Molecular cloning and characterization of a gene encoding the proline transporter protein in common bean(Phaseolus vulgaris L.)
2016-10-24JioChenJingWuYunfengLuYunnnCoHuiZengZhoyunZhngLnfenWngShuminWng
Jio Chen,Jing Wu,Yunfeng Lu,Yunnn Co,Hui Zeng,Zhoyun Zhng, Lnfen Wng,Shumin Wng,*
aNanyang Normal University,Nanyang,Henan 473061,China
bInstitute of Crop Science,The Chinese Academy of Agricultural Sciences,Beijing 100081,China
Molecular cloning and characterization of a gene encoding the proline transporter protein in common bean(Phaseolus vulgaris L.)
Jibao Chena,1,Jing Wub,1,Yunfeng Lua,Yuannan Caoa,Hui Zenga,Zhaoyuan Zhanga, Lanfen Wangb,Shumin Wangb,*
aNanyang Normal University,Nanyang,Henan 473061,China
bInstitute of Crop Science,The Chinese Academy of Agricultural Sciences,Beijing 100081,China
A R T I C L E I N F O
Article history:
Available online 30 June 2016
Common bean
Proline
Proline transporter
Drought stress
PvProT
As a typical compatible solute,proline is accumulated in plants under environmental stresses.Proline transporter(ProT)plays an important role in proline distribution between plant organs.Using a candidate gene approach,we cloned a cDNA sequence for ProT from common bean(Phaseolus vulgaris L.)and designated the gene PvProT.The deduced amino acid sequence of PvProT showed high similarity to Bet/ProT proteins from other leguminous plants,and the highest similarity was observed with mothbean(Vigna aconitifolia L.)VuProT. Relative quantification of the mRNA level of PvProT using real-time PCR analysis showed that the PvProT transcript level was higher in leaves than in stems and roots of common bean plants subjected to drought and salt stress.Under 20%(w/w)PEG-6000 treatment,drought-resistant plants expressed a higher level of PvProT transcripts than droughtsensitive plants.Although heterologous expression of PvProT in the Escherichia coli mutant
mkh13 showed that PvProT exhibited uptake activities for proline and betaine,no betaine content was detected in the common bean.These findings suggest that PvProT plays an important role in the transportation of proline in common bean plants exposed to drought and salt stress.
©2016 Crop Science Society of China and Institute of Crop Science,CAAS.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
Proline is one of the most widely distributed compatible solutes.Accumulation of proline does not interfere with normal biochemical reactions.It increases cellular osmotic potential to maintain a stable intracellular environment and protects plants from oxidative damage andmembraneintegrity under osmotic treatment[1,2].Two proline-synthetic pathways are known in higher plants.One is the conversion of glutamate and the other is the conversion of ornithine[3].In the glutamate pathway,proline is synthesized mainly in the cytosol from glutamate via pyrroline-5-carboxylate by the sequential action of pyrroline-5-carboxylate synthetase(P5CS)andpyrroline-5-carboxylatereductase.Yoshibaetal.[4]reportedthat dehydration and salt stress induced increased expression of P5CS in Arabidopsis thaliana.Kavi Kishor et al.[5]postulated that P5CS is the rate-limiting enzyme in proline biosynthesis in plants.In the ornithine pathway,δ-ornithine aminotransferase(δ-OAT)plays a key role.δ-OAT transfers the δ-amino group of ornithine to α-ketoglutarate or related α-keto acids,thereby forming glutamate semialdehyde and glutamate. Glutamate semialdehyde is spontaneously cyclized to pyrroline-5-carboxylate[5].For degradation,proline is imported into mitochondria,whereitisconvertedbacktoglutamatebyproline dehydrogenase and pyrroline-5-carboxylate dehydrogenase[2].
Under abiotic stress,proline accumulates differently in different plant organs[6-8].This difference results not only from increased synthesis and decreased degradation but also from transportation.In plants,proline transportation is mediated by a proline transporter(ProT)[9].Proline transporters were first isolated from Arabidopsis as highly selective transporters for proline[10].In barley(Hordeum vulgare),HvProT is the only known transporter that recognizes proline but not betaine.As a salt-inducible gene,HvProT is highly expressed under salt stress in root tips,especially the root cap and cortex cells[11].The EgProT1 protein from oil palm(Elaeis guineensis)is involved in the uptake of betaine,choline,and proline.LeProT1 expression in a yeast mutant showed that LeProT1 transports proline and γ-amino butyric acid with low affinity and glycine betaine with high affinity[12].These findings indicate that ProT proteins show different substrate specificities in different plants[13].
