Evaluation of common bean(Phaseolus vulgaris L.)genotypes for drought stress adaptation in Ethiopia
2016-10-24KwaenaDarkwaDanielAmachewHusseinMohammedAsratAsfawMatthewBlair
Kwaena Darkwa,Daniel Amachew*,Hussein Mohammed, Asrat Asfaw,Matthew W.Blair*
aSouthern Agricultural Research Institute,Ethiopia
bCollege of Agriculture,Hawassa University,Ethiopia
cTennessee State University,Nashville,TN,USA
dInternational Institute of Tropical Agriculture,Abuja,Nigeria
eInternational Potato Center(CIP),Nyankpala,Ghana
Evaluation of common bean(Phaseolus vulgaris L.)genotypes for drought stress adaptation in Ethiopia
Kwabena Darkwab,e,Daniel Ambachewa,c,*,Hussein Mohammedb, Asrat Asfawa,d,Matthew W.Blairc,*
aSouthern Agricultural Research Institute,Ethiopia
bCollege of Agriculture,Hawassa University,Ethiopia
cTennessee State University,Nashville,TN,USA
dInternational Institute of Tropical Agriculture,Abuja,Nigeria
eInternational Potato Center(CIP),Nyankpala,Ghana
A R T I C L E I N F O
Article history:
Available online 22 July 2016
Climate resilient varieties
Correlation analysis
Drought-adapted common bean genotypes
Multiple adaptive traits
Drought stress linked with climate change is one of the major constraints faced by common bean farmers in Africa and elsewhere.Mitigating this constraint requires the selection of resilient varieties that withstand drought threats to common bean production. This study assessed the drought response of 64 small red-seeded genotypes of common bean grown in a lattice design replicated twice under contrasting moisture regimes,terminal drought stress and non-stress,in Ethiopia during the dry season from November 2014 to March 2015.Multiple plant traits associated with drought were assessed for their contribution to drought adaptation of the genotypes.Drought stress determined by a drought intensity index was moderate(0.3).All the assessed traits showed significantly different genotypic responses under drought stress and non-stress conditions.Eleven genotypes significantly(P≤0.05)outperformed the drought check cultivar under both drought stress and non-stress conditions in seed yielding potential.Seed yield showed positive and significant correlations with chlorophyll meter reading,vertical root pulling resistance force,number of pods per plant,and seeds per pod under both soil moisture regimes,indicating their potential use in selection of genotypes yielding well under drought stress and non-stress conditions.Clustering analysis using Mahalanobis distance grouped the genotypes into four groups showing high and significant inter-cluster distance,suggesting that hybridization between drought-adapted parents from the groups will provide the maximum genetic recombination for drought tolerance in subsequent generations.
©2016 Crop Science Society of China and Institute of Crop Science,CAAS.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
Common bean(Phaseolus vulgaris L.)is one of Africa's most essential pulses[1].Among the grain legumes cultivated in Ethiopia,dry beans are regarded as the most important crop for food security and wealth creation[2].An overview of four years'data from 2011 to 2014 indicates that more than 337,000 ha were dedicated to production of 455,000 tons of common beans annually[3].Common bean contributes to the national economy as both a food and an export commodity,in both cases serving as a source of income and employment to a large supply chain[4].The crop provides vital nutrients as a food including vitamins,proteins,and minerals and the stems are also used as fodder for livestock,especially in the dry spell following the main cropping season[5].As a legume,common bean plants also contribute to soil fertility enhancement through atmospheric nitrogen fixation[1].
Drought stress,both as a seasonal phenomenon and as part of climate change,is currently the leading threat to the world's food supply[6].This stress is more severe than other abiotic stresses in common beans,making it the main challenge to the livelihood of bean farmers in marginal,unfavorable environments[2,7].Most common bean production in the developing world occurs under conditionswhere the risk of drought is high[7,8].Numerousregions where drought is already a challenge in Africa,such as Ethiopia,will suffer from warmer and successively drier weather as a result of climate change over the next few decades[9].
Several studies have revealed the radical effect of drought stress on common bean performance.Exposure to drought affects total biomass and seed yield,photosynthate translocation and partitioning,number of pods and seeds per plant,root length and mass,and maturation time[2,10-12].In common bean,droughtstressduringfloweringandpost-floweringcaused reductions of 60-99%in yield[13,14],25.4%in number of pods per plant,20.3%in numbers of seed per pod[15],and 11%in seed size[2].
The average national yield of common bean in Ethiopia is estimatedat1300 kg ha-1onsmallholderfarmsand1700 kg ha-1on commercial farms[16]in contrast to a production potential of 3000 to 4000 kg ha-1in research fields[7,17,18].It is generally assumed that drought problems in crop production can be resolved by applying irrigation,but most African farmers are resource-constrained and lack access to irrigation water[14].In addition,many farmers grow beans in uneven terrain not suitable for irrigation[7,14].The best option for reducing such yield gaps and realizing yield stability under unfavorable environments is thus the development and deployment of drought-tolerant varieties.Drought tolerance,once genetically encoded in the seed of a variety,can be used readily by many farmers for combating drought effects in common bean production[2].Availability and use of highyielding drought-tolerant varieties of common bean would decrease dependence on irrigation water and thereby reduce cost of production,stabilize yield in drought-prone areas,and ultimately increase profit margins for farmers.
Breeding for drought-tolerant crops is challenging and time-consuming,owing to the need for simultaneously considering multiple abiotic and biotic factors modulating the levelofdrought-tolerance.Previousattemptsmadeto evaluate genotypes for drought tolerance indicated high levels of drought tolerance in Durango landraces and some Mesoamerican common bean cultivars[19-21].Genotypic evaluation studies in Ethiopia identified drought tolerant genotypes and selection traits for improving drought adaptation in common bean[2,10,14].The present study assessed multiple adaptive traits for their relative contribution to drought adaptation of the genotypes and combined this assessment with clustering analysis to identify divergent trait progenitors and candidate varieties for use in hybridization to gain maximum genetic recombination for droughttolerance in subsequent generations.
