest dry pea producer in the world,China has a history of more than 2000 years in pea cultivation[2].Atpresent,almost all pea products are consumed domestically,in contrast to their use by other leading pea exporters such as Canada.With the increase of consumer demand and land resource limitation in China,improvement by breeding has received great attention in recent years.
Field pea has adapted to a wide range of climates and altitudes.It is commonly recognized as consisting of spring,Mediterranean,and winter types[3].Both winter and spring types are grown in many countries including China.Winter pea has higher yield potential than spring pea owing to its longer growth period,higher efficiency of radiation use in early spring,and escape from drought stress at harvest stage[3,4].Pea is widely planted in 29 provinces or autonomous regions of China with complex ecological conditions.Winter pea is conventionally sown in autumn in the area south of 33° north latitude.In recent years,the northern boundary of winter pea has been moved northward in China to achieve yield increases by enlargement of the winter pea region. However,severe cold weather has affected the historical pea-producing area in recent years,leading to great yield fluctuation.Thus,cold-tolerant varieties are very important in the safe production of winter pea.
As of the end of 2015,more than 6200 pea accessions collected from 40 countries or regions have been preserved in the National Crop Genebank of China(Beijing).Part of these genetic resources was recently genotyped with DNA markers to evaluate the genetic diversity and relationships in the Chinese pea collection[5].However,phenotyping data for Chinese pea germplasm resources are incomplete,in particular for evaluation of cold tolerance.Phenotypic evaluation is the first step in identifying potential resources in breeding programs.In this study,we performed a large-scale screening experiment,evaluating the cold tolerance of 3677 pea accessions in the field in Qingdao,Shandong province.The tolerant varieties were validated in the second year.The consistent varieties are primarily recommended for use in the genetic improvement of winter pea to cold stress.
2.Materials and methods
2.1.Plant materials
A total of 3677 accessions of field pea were provided by the National Crop Genebank of China,located in the Institute of Crop Science,Chinese Academy of Agricultural Sciences,Beijing.Most of them arelandraces collected from 27 provinces of China.Five of them failed to germinate,so that 3672 accessions were evaluated for cold tolerance.Among the 2317 accessions originating in China,906 and 1411 accessions were of the winter and spring phenological groups,respectively.A total of 402 accessions were introductions from foreign countries.The remaining 953 accessions were included in an“unknown”group owing to incomplete passport data(Table 1).
2.2.Experimental design and trial management
The two-year experiment was performed in an field of the research farm of Qingdao Academy of Agricultural Sciences, Qingdao,Shandong,China(36°09′8.05″N,120°25′15.77″E,and 15 m above sea level)on October 20,2009.The previous crop was Chinese cabbage(Brassica pekinensis L.).Only the genotypessurvivinginthe2009-2010growing seasonwere evaluated again in the same field in the 2010-2011 growing season.

Table 1-Cold tolerance of pea accessions evaluated in the field experiment in the 2009-2010 growing season.
Field layout and agronomic practice were standard in both years.No irrigation water was supplied because of sufficient rainfall during the growing period in both years.Abamectin at 1.8%EC(5000×)was sprayed at seedling(April 18),initial bloom(April 25),and full-bloom(May 1)stage to control pea leaf miner(Phytomyza horticola Gourean).Weeds were removed by hand.
The 3677 pea accessions were planted by hand in a completely randomized design without replication.Eachgenotype was planted in a plot 1 m in length and 0.5 m in width,containing a single row with 25 plants.
2.3.Temperatures during experiment
Neighboring the Yellow Sea,Qingdao has a four-season,monsoon-influenced,temperate climate.January is the coldest month in the year,with an average temperature of 0.9°C.Daily temperatures during the experiment(Fig.1)were downloaded from the website of the Qingdao Meteorological Administration(http://qdqx.qingdao.gov.cn/).Temperatures in the 2009/2010 and 2010/2011 winter were lower than those in normal years in Qingdao.For example,the lowest temperatures were-13°C on January 12,2010 and-10°C on January 15-16,2011,and 40 and 66 days showed temperatures lower than 0°C in the 2009/2010 and 2010/2011 winters,respectively.These were ideal conditions for differentiating winter-hardy from non-winter-hardy accessions.
2.4.Indicators of cold tolerance
Seedlings of each genotype were counted 50 days after planting for calculation of germination percentage.A second count was performed on January 19,2010 when the extreme cold event ended.