The common bean is one of the most ancient crops of the Americas and is also the most important grain legumes worldwide for direct human consumption.Under drought and salt stress conditions,the common bean accumulates proline to resist stress damage[14].Previously,we investigated proline synthesis in common bean[15].Recently,we have cloned a δ-OAT gene from common bean[16].To comprehensively investigate the proline accumulation mechanism,we isolated a cDNA for a ProT from common bean in the present study.Expressing PvProT in the Escherichia coli mutant mkh13 showed that the common bean PvProT transports betaine and proline.
2.Materials and methods
2.1.Plant material and growth conditions
Seeds of common bean used in this study were germinated in dishes irrigated with sterile water for 4 days,and then single sprouted seeds were transplanted to a pot filled with a 30 g mixture of soil and sand(1:1)and cultured in a growth chamber under white fluorescent light(16 h light/8 h dark)for two weeks before drought treatments.
For analysis of the gene expression response to drought and salt stress,a common bean landrace,Huanghua Dou,was used.Seedlings were irrigated with 20%PEG-6000 water solution.Four drought stress treatments were imposed: 4 days of drought stress(4 d-stressed),3 days of drought stress(3 d-stressed),2 days of drought stress(2 d-stressed),and 1 day of drought stress(1 d-stressed).Seedlings 10,11,12, and 13 days old were stressed for 4,3,2,and 1 days,respectively.Untreated plants were irrigated with water for 14 days as control(0 d-stressed).As salt stress treatments,12-day-old seedlings were irrigated with 100 mL of NaCl solutionofdifferentconcentrations(50,100,150,and 200 mmol L-1)in each pot and cultured for 2 days.Seedlings 12 days old were irrigated with 100 mL water in each pot and cultured for 2 days as a salt stress control.
For analysis of gene expression in drought-tolerant and drought-sensitive common bean,12-day-old seedlings of drought-tolerant(F1372 and F5575)and drought-sensitive(F4409 and F4851)landraces of common bean were treated with 20%PEG-6000 for 24 h.
Each treatment used 60 single plants and three replicate experiments were conducted.Immediately after stress was applied,60 plant tissue samples for each independent experiment were collected,dipped in liquid nitrogen,and stored at-80°C.All tissue samples were randomly divided into three subsamples,which were used for proline content measurement and total RNA extraction.
2.2.Cloning and sequence analysis of the PvProT gene
After total RNA extraction,first-strand cDNA was synthesized using PrimeScript 1st strand cDNA Synthesis Kit following the instructions of the manufacturer(TaKaRa Bio,Inc.).For amplification of the ProT gene from common bean,a primer pair P1(U 5′-ATT CGG TGG ATT CTC GTT CAT TC-3′;D 5′-ATA TTT TCT GAT CTG AGT GAC C-3′)was designed using the sequence of GmProT(GenBank ID:NM001250221).PCR was performed at 95°C for 5 min,30 cycles of 95°C for 30 s,55°C for 1 min,72°C for 1.5 min,followed by a final step of 72°C for 7 min.Finally,the target band was sequenced by BGI Corporation after ligation and transformation.Analyses of nucleotide and amino acid sequences were performed with DNAstar(http://www.dnastar.com/blast/ncbi-blast.html)and DNAman(Lynnon Biosoft Corporation,USA)software.
2.3.Determination of proline
Proline content was estimated with the ninhydrin reaction following Bates et al. [17]. A portion of frozen leaves was homogenized with 3 mL of 3% (w/v) sulfosalicylic acid and then the mixture was incubated in a 100 °C water bath for 10 min and centrifuged at 4000×g for 5 min, after which 1 mL of the supernatant was taken for analysis and 1 mL of ninhydrin reagent and 1 mL of glacial acetic acid were added. The mixture was incubated in a 100 °C water bath for 30 min and extracted with 4 mL toluene, and the absorption of the chromophore was read with a spectrophotometer at 520 nm against toluene as a blank. Proline content was calculated using a standard curve constructed with known proline concentration standards.