2.Materials and methods
2.1.Experimental design and trial management
This study used 64 genotypes,of which one was bred locally for drought adaptation and the rest were introduced from the International Center for Tropical Agriculture(CIAT by its Spanish acronym),Cali,Colombia(Table S1).These genotypes were generated from crosses between well-known sources of drought resistance.The SCR lines are small red beans carrying drought tolerance with recessive genes for resistance to bean common mosaic virus.Hawassa Dume,a small red-seeded Mesoamerican bean type bred locally for its disease tolerance,seed color,and yield advantage under water deficit conditions and released in Ethiopia in 2008,was used as a locally adapted variety check.A few advanced breeding lines(SER16,RCB745,and SXB412)were included as additional checks.
The genotypes were evaluated in 8×8 simple lattice design experiments with two replications,each repeated under two moisture regimes for a total of four replicates evaluated.The first treatment was non-stress(NS),in which the genotypes were irrigated until maturity whenever soil moisture was depleted to 30%field capacity.The second treatment was drought stress(DS),in which the genotypes were irrigated up to the mid-pod stage when soil moisture was depleted to 30%field capacity and thereafter the irrigation was halted until maturity,thus exposing the genotypes to terminal drought stress.The plots consisted of two rows 3 m in length using 60 cm between-row and 10 cm within-row spacing.Across bothtreatments,atotalof100 kg ha-1diammoniumphosphate fertilizer was applied at planting and the plots were handweeded once before flowering.
The experiment was performed during the dry season at the Hawassa Agricultural Research Center, South Nations,Nationalities and People's Regional State (SNNPR) from November 2014 to March 2015. Hawassa is located at 7°03′ N and 38°30′ E at an elevation of 1650 m.a.s.l. with average annual rainfall of 959 mm distributed mainly in the rainy season (May to August). The site had well-drained sandy loam soil of pH 7. The daily average maximum and minimum temperatures of the site during the growing season were 26.9 °C and 12.4 °C, respectively, and the genotypes were planted in the dry season when additional moisture from rainfall was unlikely. Daily precipitation and minimumand maximum temperatures were recorded with a digital mobile weather station located at the experimental field in Hawassa to confirm the low-rainfall regime.Soil moisture content was determined with Aquaterr digital soil moisture,temperature and salinity meter(Aquaterr instruments and automation,USA)at sample points of 10,20,and 40 cm soil depth during flowering,mid-pod filling and maturity stages.
2.2.Plant trait measurements
Physiological traits of genotypes were assessed by measurement of multiple plant attributes using nondestructive sampling at different growth stages of the crop.The traits measured were 1)days from sowing to flower opening of at least one flower on 50%of plants in a plot(days to flowering,DF);2)days to maturity(DM)based on number of days from sowing to physiological maturity of at least 90%of the plants in a plot;3)leaf chlorophyll content measured by SPAD chlorophyll meter reading(SCMR)at mid-pod filling stage,about one month after flowering and before harvest maturity on 10 fully expanded young leaves of three plants in each plot using a non-destructive,portable SPAD-502 chlorophyll meter(Minolta Camera Co.,Ltd.,Japan);4)plant height(PLHT)was also measured at mid pod filling stage on five plants per plot using meter stick,and the final measurements were recorded at harvest and included;5)vertical root pulling force resistance(RPF);6)number of pods per plant(PDPL);7)number of seeds per pod(SDPD);8)100 seed weight(100 SW);and 9)seed yield per hectare(YLDH).RPF was measured on five plants per plot using IMADA-DS2 digital force gauge(Cole-Parmer instrument company LLC,U.S.A.)by tying a string to the stem of the plant just above the ground and pulling it upward.PDPL and SDPD were recorded by counting the pods and seeds of five randomly selected plants.Seed yield was recorded on a plot basis using FX3000i sensitive digital balance with a capacity of measuring up to 3200 g and 0.01 g scale(A&D Engineering LLC,U.S.A.),which was also used to determine 100 SW as a random sample of total yield.Finally,yield was corrected based on seed moisture content determined with a seed moisture meter(Dickey John corporation,U.S.A.).The plot yield was converted to yield per hectare after adjusting to 12%moisture content.
2.3.Statistical analysis
A general linear model(GLM)was used for data analysis and LSD at P≤0.05 was used for mean separation.Data from each growing environment were analyzed separately and the homogeneity of error variances was tested by Bartlett test[22]before combined analyses were performed.Simple correlation coefficients among traits were determined using the mean trait values for genotypes.All data were used in an analysis of variance(ANOVA)using the GLM procedure in SAS v.9.4 software(SAS Institute,2012).
In addition to the direct measurements,some derived variables were calculated from primary data:drought intensity index,drought susceptibility index,drought tolerance index,mean productivity,geometric mean productivity,yield reduction rate,and yield stability index[23-26].

where YDSand YNSare the mean yields of a given genotype evaluated under drought stress and non-stress conditions,respectively,and XDSand XNSare the mean seed yields over all genotypes evaluated under drought stress and non-stress conditions,respectively.Principal component analysis was employed to identify traits with more contribution in to the principal components.Clustering of genotypes was performed using the average linkage method,using the 14 phenotypic traits evaluated under drought-stress treatment and six drought indices.Traits with Eigenvectors greater than or equal to 1 were considered in the cluster formation and the ideal number of clusters was determined by looking at the agreement between cubic clustering criterion,pseudo F and pseudo-t2statistics between groups[28].Genetic distances between the centroids of clusters were calculated as standardized D2,based on suggestions of Mahalanobis[29].