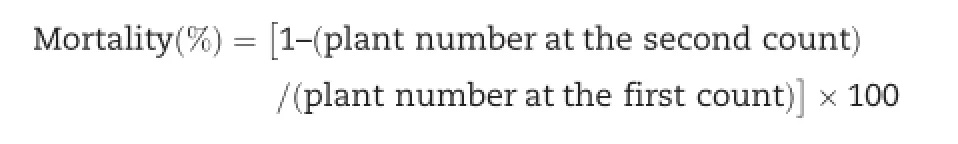
Cold tolerance of genotypes was assessed visually at three levels.In a highly resistant(HR)genotype the shoots and leaflets remained green without damage and seeds could be harvested;in a moderately resistant(MR)genotype,some shoots and leaflets were killed,but the roots were alive and generated new branches in spring,and seeds could be harvested;in a susceptible(S)genotype,the shoots and roots were completely killed with no seed harvest.
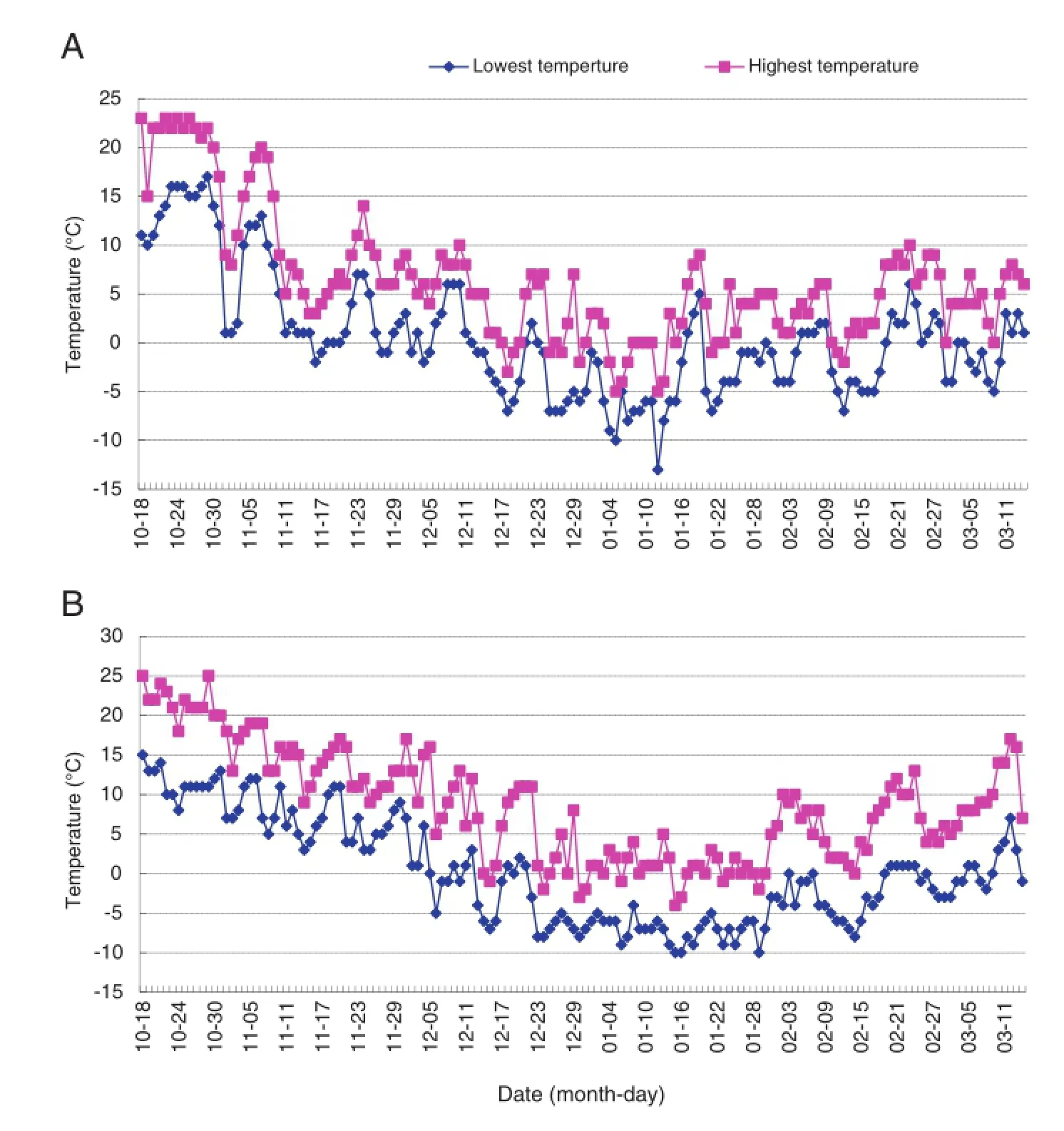
Fig.1-Daily temperatures in Qingdao,Shandong during the experiment.A:2009-2010 growing season;B:2010-2011 growing season.Data source:Qingdao Meteorological Administration(http://qdqx.qingdao.gov.cn/).