2.4.Real-time quantitative PCR
To examine the expression pattern of PvProT in response to drought stress,real-time quantitative PCR was performed using a C1000 thermal cycler(Bio-Rad Corporation,USA)with the following primers:PvProT(U 5′-GAC GCT GCT CTC AAC GAC AA-3′;D 5′-AGC GAT ATT ATG GTA GCA AGA ACT-3′),with an actin primer pair(U 5′-GAA GTT CTC TTC CAA CCA TCC-3′;D 5′-TTT CCT TGC TCA TTC TGT CCG-3′)used as an internal control.The program consisted of denaturation for 2 min at 95°C followed by 40 cycles of 95°C for 15 s,66.5°C for 30 s,and 72°C for 45 s.The relative expression levels were calculated as 2-ΔΔCTfollowing Livak et al.[18].
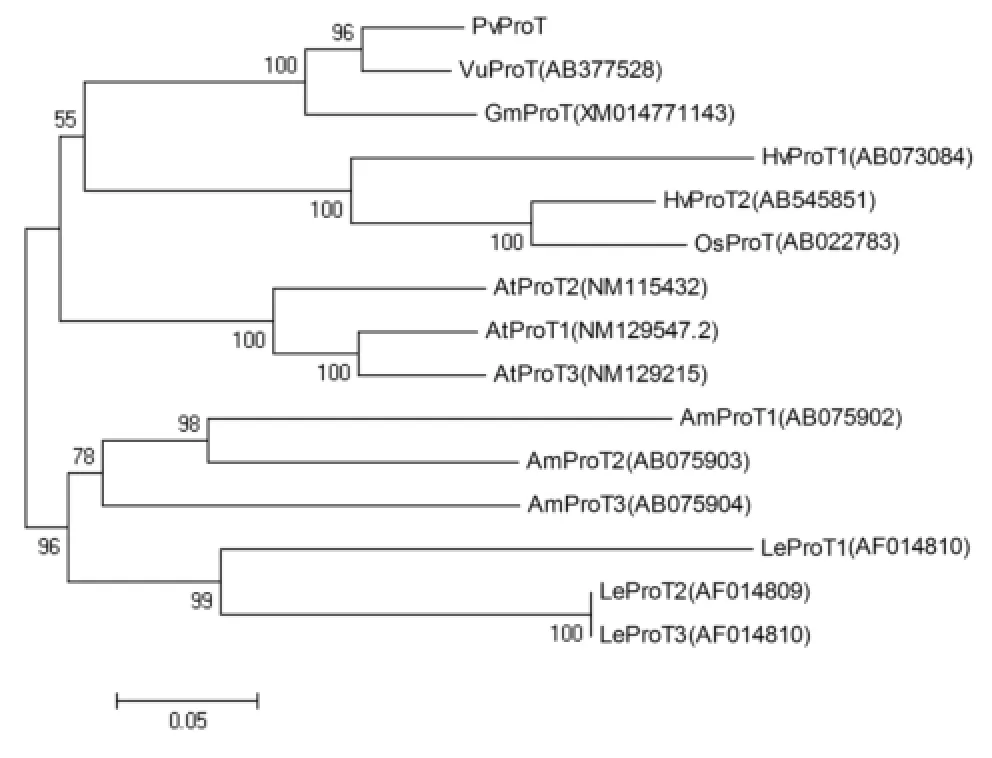
Fig.1-Phylogenetic analysis of angiosperm ProT proteins. Amino acid sequences of ProTs from barley(HvProT1-2),rice(OsProT1),Arabidopsis(AtProT1-3),tomato(LeProT1-3),and mangrove(AmBet/ProT1-3)were aligned with ClustalW.The trees were constructed with MEGA 4.0 by neighbor joining and 1000 bootstrap replications.
2.5.Construction of expression vector for PvProT in E.coli
The coding region of PvProT was amplified from the cDNA of common bean with primer pairs P2-BamH I(U 5′-GGA TCC ATT CGG TGG ATT CTC GTT CAT TC-3′)and P2-EcoR I(D 5′-GAA TTC ATA TTT TCT GAT CTG AGT GAC C-3′).PCR products were subcloned into pGETM and sequenced.The DNA fragments coveringthecodingregionof PvProT were prepared by digestion with BamH I and EcoR I and ligated into the corresponding site of the pRSETB vector.The resulting plasmid pRSETB-PvProT was transformed into E.coli MKH13 cells.Transformants were selected on LB agar containing 50 μg mL-1ampicillin.
2.6.Complementation test
Complementation testing on agar plates followed Waditee etal.[19]withslightmodification.E.colimkh13cellstransformed with pRSETB empty vector and pRSETB-PvProT were grown overnight at 37°C in LB medium(pH 7.0)containing ampicillin(100 μg mL-1).Cells were then spread on a 0.8%agar plate containing 0.8 mol L-1NaCl,1 mmol L-1IPTG,and 1 mmol L-1betaine or 1 mmol L-1proline and incubated at 37°C for 7 days.