3.Results
3.1.Weather and soil moisture
The maximum and minimum daily temperatures and the daily rainfall received during the crop-growing period are presented in Fig.1.The DS and NS treatments received a total of 51.8 mm rainfall during the growing season in only three rain events,creating moisture-stress conditions for the DS treatment.The amounts of water in the soil profile throughout the crop growth period are shown in Fig.2 for DS and NS conditions,respectively.Drought stress resulted in 29.8% reduction in YLDH,26.1%reduction in SCMR,and 19.1% reduction in PDPL(Table 1).
3.2.Yield-related traits
Data from NS and DS treatments were compared to assess the effect of drought stress on yield-related traits and the datasets were combined after a test for homogeneity of error variance confirmed the appropriateness of a global ANOVA treating genotypes as fixed and environments as random.The meansquare values for DM,PLHT,and SCMR were highly significant(P≤0.01)between genotypes,between treatments,and for genotype-by-treatment interaction(Table 1).A significant difference(P≤0.01)was also observed between genotypes for RPF.DM ranged from 94 to 107 days with mean of 102 days in the NS treatment and from 82 to 99 days with mean of 89 days in the DS treatment.

Fig.1-Rainfall distribution and maximum and minimum temperatures during crop growing period at Hawassa. Source:Agro-Meteorology Department,Hawassa Agricultural Research Center.
Exposure to drought caused a mean reduction of 13 days(12.7%)in DM compared to the NS treatment.SCR16 and BSF10 were the earliest to mature(95 days)in the NS treatment,while Hawassa Dume(106 days)and BFS29(107 days)were late to reach physiological maturity in the NS treatment. Under drought stress,BFS10 and BFS55 were earliest to reach physiological maturity(82 days)and were the highest-yielding varieties.The genotypes SCR27(98 days)and SCR25(99 days)were late to reach physiological maturity in the DS treatment.

Fig.2-Soil moisture content measured at three different soil depths across three time periods under drought stress and non-stress conditions at Hawassa from November 2014 to March 2015.(a):drought stress;(b):non-stress.FL:flowering stage;MPF:mid-pod-filling stage;PM:physiological maturity stages.
With respect to plant architecture,PLHT ranged from 24.0 to 47.5 cm with mean of 37.3 cm for the NS and from 24.8 to 43.8 cm with a mean of 31.8 cm for the DS treatments.A mean reduction of 5.5 cm in PLHT was observed when the NS compared with the DS treatment.The shortest plant in the NS was SCR1 and the tallest was BFS35.Genotypes SCR13 and BFS67 showed the minimum and maximum plant heights,respectively,in the DS treatment.
With respect to photosynthesis in the DS treatment,the highest SCMR(39.7)was measured for SCR5 and the lowest(15.6)for SCR15.This trait was also highly affected by drought stress,with a 26.1%reduction from the non-stress treatment. BFS34 and SCR34 showed the highest and lowest RPR of 11.3 and 25.4,respectively,in the NS treatment.RPF ranged from 9.4 to 29.8 with a mean of 21.1 for the DS treatment,BF54 and BFS55 showing the highest values and SCR15 the lowest.Drought stress caused a 1.4%increase in RPF in comparison with the NS treatment.
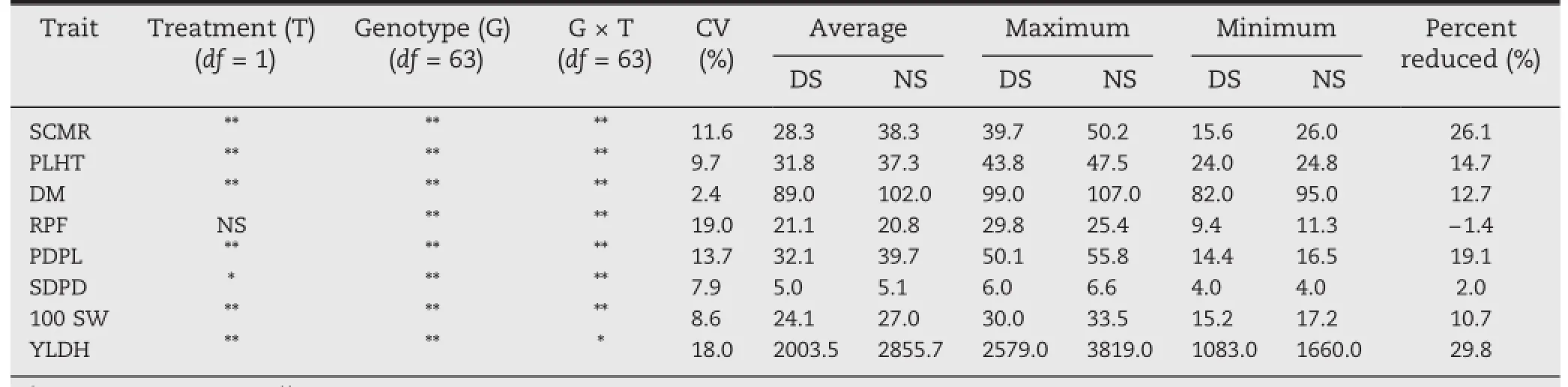
Table 1-Significance of treatment,genotype,and genotype-by-treatment effects for traits evaluated in 64 common bean genotypes grown under drought-stress and non-stress treatments at Hawassa,November 2014 to March 2015.
Among yield components,highly significant differences(P≤0.01)between the treatments,genotypes,and genotypeby-treatment interactions were also observed for the variables PDPL,SDPD,and 100 SW,all measured at or after harvest. PDPL ranged from 16.6 to 55.8 with mean of 39.6 for the NS treatment and from 14.4 to 50.1 with a mean of 32.1 for the DS treatment.The mean PDPL was 18.9%higher in the NS than in the DS treatment.Genotypes BFS35 and SCR1 showed the highest and lowest PDPL,respectively,in the NS treatment,whereas BFS55 and SCR35 showed the highest and lowest in the DS treatment.Exposure to drought caused 100 SW to decrease by 10.7%from the NS treatment.SCR18 showed the highest 100 SW and SCR6 the lowest in the NS. SCR2 and SEC24 showed the highest and lowest 100 SW,respectively,in the DS treatment.