3.Results
3.1.Germination percentage of seeds planted in the field
The distribution of germination percentage of the 3677 accessions is shown in Fig.2.Great variation in germination rate was observed,ranging from 0 to 100.0%with an averageof 71.2%.Among the accessions,3230(87.84%)showed more than 50%germination or seedling development,442(12.02%)showed low germination percentage(<50%),and five(0.14%)showed zero germination(Fig.2).This result indicated that the pea seeds preserved in the National Crop Genebank of China were vigorous and suitable for the study.
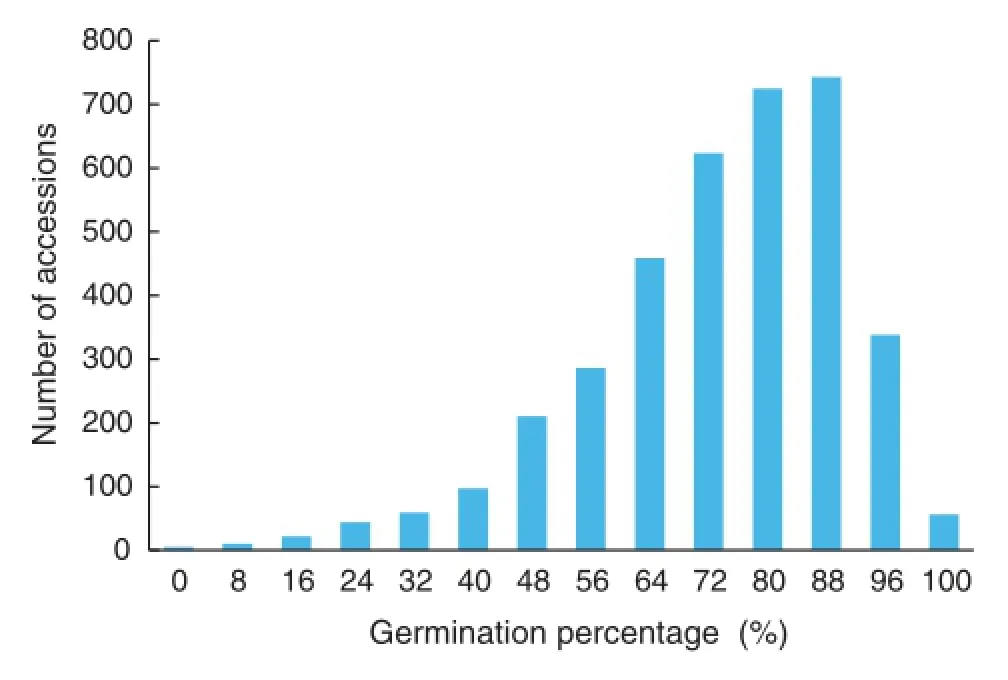
Fig.2-Distribution of germination percentage in the 3677 accessions 50 days after planting.
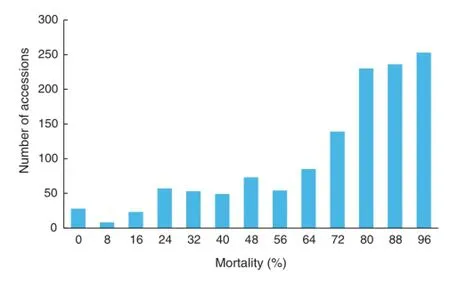
Fig.4-Mortality distribution among the 1278 accessions.Of 3672 accessions in the field,2394 were completely killed by the cold.