2.7.Statistical analysis
Analyses of nucleotide and amino acid sequences were performed with DNAstar and DNAman.Statistical analysis was performed using SPSS statistical computer package(version 16.0;SPSS Inc.Chicago,USA).Mean and standard error(SD)values of three replicates were calculated and compared with those of the control or among treatments by analysis of variance to identify significant differences at P<0.01,followed by least significant difference tests.
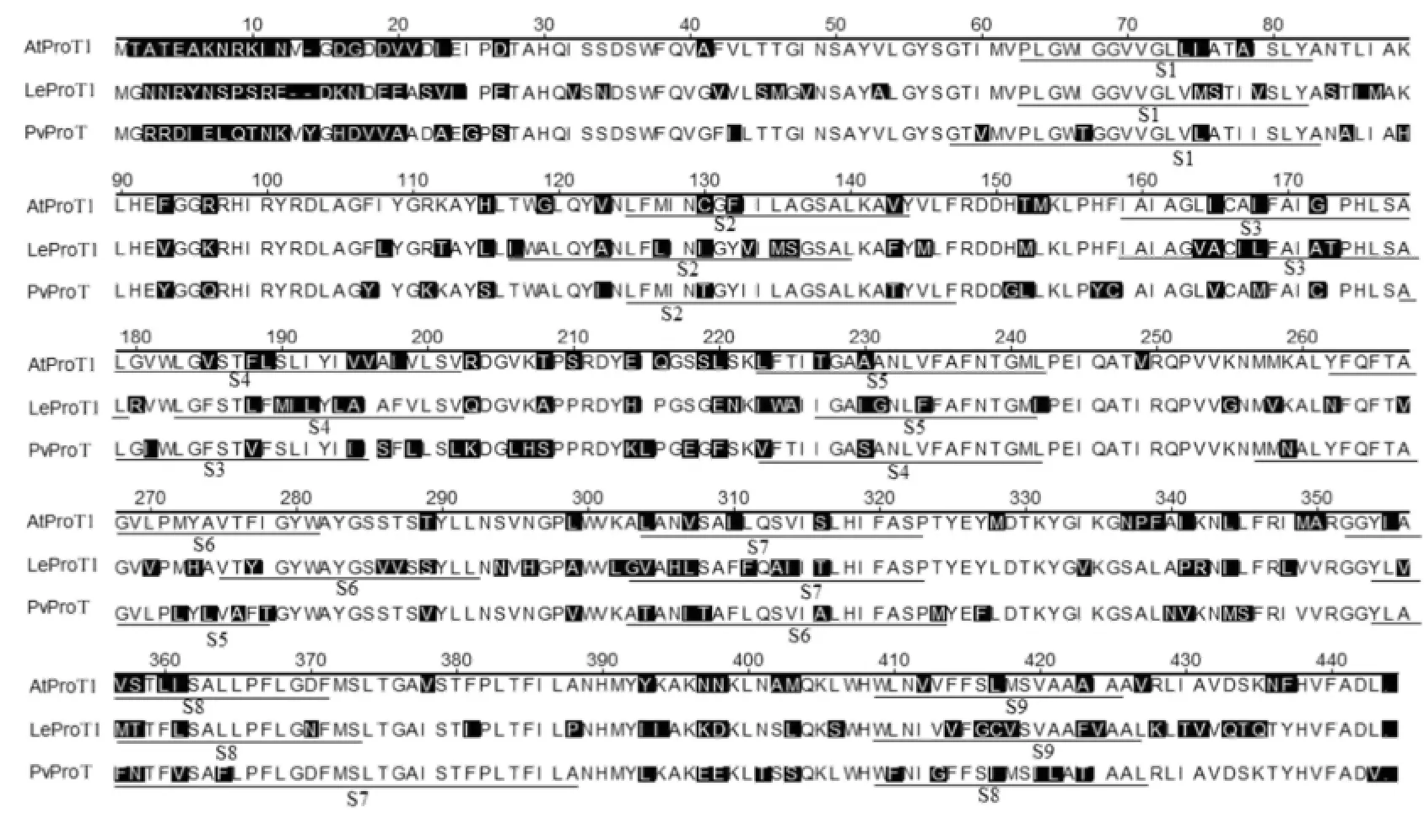
Fig.2-Prediction of putative membrane-spanning regions in the amino acid sequences of PvProT,AtProTl,and LeProTl. Underlined sequences labeled S1 to S9 represent predicted transmembrane regions.