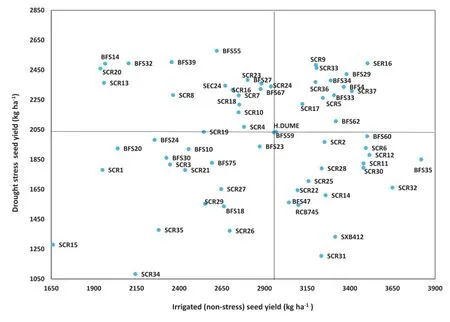
Fig.3-Scattergram showing the identification and categorization of common bean genotypes based on their seed yield in a comparison of drought-stress(DS)and non-stress(NS)growing conditions.Horizontal and vertical lines indicate mean values in DS and NS for the check,Hawassa Dume.
For YLDH,the mean squares of genotypes,treatments,and genotype-by-treatment interaction also showed highly significant differences(P≤0.001).Exposure to drought stress caused a yield penalty of 29.8%in the DS relative to the NS treatment.On the basis of seed yield under DS and NS conditions,the 64 genotypes could be classified into four differential categories(Fig.3 and Table S1).In the firstcategory were genotypes with higher yield than the check in both treatments,namely SCR5,SCR9,SCR17,SCR33,SCR36,SCR37,SER16,BFS4,BFS29,BkFS33,BFS34,BFS59,and BFS62. Two of these 13 genotypes,namely BFS29 and SER16,showed significantly(P≤0.05)superior yield performance relative to the check under both DS and NS treatments,with the highest mean productivities of 2902 and 2999.5 kg ha-1,respectively,versus 2493.5 kg ha-1for the control variety Hawassa Dume,with 16.4%and 20.3%yield advantages.
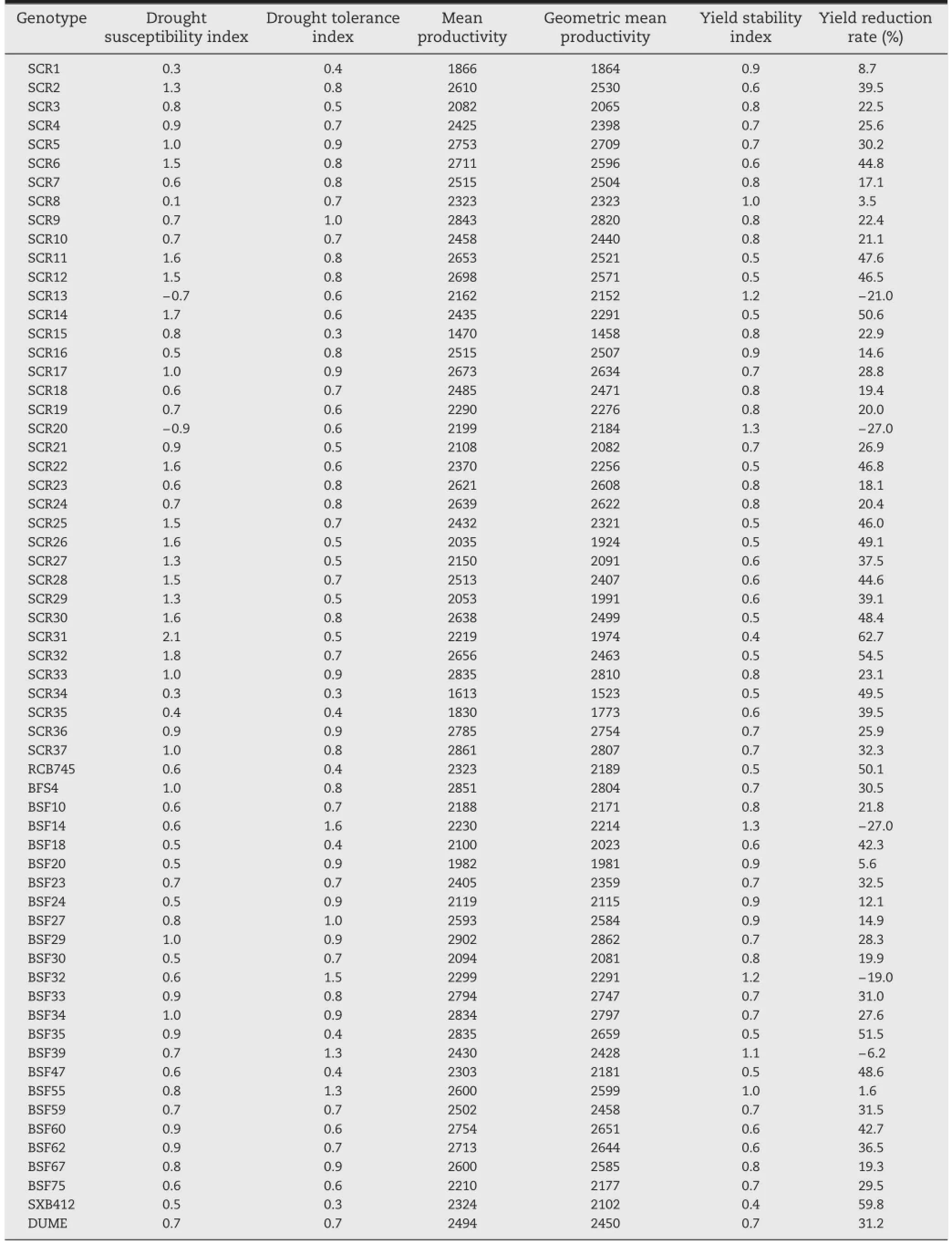
Table 2-Drought tolerance indices of 64 common bean genotypes grown under non-stress and drought-stress conditions at Hawassa,November 2014 to March 2015.