3.2.Mortality calculated based on plant stand count after the cold period
The cold weather in winter 2009/2010 resulted in partial plant death in the field.The field scenes on December 10,2009 and January 19,2010 differed markedly(Fig.3).On January 19,2010,2394 accessions showed no surviving plants in the field and mortalities were as high as 100%.In contrast,28 accessions showed zero mortality.The remaining 1250 accessions showed mortality ranging from 2%to 96%(Fig.4).The mean mortality was 88.4%,implying that the cold stress was sufficiently strong for selection.

Fig.3-Overview of the experimental plots.A:Field scene on December 10,2009;B:field scene on January 19,2010.
3.3.Scale of cold tolerance based on phenotype
Mortality showed a typical continuous distribution(Fig.4),leaving it difficult to define criteria for separating germplasm groups.We suggest using quality traits for cold-tolerance scoring,because there were two distinct kinds of surviving plants:green,healthy and intact plants or plants surviving but appearing yellow or brown.Accordingly,we defined these two groups as highly resistant(HR)and moderately resistant(MR)genotypes,respectively,and completely dead genotypes as susceptible(S).Fig.5 shows a representative plot in each group.Given the presence of different kinds of plants within many plots(genotypes),the presence of healthy green plants on January 19,2010 was the criterion for assigning the accession as HR.In some extreme cases,when 60%of the plants in the plot were dead but the remaining 40%were green and intact,the accessions were still placed in the HR group.
As a result,the 3672 accessions were assigned to three classes,with 214,835,and 2623 accessions assigned to the HR,MR,and S classes,respectively.There was high variation in mortality percentage within each class:in the HR class,mortality ranged from 0 to 60%and in the MR class,0 to nearly 100%.In the S class,100%of the plants appeared dead on January 19,2010.However,one to several plants did not die completely and shoots grew from underground upon warming. Thus,434 of the 2623(16.5%)S accessions produced one to seven plants later in the season.

Fig.5-Scoring cold tolerance of pea by phenotype after cold stress in the filed(January 19,2010).A:susceptible phenotype,showing death of all seedlings after cold stress;B:moderately resistant phenotype,showing recovery growth of seedlings after cold stress;C:highly resistant phenotype,showing seedlings with normal growth.
3.4.Cold-resistance scores and phenological groups of the accessions
Table 1 presents a summary of the evaluation results,the numbers of accessions in each of the cold-tolerance classes,and information for the regions where the accessions were collected,Assuming that the accessions collected from the winter growing zone are in the winter phenological group and those from the spring growing zone are in the spring phenological group,it is apparent that the frequency(11.3%,102/906)of HR accessions in the winter group is higher than that(3.1%,44/1411)in the spring group.The same trend is seen for the frequency of MR accessions(35.1%in the winter and 17.4%in the spring group).The frequency of S accessions(79.5%,1122/1411)in the spring group is much higher than that(53.6%,486/906)in the winter group.In addition to the winter and spring groups,there were 953 accessions of unknown origin and 402 accessions that were acquired from foreign countries.The phenological groups of these accessions could not be determined.The percentages of HR,MR,and S accessions in this unknown group were approximately 7.0%,25.9%,and 67.1%,respectively.
3.5.Validation of cold-tolerant accessions
In winter 2009/2010,214 and 835 accessions were identified as respectively HR and MR to cold stress.These genotypes were planted in a replicated field test in the 2010/2011 winter.The 214 HR accessions segregated as 66(~30.8%)HR,125(~58.4%)MR,and 23(~10.8%)S and the 835 MR accessions segregated as 188(~22.5%)HR,410(~49.1%)MR,and 237(~28.4%)S.Most accessions showed similar resistance categories across years,indicating that the evaluation method used is effective for mass selection of pea.
4.Discussion
4.1.Cold tolerance of cool-season pulses
Pea is among the most important cool-season pulse crops,which also include chickpea(Cicer arietinum L.),lentil(Lens culinaris Medikus)and faba bean(Vicia faba L.),that have a wide geographical distribution.When these crops are planted in the autumn,they face several environmental challenges including low-temperature stress.Classified into chilling(0-15°C)and freezing(<0°C)stresses,low-temperature stress is a major environmental factor limiting the growth,productivity,and geographical distribution of crops[6]and has been extensively studied.Methods have been reported for cold-stress screening in many crops[7-9].There are numerous publications describing the cold tolerance of chickpea[10,11],faba bean[12-16],and lentil[17-21].A quantitative method was developed to assess the freezing resistance of faba bean using artificial freezing stress with limited numbers of plants[22].Inci and Toker[15]evaluated 114 faba bean accessionsfor cold tolerance ona scale of 1 to 5 at seedling stage and identified promising lines to be growninatargetproductionarea.Physiological,morphological,physio-biochemical and proteome changes during short-term cold stress were characterized in chickpea[10,23].