3.Results
3.1.Cloning of ProT gene from common bean
cDNA was cloned from common bean using a candidate gene approach.The isolated cDNA was designated as PvProT.Thenucleotide sequence of the cDNA was 1675 bp in length and contained a 164-bp 3′untranslated region,a 180-bp 5′untranslated region,and a 1332-bp open reading frame encoding a polypeptide of 443 amino acid residues.The native molecular weight of the PvProT protein was estimated as 58.7 kDa.
In an alignment of the deduced amino acid sequence with those of 14 amino acid transporters from other angiosperms,PvProT showed higher identity with ProT proteins from two other leguminous plants,namely cowpea(Vigna unguiculata)VuProT(93.5%)and soybean(Glycine max)GmProT(89.8%).The PvProT protein showed 64.9%sequence similarity with OsProT,65.5%with HvProT2,and 59.7%with HvProTl.Given that common bean,mothbean,and soybean are leguminous dicotyledons,the above results suggest that ProTs evolved after the divergence of monocotyledonous and dicotyledonous plants. As reported previously[13,20],ProT members isolated from a single plant species always cluster together,indicating that duplication of the genes was a relatively recent event(Fig.1).
A hydropathy plot[21]indicated that the predicted PvProT protein was highly hydrophobic.The pattern of the hydropathy plot of PvProT was similar to those of AtProTl and LeProTl(Fig.2).Transmembrane prediction with the TMpred software[22]indicated that PvProT contained eight putative membranespanning regions.Both AtProTl and LeProTl were predicted tocontain nine transmembrane regions,asin previous reports[10,12].The third and fourth domains of AtProTl and LeProTl may represent a single region.Thus,PvProT,AtProTl,and LeProTl may be similar in containing eight membranespanning regions.
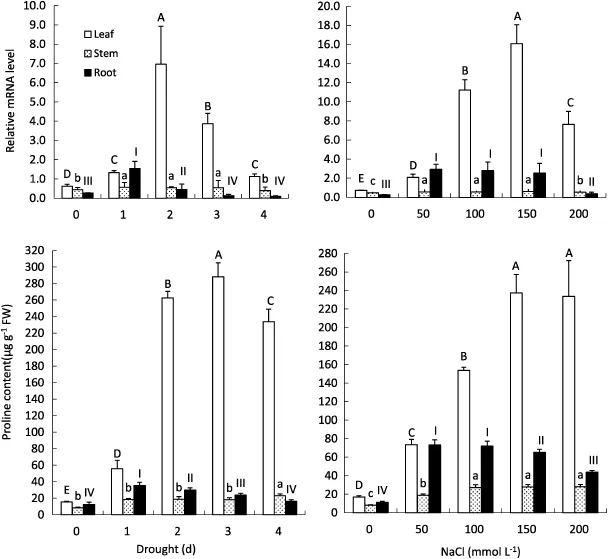
Fig.3-Changes in PvProT mRNA levels and proline concentration in common bean under salt and drought stress.The levels of PvProT mRNA and proline concentration were measured in the leaf,stem,and root.Data are shown as means±SE of three different measurements.Different lowercase letters,uppercase letters,and Roman numbers above different bars represent significance at the 0.01 probability level,respectively.
3.2.Effects of drought and salt stress on PvProT transcription
Real-time PCR analysis was performed to investigate the expression of PvProT in the leaf,stem and root of common bean plants under drought and salt stress.Upregulated expression of PvProT was observed during the drought stress period(Fig.3).
At all time points of drought stress,the level of PvProT transcripts in leaves of drought-stressed plants was significantly higher(P<0.01)than that of control plants(0 days).In drought-stressed plants treated for 1,2,3,and 4 days,the relative levels of PvProT mRNA in leaves were 2.1,11.0,6.1,and 1.8 times higher than that of the control(0 days).Significant(P<0.01)induction of PvProT expression(up to 5.7-fold)was detected in the roots of drought-stressed plants after 1 dayand then slowly decreased to 1.7 times that of control plants(2 days),and then decreased further to 0.4 times at 4 days. No significant accumulation of PvProT mRNA was detected in the stem during drought stress.