Table 2(continued)
In the second yield category were genotypes with the lowest degree of adaptation and yield in the DS and NS treatments,including SCR1,SCR3,SCR15,SCR21,SCR26,SCR27,SCR29,SCR34,SCR35,BFS10,BFS18,BFS20,BFS23,BFS24,BFS30,and BFS75.The third category contained genotypes that showed high yield(higher than the check)in the DS treatment but low yields(lower than the check)in the NS treatment,including SCR4,SCR7,SCR8,SCR10,SCR13,BFS27,SCR16,SCR18,SCR19,SCR20,BFS14,SCR23,SCR24,BFS32,BFS39,BFS55,BFS67,and SEC24.Category 4 included genotypes that yielded well in the NS treatment but showed correspondingly loweryield in theDS treatment.ThesewereSCR2,SCR6,SCR11,SCR12,SCR14,SCR22,SCR25,SCR28,SCR30,SCR31,SCR32,RCB745,BFS35,BFS47,BFS60,and SXB412.In total,10 genotypes from group 4 had significantly higher yields than Hawassa Dume in the NS treatment.These were genotypes BFS35,SCR32,SCR12,SER16,BFS60,SCR6,SCR11,SCR30,SCR37,and BFS29.The yield advantage of these genotypes ranged from 29.2%for BFS35(3819 kg ha-1)to 14.4%for BFS29(3381 kg ha-1).Finally,nine genotypes(BFS55,BFS39,SER16,BFS32,BFS14,SCR9,SCR33,SCR20,and BFS29),gave yields significantly(P≤0.05)higher thanthatofthecheckintheDS.YieldadvantagesoverHawassa Dume ranged from 27%for BFS55(2579 kg ha-1)to 19.2%for BFS29(2423 kg ha-1).

Table 3-Simple correlation coefficients between seed yield and other traits of 64 common bean genotypes evaluated under drought-stress(upper diagonal)and non-stress(lower diagonal)conditions at Hawassa from November 2014 to March 2015.
3.3.Effect of drought stress on seed yield
The severity of drought stress effects on seed yield over all of the experiments,expressed as a drought intensity index,was moderate at 0.3.The drought tolerance indices for individual genotypes were also estimated(Table 2).Based on mean productivity and geometric mean productivity,genotypes SER16,BFS29,SCR37,BFS54,SCR9,and SCR33 were higher-yielding under the two watering regimes.
In contrast,the genotype rankings by the indices of drought susceptibility,drought tolerance,and yield stability and yield reduction rate were different from those by mean productivity and geometric mean productivity.Accordingly,genotypes SCR20,BFS14,SCR13,BFS32,BFS39,BFS55,and SCR8 were considered tolerant to drought stress because of their low values for drought susceptibility index and yield reduction rate and high values for drought tolerance index and yield stability index.However,these genotypes were not among the highest-yielding lines under the NS condition. In contrast,genotypes SCR14,BFS47,SCR29,RCB745,BFS18,SCR35,SCR26,SXB412,SCR15,SCR31,and SCR34 were considered susceptible to drought stress,although some yielded well under NS condition.
3.4.Correlation between traits
As shown in Table 3,YLDH was positively correlated(P≤0.01)with SCRM (r=0.5),RPF(r=0.6),PDPL(r=0.7)and SDPD(r=0.6)under DS conditions.However,the significant correlation between YLDH and DM was negative(r=-0.6).DM was also significantly negatively correlated with RPF,PDPL and SDPD.SCMR showed a positive significant correlation with RPF,PDPL,and SDPD.Under theNS condition,correlations between YLDH and all the other traits measured except for 100 SW(SCMR,PLHT,DM,RPF,PDPL,and SDPD)were positive and highly significant. Positive,significant correlations were also observed between SCMR,DM,RPF,PDPL,and SDPD.100 SW showed no significant correlation with YLDH under NS and DS conditions.
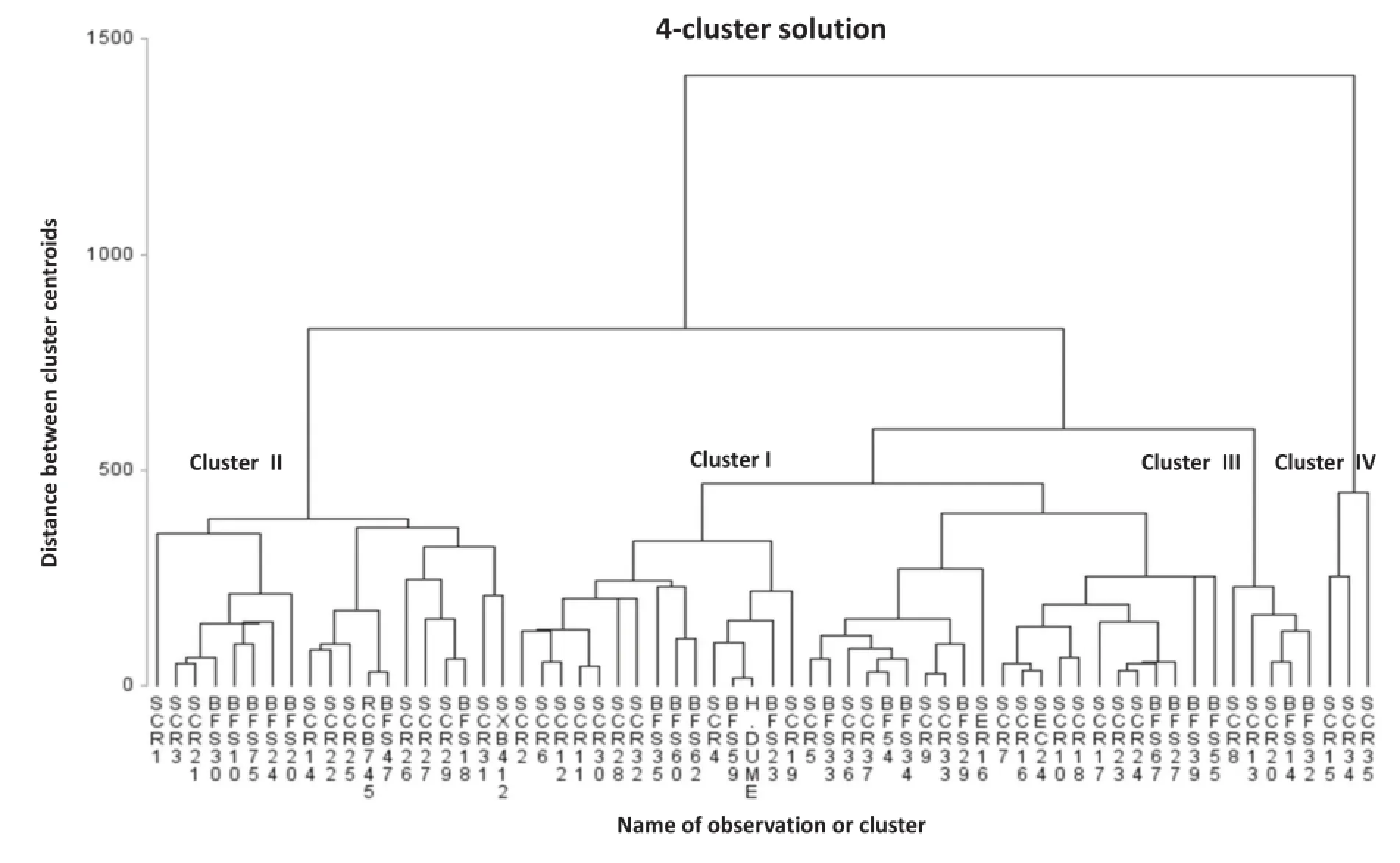
Fig.4-Average linkage-based dendrogram showing hierarchical grouping patterns of 64 common bean genotypes in four clusters based on 14 quantitative traits under drought-stress condition.
3.5.Clustering of genotypes
The average linkage grouping method using the DS variables identified by PCA produced four clusters of the 64 genotypes(Fig.4).Cluster III was the largest,containing 35 genotypes(54.69%)followed by cluster I,which contained 23 genotypes(35.94%).Clusters II and IV were small groups containing four(6.25%)and two genotypes(3.12%),respectively(Table S1).Genotypes with high degrees of yield adaptation under DS were grouped in cluster I(Fig.3,Table S1)and those with low adaptation under DS were grouped in cluster III.Genotypes SCR 15 and SCR 34 were grouped in cluster IV.These genotypes showed the least yield adaptation under both NS and DS conditions.Genotypes BFS14,BFS32,SCR13 and SCR20 were grouped in cluster II as genotypes that were not adapted under NS and highly adapted under DS conditions.