Pea genetic resources have been extensively evaluated for coldtoleranceinthefieldandbylaboratorymethods[24]aswell as by the emerging automated,integrative high-throughput phenotyping approach[25].In the United States.,14 lines of P. sativum and four lines of P.sativum var.arvense were identified by Auld et al.[26]as showing good winter survival at Moscow,Idaho.In Europe,as summarized by Cousin[27],most pea cultivarsareverysusceptibletocoldandtheperformanceofthe pea crop isconsiderably affectedby cold.Only a fewFrenchand Austrian lines from wild Pisum species or forage winter pea,probably derived from P.arvense,are cold-resistant.Resistance to cold in peas is a quantitatively inherited trait,and it is possible to conduct recurrent selection using crosses between wild accessions and winter or spring cultivated types[28].More recently,French scientists have reported the identification of twoclusters ofquantitativetraitloci(QTL)onlinkagegroup(LG)III and one cluster on LG VI for frost tolerance in winter pea using a recombinant inbred line(RIL)population derived from acrossbetweena Frenchspringgarden peacultivar,Caméor,and anewlyidentifiedsourceoffrostresistanceoriginatinginChina[29].
4.2.A simplified cold-tolerance scoring method for germplasm evaluation
Differentcoldtolerancescoringmethodsareuseddependingon experimental goals.Auld[26]used only survival percentage in the field for identifying cold-tolerant cultivars and germplasm,and Klein used a scale of 0 to 5(with 0 for no damage,5 for dead plants,and1-4forvariousdegreesoffrostdamage)inratingthe cold tolerance in a segregating RIL population for QTL detection[29].Weusedascoringmethodforevaluatingthecoldtolerance of our pea germplasm.The method not only discriminated cold-tolerant and cold-sensitive genotypes,but also discriminated superior cold-tolerant(HR,surviving without damage or injury)from moderately cold-tolerant genotypes(MR,surviving with damage or injury).The scoring results for the accessions were in partial concordance with the phenological group information.
The pea is considered to be a strictly self-pollinated(cleistogamous)species and each pea accession is expected to consist of genetically uniform plants,forming a pure line[27]. However,we observed segregation in a small proportion of the accessions under evaluation.For cold tolerance,there were plantsinsomeaccessionsthatcouldbeassignedtotwoorthree phenotypic classes.In addition,a few accessions segregated for morphological traits such as seed coat color or flower color. These segregating accessions must contain individuals with different genotypes.This intra-accession heterozygosity could result from 1)collection of these accessions as a mixture of genotypes,2)admixture of seeds from different accessions by human error during regeneration,or 3)occasional intercrosses between accessions mediated by natural pollinators such as leafcutter bees,which visit pea flowers.Considering this observation,we discourage the use of survival percentage as a measure of cold tolerance of an accession,given that it may underestimate the cold tolerance of accessions with mixed genotypes.
4.3.The significance of the presence of cold-tolerant accessions from the spring pea production region
Improving pea cold tolerance has been a breeding objective for many pea production regions.Plant breeders have always searched for cold-tolerant germplasm in historical winter pea production regions.Our result confirmed the appropriateness of this approach,with germplasm collected from the winter production region consisting of a higher percentage of HR accessions.It is interesting that we identified 44 HR accessions collected from the spring pea production region.It seems possible that these accessions were transferred from other regions by growers or breeders.It is also possible that these genotypes reflect natural variation that has occurred in populations.Further investigation is needed to identify the genetic basis of cold tolerance of accessions from different productionregions,usingmoderngenomicstools.Recombining genes from different accessions to enhance cold tolerance is possible if these accessions have different genetic bases.