Transcription of PvProT in the leaves and roots of saltstressed plants was significantly(P<0.01)higher than that of control plants.The highest level of PvProT transcripts in the leaf was observed with 150 mmol L-1NaCl treatment,in which the relativetranscriptlevel was22.3-fold higherthanthatofcontrol plants.Under 100-150 mmol L-1NaCl treatment,transcription of PvProT in the roots was stable and was 10.5-fold higher than that of control plants.Although the PvProT mRNA level in the stems of salt-stressed plants was slightly higher than that of control plants,the difference was not significant.
In both drought-and salt-stressed plants,proline concentrations in leaves,roots,and stems of stressed plants were significantly(P<0.01)higher than that of the control plants. However,proline accumulation was not stable under stress treatment.In the later stages of drought and salt stress treatment,the proline concentration in leaves and roots,though not in stems,began to decrease(Fig.3).
3.3.Transcription of PvProT in drought-tolerant and droughtsensitive common bean
Two drought-tolerant landraces,F1372 and F5575,and two drought-sensitive landraces,F4409 and F4851,of common bean were used for further analysis of the relative expression level of PvProT(Fig.4).Following treatment with 20%PEG-6000 for 24 h,F4409 and F4851 wilted more severely than F1372 and F5575.The dehydration rate of F4409(21.10%)and F4851(24.95%)was higher than that of F1372(4.61%)and F5575(11.23%).Under 20%PEG-6000 treatment,proline concentration was higher in leaves of the drought-tolerant than in those of the drought-sensitive landraces.Drought stress also induced a significantly higher relative level of PvProT transcripts in the drought-tolerant than in the drought-sensitive landraces.These results suggested that drought-resistant genotypes might transport proline more frequently and efficiently than drought-sensitive genotypes.
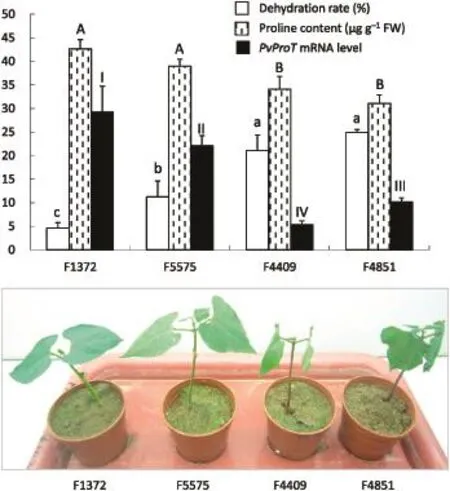
Fig.4-Relative level of PvProT mRNA,proline concentration,and the phenotypic response of 12-day-old seedlings of drought-tolerant(F1372 and F5575)and drought-sensitive(F4409 and F4851)landraces of common bean treated with 20%PEG-6000 for 24 h.
3.4.PvProT transports betaine and proline
The E.coli mutant mkh13,which is deficient in betT,putPA,proP,and proU genes,is unable to grow with proline or glycine betaine(GB)as the sole nitrogen source[23].Transformation of mkh13 with the empty expression vector(pRSETB)and the PvProT cDNA inserted in pRSETB(pRSETB-PvProT)showed that only PvProT permitted growth in the presence of selective concentrationsofprolineorglycinebetaine(Fig.5).Incontrast,mkh13 cells transformed with vector-alone pRSETB did not recover growth under either condition.These findings suggested that PvProT is a transporter for betaine and proline.
4.Discussion
Proline is an organic molecule that accumulates in plants exposed to stress conditions such as drought and salinity[24]. A correlation between proline accumulation and abiotic stress tolerance is indicated,based on findings for different plants and cell cultures[25].In accord with these previous investigations,the present study showed that drought-resistant strains(F1372 and F5575)of common bean accumulate higher proline concentrations than drought-sensitive landraces(F4409 andF4851)underdroughtstress.Thus,F1372andF5575,withthe higher concentration of proline acting as an osmoprotectant,showed superior capability to tolerate the resulting high osmotic potential.F4409 and F4851 wilted more severely than F1372 and F5575 when exposed to PEG for 24 h.