The Mahalanobis distance between clusters is presented in Table 4.The highest inter-cluster distance appeared between clusters I and II(D2=1837.8)followed by clusters I and IV(D2=1482.5)and clusters II and III(D2=941.1).The lowest inter-cluster distance was found between clusters II and IV(469.9)followed by that between clusters III and IV(654.2).
4.Discussion
Dry-season weather conditions imposed the main stress in the experiments in this trial,especially in the non-irrigated treatment.The daily average maximum and minimum temperatures during the season were 30.7°C and 11.3°C,respectively.These temperatures are within the favorable range for common bean growth[7,28].However,the total amount of rainfall received throughthegrowingperiodwasmuchlowerthanthe 350-500 mm rainfall required by the crop,indicating moderate to high drought stress.Terminal drought stress,as experienced in this study,is the most important problem for common bean production in much of the developing world[29].

Table 4-Mahalanobisdistancebetweengroupsofcommon bean genotypes.
In this study,the genotypes were evaluated under moderately high drought stress(corresponding to a drought stress index of 0.3),which was adequate to reveal genotypic differences,as seen by the differential response of the genotypes for the various traits measured.Ambachew et al.[14]and Beebe et al.[21]reported that evaluation of genotypes underconditions of extreme drought stress reduces seed yields to very low levels that could nullify the genotypic differences among test materials.However,high to moderate stress is useful for genotypic selection.It is also worth noting that because insufficient stress could result in selection of non-resistant genotypes,evaluation of common bean under high to moderate stress is considered ideal[2,29].
The significant effect of the genotypes,treatments and the genotype-by-treatment interaction for the various traits indicated that the expressions of the genotypes across the two growing moisture regimes was not static and nonresponsive but rather adaptable.This result is in accord with those of Asfaw and Blair[2],Porch et al.[30],and Rezene et al.[31],who reported differential response of common bean varieties to drought-stressed and non-stressed conditions.
The influence of drought stress on trait expression of the genotypes varied.Some characters were more sensitive to drought stress effects than others.Seed yield,days to maturity,plant height,100 seed weight,leaf chlorophyll content,and pods per plant were highly sensitive to drought stress,whereas seeds per pod and vertical root pulling force resistance were the least sensitive.This difference could be attributed to differences between genotypes or to the nature of the traits.Significant reduction in days to physiological maturity as a result of drought stress was observed in the present study and previously[20,21].Phenotypic plasticity has been reported in common beans subjected to drought stress[32]as a mechanism for adaptation.For example,some common bean genotypes respond to drought stress by hastening their maturity[7,19].Earliness to harvest can be linked to drought escape and,as such,is a mechanism of drought tolerance[33].Rao et al.[20]reported drought tolerance of early-maturing genotypes,given their lower net water requirement throughout their plant life cycle compared with late-maturing genotypes.Rezene et al.[31]and Singh[33]found that late-maturing genotypes suffer greater reduction in performance under drought stress than do early ones.
Drought is known to affect plant photosynthesis[32].The higher leaf chlorophyll content observed in the non-stressed treatmentinthisstudywasaresultoftheavailabilityofmoisture in the soil throughout the entire life cycle of the crop,which favors the vegetative growth and induced the plants to grow taller and produce more chlorophyll.Chaves et al.[34]reported that drought stress reduces leaf chlorophyll content.However,a smallchlorophyllincrease(4%)hasbeenobservedunderdrought as well[35].Drought stress during the mid-pod fill stage can decrease leaf chlorophyll content,resulting in a progressive decline in photosynthetic capacity,although in our previous study[14],genotypes with higher leaf chlorophyll content produced higher seed yield than those with lower content.