5.Conclusions
With increasing consumer demand in China,pea planting tends to be shifting northward,leading to frequent cold stress in pea production.We have identified a large number of Chinese pea accessions with a high level of cold tolerance that are of potential use in pea improvement.Incorporation of genes for cold tolerance into pea cultivars would help expand the winter pea production area and increase productivity.It would also help stabilize crop yield by mitigating the loss caused by seasonal or episodic freezing in the established historical production regions.Research in developing and applying genetic and molecular genomics tools is needed to expedite the process of genetic improvement of the pea crop.
Acknowledgments
This study was supported by the China Agriculture Research System(No.CARS-09),the National Natural Science Foundation of China(No.31371695),the Qingdao Municipal Project for Science and Technology in Public Benefit(No.14-2-3-35-nsh),and the Shandong Elite Variety Project(No.2016LZ01-01-02).
R E F E R E N C E S
[1]FAOSTAT Databases,Food and Agriculture Organization of the United Nations 2012,Available at http://faostat.fao.org/ site/567/DesktopDefault.aspx?PageID=567#ancor.
[2]X.L.Sun,T.Yang,J.J.Hao,X.Y.Zhang,R.Ford,J.Y.Jiang,F. Wang,J.P.Guan,X.X.Zong,SSR genetic linkage map construction of pea(Pisum sativum L.)based on Chinese native varieties,Crop J.2(2014)170-174.
[3]F.L.Stoddard,C.Balko,W.Erskine,H.R.Khan,W.Link,A. Sarker,Screening techniques and sources of resistance to abiotic stresses in cool-season food legumes,Euphytica 147(2006)167-186.
[4]P.Urbatzka,R.Graß,T.Haase,C.Schüler,D.Trautz,J.Heß,Grain yield and quality characteristics of different genotypes of winter pea in comparison to spring pea for organic farming in pure and mixed stands,Org.Agric.1(2011)187-202.
[5]X.X.Zong,R.J.Redden,Q.C.Liu,J.P.Guan,Y.H.Xu,X.J.Liu,J. Gu,L.Yan,P.Ades,R.Ford,Analysis of a diverse global Pisum sp.collection and comparison to a Chinese local P.sativum collection with microsatellite markers,Theor.Appl.Genet. 118(2009)193-204.
[6]J.Zhu,C.H.Dong,J.K.Zhu,Interplay between cold-responsive gene regulation,metabolism and RNA processing during plant cold acclimation,Curr.Opin.Plant Biol.10(2007)290-295.
[7]N.Mantri,R.Ford,T.E.Coram,E.C.K.Pang,Evidence of unique and shared responses to major biotic and abiotic stresses in chickpea,Environ.Exp.Bot.69(2010)286-292.
[8]N.Mantri,E.C.K.Pang,R.Ford,Molecular Biology for Stress Management,in:S.Yadav,D.McNeil,R.Redden,S.Patil(Eds.),Climate Change And Management of Cool Season Grain Legume Crops,Springer,Science+Business Media,NY,USA 2010,pp.377-408.
[9]N.Mantri,V.Patade,S.Penna,R.Ford,E.C.K.Pang,Abiotic Stress Responses in Plants-Present and Future,in:P.Ahmad,M.N.V.Prasad(Eds.),Abiotic Stress Responses in Plants: Metabolism to Productivity,Springer New York,New York,USA 2012,pp.1-19.
[10]H.Leila,M.A.Reza,Physio-biochemical and proteome analysis of chickpea in early phases of cold stress,J.Plant Physiol.170(2013)459-469.
[11]K.S.Seyyedeh-Sanam,M.A.Reza,Z.Hassan,K.Mona,T. Alireza,R.Seyyedeh-Sanaz,Effect of short-term cold stress on oxidative damage and transcript accumulation of defense-related genes in chickpea seedlings,J.Plant Physiol. 171(2014)1106-1116.
[12]H.Herzog,Freezing resistance and performance of faba bean populations during winter seasons in Northern Germany,J. Agron.Crop Sci.162(1989)225-235.
[13]H.Herzog,Influence of pre-hardening duration and dehardening temperatures on varietal freezing resistance in faba beans(Vicia faba L.),Agronomie 9(1989)55-61.
[14]F.Sau,M.I.Minguez,Adaptation of indeterminate faba beans to weather and management under a Mediterranean climate,Field Crops Res.66(2000)81-99.
[15]N.E.Inci,C.Toker,Screening and selection of faba beans(Vicia faba L.)for cold tolerance and comparison to wild relatives,Genet.Resour.Crop.Evol.58(2011)1169-1175.