Among the leaf,root,and stem of common bean seedlings,the proline concentration and the level of PvProT mRNA were highest in the leaf.In addition,evaluation of PvProT gene expression profiles indicated that drought-tolerant genotypes showed higher expression levels of PvProT than did droughtsensitive landraces.These results suggested that following the expression increase of PvProT,proline transportation may increase rapidly.Fujiwara et al.[26]showed that HvProT2 functions as an influx transporter of proline localized in the mestome sheath and lateral root cap cells in barley.In tomato(Solanum lycopersicum),however,expression of LeProT1 was confined to pollen grains and was correlated with elevated proline concentrations[18].Raymond and Smirnoff[27]verified that increased accumulation of proline in the tip region of maize(Zea mays)roots was achieved by proline transport but not by de novo synthesis of proline.Although AtProT1 was expressed in all organs of the plant,the highest quantities were detected in the peduncle phloem,which enters the carpels,and expression was downregulated after fertilization[10].
Glycine betaine(betaine)is an important osmoprotectant in bacteria,algae,plants,and animals.In plants,betaine issynthesized in chloroplasts from choline via a two-step oxidation process and must be transported for a long distance in response to environmental stresses[20].Waditee et al.[19]reported that betaine-accumulating mangrove harbors betaine/ proline transporters that can efficiently take up betaine and proline with similar affinities.In the present study,although betaine content in common bean was not measured,complementation testing using E.coli mkh13 indicated that PvProT transports both proline and betaine.Three proline transporters of Arabidopsis transport the compatible solutes proline and glycine betaine as well as the stress-induced compound γ-aminobutyric acid,when expressed in heterologous systems[28].Expression of cDNA of EgProT1 in the E.coli mutant mkh13 indicates that the protein exhibits uptake activities for glycine betaine,choline,and proline[13].Barley HvProT1 is the only known transporter that recognizes proline but not betaine[29]. These findings imply that transportation of proline is the main function of proline transporters in plant organs.
Limited information about proline transportation in common bean ispresentlyavailable.The studyofPvProT expression in response to drought and salt stress thus contributes to the elucidation of the network of proline metabolic mechanisms in this important crop.The insights into proline transportation obtained in this study provide a basis for future studies on the improvement of drought and salt tolerance in common bean.
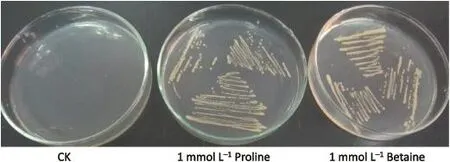
Fig.5-Complementation test of proline/betaine-deficient E.coli mkh13 mutant cells with PvProT.mkh13 mutant cells were grown for 3 days on agar medium supplemented with 0.8 mol L-1NaCl and 1 mmol L-1proline or 1 mmol L-1betaine as described in Materials and methods.The control represents mkh13 cells transformed with the pRSETB vector.
Acknowledgments
This work was supported by an earmarked fund for China Agriculture Research System(No.CARS-09),the Agricultural Science and Technology Innovation Program(ASTIP)of CAAS,and the Higher Education Institution Key Research Project Plan of Henan Province(No.15A210042).
R E F E R E N C E S
[1]Z.F.Pei,D.F.Ming,D.Liu,G.L.Wan,X.X.Geng,H.J.Gong,W.J. Zhou,Silicon improves the tolerance to water-deficit stress induced by polyethylene glycol in wheat(Triticum aestivum L.)seedlings,J.Plant Growth Regul.29(2010)106-115.
[2]P.B.Kavi Kishor,S.Sangam,R.N.Amrutha,P.S.Laxmi,K.R. Naidu,K.Rao,S.Rao,K.J.Reddy,P.Theriappan,N. Sreenivasulu,Regulation of proline biosynthesis,degradation,uptake and transport in higher plants:its implications in plant growthand abiotic stresstolerance,Curr.Sci.88(2005)424-438.
[3]Y.L.Yang,F.Yang,X.N.Li,R.X.Shi,J.Lu,Signal regulation of proline metabolism in callus of the halophyte Nitraria tangutorum Bobr grown under salinity stress,Plant Cell Tissue Organ Cult.112(2013)33-42.
[4]Y.Yoshiba,T.Kiyosue,T.Katagiri,H.Ueda,T.Mizoguchi,K. Yamaguchi-Shinozaki,Y.Harada,K.Shinozaki,Correlation between the induction of a gene for Δ1-pyrroline-5-carboxylate synthase and the accumulation of proline in Arabidopsis thaliana under osmotic stress,Plant J.7(1995)751-760.
[5]P.B.Kavi Kishor,Z.L.Hong,G.H.Miao,C.A.A.Hu,D.P.S.Verma,Overexpression of Δ1-pyrroline-5-carboxylate synthetase increases proline production and confers osmotolerance in transgenic plants,Plant Physiol.108(1995)1387-1394.