The higher mean performance of genotypes for vertical root pulling force resistance under drought stress conditions suggests that common bean responds to drought stress by increasing root growth.The role of vertical root pulling force resistance in common beans was first reported by Ambachew et al.[14]as a proxy root trait for measuring the roots'ability to obtain water.The higher the resistance to the upward pulling force,the greater was expected to be the root system attachment to the soil in which it was growing,suggesting higher root density and deeper rooting system.The same study found a significant correlation between vertical root pulling force resistance and seed yield.
Yield-component traits are generally good indicators of overall drought stress,and our study showed significant reductions in number of pods per plant,100 seed weight,and seed yield under drought conditions.Similarly,Asfaw and Blair[2]reported significant reductions in pod number per plant,seed number per pod,100 seed weight and seed yield of common beans under similar drought-stressed conditions.The higher reduction in number of pods per plant in drought-stress as compared to the non-stress condition,may have been due to a reduction in flower fertilization under drought-stress conditions[14].
Thereductioninseedyieldand100 seedweight associated with drought is thought to be caused by a decrease in photosynthate assimilation and poor carbohydratepartitioningtothedevelopinggrainbecauseof drought stress[20,31,35].The strong association between photosynthate assimilation and better remobilization of carbohydrates by drought-tolerant genotypes permits them tomaintainhigh100 seedweightirrespectiveofthe moisture content of the soil[33].
Thisstudyhasimplicationsforplantbreeding.Understanding of the relationships among plant traits under drought-stress should prompt common bean breeders to make better yield measurements and record drought-response characteristics in more detail.Among the yield traits,we found,as also previously reported[2,14,21,31],a positive significant correlation between seed yield and pods per plant and seeds per pod under drought and non-stressed conditions.The success of hybridization in a breedingprogramdependsonthechoiceofdistantparentallines. Crosses that involve parents selected from the clusters characterized by maximum genetic distance in this study are expected to result in maximum genetic recombination and variation in subsequent generations once the lines are introduced into small red bean breeding for Ethiopia or other countries.
5.Conclusions
The adaptation of genotypes to drought-stress conditions and their good performance in a well-watered environment were associated with leaf chlorophyll content,vertical root pulling force resistance,number of pod per plant,and number of seeds per pod.Most of the genotypes showed adaptation to drought stress by reducing their days to physiological maturity,thereby minimizingtheeffectofdrought.GenotypesBFS55,BFS39,BFS32,BFS14,SCR9,SCR33,and SCR20,which yielded well under the drought-stressedcondition,maybegoodsourcesofresistanceto this stress.Hybridization between genotypes selected from clusters I and II will provide the maximum genetic recombination and variation for drought tolerance.
Acknowledgments
We acknowledge funding to D.Ambachew,A.Asfaw,and M.W.Blair by the Tropical Legumes project of the Generation Challenge Program(C-086-13)with support from the Bill andMelinda Gates Foundation.The Evans Allen Fund is recognized for funding Matthew W.Blair and Daniel Ambachew at Tennessee State University.We also thank the South Agricultural Research Institute(SARI)for hosting this research.We acknowledge CIAT(S.Beebe and B.Raatz)for supplying germplasm and previous SARIstafffor development of Hawassa Dume.
Supplementary data
Supplementary data for this article can be found online at http://dx.doi.org/10.1016/j.cj.2016.06.007.
R E F E R E N C E S
[1]W.J.Broughton,G.Hernandez,M.W.Blair,S.E.Beebe,P.Gepts,J.Vanderleyden,Beans(Phaseolus spp.)model food legumes,Plant Soil 252(2003)55-128.
[2]A.Asfaw,M.W.Blair,Quantification of drought tolerance in Ethiopian common bean varieties,Agric.Sci.5(2014)124-139.
[3]FAOSTAT,http://faostat3.fao.org/browse/Q/QC/E Accessed on June 28,2016.
[4]K.Tumsa,R.Buruchara,S.E.Beebe,Common Bean Strategies and Seed Roadmaps for Ethiopia,in:E.S.Monyo,G.C.L. Laxmipathi(Eds.),Grain Legumes Strategies and Seed Roadmaps for Selected Countries in Sub Saharan Africa and South Asia,TL-II Project Report,ICRISAT,India 2014,pp.3-11.
[5]Z.Wondatir,Y.Mekasha,Feed resources availability and livestock production in the central rift valley of Ethiopia,Int. J.Livest.Prod.5(2014)30-35.
[6]H.Budak,M.Kantar,K.Y.Kurtoglu,Drought tolerance in modern and wild wheat,Sci.World J.10(2013)548246.
[7]S.E.Beebe,I.M.Rao,M.W.Blair,J.A.Acosta-Gallegos,Phenotyping common beans for adaptation to drought,Front. Physiol.5(2013)123-138.
[8]S.P.Singh,Broadening the genetic base of common bean cultivars,Crop Sci.4(2001)1659-1675.
[9]P.G.Jones,P.K.Thornton,The potential impacts of climate change on maize production in Africa and Latin America in 2055,Glob.Environ.Chang.13(2003)51-59.
[10]A.Asfaw,C.Almekinders,M.W.Blair,P.Struik,Participatory approach in common bean breeding for drought tolerance for southern Ethiopia,Plant Breed.131(2012)125-134.
[11]D.C.Nielsen,N.Nelson,Black bean sensitivity to water stress at various growth stages,Crop Sci.38(1998)422-427.
[12]P.Ramirez-Vallejo,J.D.Kelly,Traits related to drought resistance in common bean,Euphytica 99(1998)127-136.