[16]W.Link,C.Balko,F.L.Stoddar,Winter hardiness in faba bean: physiology and breeding,Field Crops Res.115(2010)287-296.
[17]A.Ali,D.L.Johnson,Heritability estimates for winter hardiness in lentil under natural and controlled conditions,Plant Breed.119(2000)283-285.
[18]I.Eujayl,W.Erskine,M.Baum,E.Pehu,Inheritance and linkage analysis of frost injury in lentil,Crop Sci.39(1999)639-642.
[19]A.Hamdi,I.Kusmenoglu,W.Erskine,Sources of winter hardinessinwildlentil,Genet.Resour.Crop.Evol.43(1996)63-67.
[20]A.Kahraman,I.Kusmenoglu,N.Aydin,A.Aydogun,W. Erskine,F.J.Muehlbauer,Genetics of winter hardiness in 10 lentil recombinant inbred line populations,Crop Sci.44(2004)5-12.
[21]A.Kahraman,I.Kusmenoglu,N.Aydin,A.Aydogun,W. Erskine,F.J.Muehlbauer,QTL mapping of winter hardiness genes in lentil,Crop Sci.44(2004)13-22.
[22]H.Herzog,A quantitative method to assess freezing resistance in faba beans,J.Agron.Crop Sci.158(1987)195-204.
[23]H.Leila,M.A.Reza,M.R.Naghavi,Y.Farayedi,B. Sadeghzadeh,K.Alizadeh,Physiological and morphological characteristics of chickpea accessions under low temperature stress,Russ.J.Plant Physiol.58(2011)157-163.
[24]J.Wery,S.N.Silim,E.J.Knights,R.S.Malhotra,R.Cousin,Screening techniques and sources of tolerance to extremes of moisture and air temperature in cool season food legumes,Euphytica 73(1993)73-83.
[25]J.F.Humplík,D.Lazár,T.Fürst,A.Husičková,M.Hýbl,L. Spíchal,Automated integrative high-throughput phenotyping of plant shoots:a case study of the cold-tolerance of pea(Pisum sativum L.),Plant Methods 11(2015)20.
[26]D.L.Auld,R.L.Ditterline,G.A.Murray,J.B.Swensen,Screening peas for winterhardiness under field and laboratory conditions,Crop Sci.23(1983)85-88.
[27]R.Cousin,Peas(Pisum sativum L.),Field Crops Res.53(1997)111-130.
[28]R.Cousin,A.Burghoffer,P.Marget,A.Vingére,G.Etévé,Morphological,physiological and genetic bases of resistance in pea to cold and drought,in:K.B.Singh,M.C.Saxena(Eds.),Breeding for Stress Tolerance in Cool-Season Food Legumes,John Wiley,Chichester,United Kingdom 1993,pp.311-320.
[29]A.Klein,H.Houtin,C.Rond,P.Marget,F.Jacquin,K. Boucherot,M.Huart,N.Rivière,G.Boutet,I.Lejeune-Hénaut,J.Burstin,QTL analysis of frost damage in pea suggests different mechanisms involved in frost tolerance,Theor. Appl.Genet.127(2014)1319-1330.
29 April 2016
in revised form 8 June 2016 Accepted 20 June 2016
.
E-mail address:zongxuxiao@caas.cn(X.Zong).
Peer review under responsibility of Crop Science Society of China and Institute of Crop Science,CAAS.
1These authors contributed equally to this work.
http://dx.doi.org/10.1016/j.cj.2016.06.016
2214-5141/©2016 Crop Science Society of China and Institute of Crop Science,CAAS.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
杂志排行
The Crop Journal的其它文章
- Development of a panel of unigene-derived polymorphic EST-SSR markers in lentil using public database information
- Characterization of chickpea germplasm conserved in the Indian National Genebank and development of a core set using qualitative and quantitative trait data
- Achievements and prospects of grass pea(Lathyrus sativus L.)improvement for sustainable food production
- Nutritional composition and antioxidant activity of twenty mung bean cultivars in China
- Genetics of seed flavonoid content and antioxidant activity in cowpea(Vigna unguiculata L.Walp.)
- Molecular cloning and characterization of a gene encoding the proline transporter protein in common bean(Phaseolus vulgaris L.)