[13]P.Manjeru,T.Madanzi,B.Makeredza,A.Nciizah,M.Sithole,Effect of Water Stress at Different Growth Stage on Components and Grain Yield of Common Bean(Phaseolus vulgaris L.),Afr.Crop Science Conference Proceedings,Vol.8,2007,pp.299-303.
[14]D.Ambachew,F.Mekbib,A.Asfaw,S.E.Beebe,M.W.Blair,Trait associations in common bean genotypes grown under drought stress and field infestation by BSM bean fly,Crop J.3(2015)305-316.
[15]S.Khaghani,M.R.Bihamata,F.Rahim,H.R.Dorry,Study of qualitative and quantitative traits in red bean in non-stress and drought condition,Asian J.Plant Sci.7(2008)563-568.
[16]Central Statistical Agency(CSA)of Ethiopia,Agricultural Sample Survey,Addis Ababa,Ethiopia,http://www.csa.gov.et 2013 Accessed on January 5,2016.
[17]IFPRI(International Food Policy Research Institute),Pulses value chain potential in Ethiopia:constraints and opportunities for enhancing exports.Pulses diagnostics,http://www.ethiopiam.agriculture.file.wordpress.com 2010 Accessed on December 20,2015.
[18]M.W.Blair,C.H.Galeano,E.Tovar,M.C.Muñoz-Torres,A.V. Castrillón,Development of a Mesoamerican intra-genepool genetic map for quantitative trait detection in a drought tolerant×susceptible common bean(Phaseolus vulgaris L.)cross,Mol.Breed.29(2012)71-88.
[19]H.Terán,S.P.Singh,Comparison of sources and lines selected for drought resistance in common bean,Crop Sci.41(2002)64-70.
[20]C.G.Muñoz-Perea,R.G.Allen,D.T.Westermann,J.L.Wright,S.P.Singh,Water use efficiency among dry bean landraces and cultivars in drought-stressed and non-stressed environments,Euphytica 155(2007)393-402.
[21]S.E.Beebe,I.M.Rao,C.Cajiao,C.M.Grajales,Selection for drought resistance in common bean also improves yield in phosphorus limited and favorable environments,Crop Sci.48(2008)582-592.
[22]M.S.Bartlett,Properties of sufficiency and statistical tests,Proc.R.Soc.London,Ser.A 160(1937)268-282.
[23]R.A.Fischer,R.Maurer,Drought resistance in spring wheat cultivars:I.Grain yield response,Aust.J.Agric.Res.29(1978)897-907.
[24]G.C.J.Fernandez,Effective Selection Criteria for Assessing Plant Stress Tolerance,in:C.G.Kuo(Ed.),Adaptation of Food Crops to Temperature and Water-Stress,AVRDC,Shanhua,Taiwan,China 1992,pp.257-270.
[25]A.A.Rosielle,J.Hamblin,Theoretical aspects of selection for yield in stress and non-stress environment,Crop Sci.21(1981)943-945.
[26]M.Bouslama,W.T.Schapaugh,Stress tolerance in soybean:1. Evaluation of three screening techniques for heat and drought tolerance,Crop Sci.24(1984)933-937.
[28]P.C.Mahalanobis,The Generalized Distance in Statistics,Proceedings of the National Institute of Sciences(Calcutta),Vol.2,1936,pp.49-55.
[29]I.M.Rao,S.Beebe,J.Polania,J.Ricaurte,C.Cajiao,R.Garcia,M. Rivera,Can tepary bean be a model for improvement of drought resistance in common bean?Afr.Crop.Sci.J.21(2013)265-281.
[30]T.G.Porch,V.H.Ramirez,D.Santana,E.W.Harmsen,Evaluation of common bean for drought tolerance in Juana Diaz,Puerto Rico,J.Agron.Crop Sci.195(2009)328-334.
[31]Y.Rezene,S.Gebeyehu,H.Zelleke,Genetic variation for drought resistance in small red seeded common bean genotypes,Afr.Crop.Sci.J.19(2011)303-312.
[32]S.Gebeyehu,Physiological Response to Drought Stress of Common Bean(Phaseolus vulgaris L.)Genotypes Differing in Drought ResistancePhD Dissertation Universidad de Liebig-Giessen,Giessen,Germany,2006.
[33]S.P.Singh,Selection for water-stress tolerance in interracial population of common bean,Crop Sci.35(1995)153-165.
[34]M.M.Chaves,J.Flexas,C.Pinheiro,Photosynthesis under drought and salt stress:regulation mechanisms from whole plant to cell,Ann.Bot.103(2009)551-560.
[35]A.Asfaw,M.W.Blair,P.Struick,Multi-environment quantitative trait locus analyses for photosynthate acquisition,accumulation and remobilization traits in a common bean,Genes Genomes Genet.2(2012)579-595.
3 May 2016
in revised form 28 June 2016 Accepted 11 July 2016
s at:Tennessee State University,Nashville,TN,USA.
E-mail addresses:ddemissi@my.tnstate.edu(D.Ambachew),mblair@tnstate.edu(M.W.Blair).
Peer review under responsibility of Crop Science Society of China and Institute of Crop Science,CAAS.
http://dx.doi.org/10.1016/j.cj.2016.06.007
2214-5141/©2016 Crop Science Society of China and Institute of Crop Science,CAAS.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
杂志排行
The Crop Journal的其它文章
- Editorial:Food Legume Diversity and Legume Research Policies
- QTL and candidate genes associated with common bacterial blight resistance in the common bean cultivar Longyundou 5 from China
- Two major er1 alleles confer powdery mildew resistance in three pea cultivars bred in Yunnan Province,China
- Construction of an integrated map and location of a bruchid resistance gene in mung bean
- Large-scale evaluation of pea(Pisum sativum L.)germplasm for cold tolerance in the field during winter in Qingdao
- Molecular cloning and characterization of a gene encoding the proline transporter protein in common bean(Phaseolus vulgaris L.)