[6]E.Cominelli,C.Tonelli,Transgenic crops coping with water scarcity,New Biotechnol.27(2010)473-477.
[7]R.Pratelli,G.Pilot,Regulation of amino acid metabolic enzymes and transporters in plants,J.Exp.Bot.65(2014)5535-5556.
[8]J.Sánchez-Martín,J.Heald,A.Kingston-Smith,A.Winters,D. Rubiales,M.Sanz,L.A.J.Mur,E.Prats,A metabolomic study in oats(Avena sativa)highlights a drought tolerance mechanism based upon salicylate signalling pathways and the modulation of carbon,antioxidant and photo-oxidative metabolism,Plant Cell Environ.38(2015)1434-1452.
[9]T.Kiyosue,Y.Yoshiba,K.Yamaguchi-Shinozaki,K.Shinozaki,Anucleargeneencodingmitochondrialprolinedehydrogenase,an enzyme involved in proline metabolism,is up-regulated by proline but down-regulated by dehydration in Arabidopsis,Plant Cell 8(1996)1323-1335.
[10]D.Rentsch,H.Brigitte,S.Elmon,B.F.Wolf,Salt stress-induced proline transportem and salt stress-repressed broad specificity amino acid permeases identified by suppression of a yeast amino acid permease-targeting mutant,Plant Cell 8(1996)1437-1446.
[11]A.Ueda,W.Shi,T.Shimada,H.Miyake,T.Takabe,Altered expression of barley proline transporter causes different growth responses in Arabidopsis,Planta 227(2008)277-286.
[12]R.Schwacke,S.Grallath,K.E.Breitkreuz,E.Stransky,H. Stransky,W.B.Frommer,D.Rentsch,LeProT1,a transporter for proline,glycine betaine,and gamma-amino butyric acid in tomato pollen,Plant Cell 11(1999)377-391.
[13]N.Yamada,C.U.Suriyan,K.Hakuto,P.Worrawat,T.Yoshito,K.Chalermpol,T.Teruhiro,Isolation and characterization of proline/betaine transporter gene from oil palm,Tree Physiol. 31(2011)462-468.
[14]J.B.Chen,S.M.Wang,R.L.Jing,X.G.Mao,CloningthePvP5CSgene from common bean(Phaseolus vulgaris L.)and its expression patterns under abiotic stresses,J.Plant Physiol.166(2009)12-19.
[15]J.B.Chen,X.Y.Zhang,R.L.Jing,W.B.Matthew,X.G.Mao,S.M. Wang,Cloning and genetic diversity analysis of a new P5CS gene from common bean(Phaseolus vulgaris L.),Theor.Appl. Genet.120(2010)1393-1404.
[16]J.B.Chen,Y.N.Cao,Z.Y.Zhang,S.M.Wang,J.Wu,L.F.Wang,Cloning of the OAT gene and the correlation between its expression and drought tolerance in Phaseolus vulgaris L. J.Integr.Agric.5(2016)60345-60347.
[17]L.S.Bates,S.P.Waldren,I.D.Teare,Rapid determination of free proline for water stress studies,Plant Soil 39(1973)205-207.
[18]K.J.Livak,T.D.Schmittgen,Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCTmethod,Methods 25(2001)402-408.
[19]R.Waditee,T.Hibino,Y.Tanaka,T.Nakamura,A. Incharoensakdi,S.Hayakawa,S.Suzuki,Y.Futsuhara,Y. Kawamitsu,T.Takabe,T.Takabe,Functional characterization of betaine/proline transporters in betaine-accumulating mangrove,J.Biol.Chem.277(2002)18373-18382.
[20]S.Grallath,T.Weimar,A.Meyer,C.Gumy,M.Suter-Grotemeyer,J.M.Neuhaus,D.Rentsch,The AtProT family,compatible solute transporters with similar substrate specificity but differential expression patterns,Plant Physiol.137(2005)117-126.
[21]J.Kyte,R.F.Doolittle,A simple method for displaying the hydropathic character of a protein,J.Mol.Biol.157(1982)105-132.
[22]K.Hofmann,W.Stoffel,PROFILEGRAPH:an interactive graphical tool for protein sequence analysis,Bioinformatics 8(1992)331-337.
[23]M.Haardt,B.Kempf,E.Faatz,E.Bremer,The osmoprotectant proline betaine is a major substrate for the binding-protein-dependent transport system ProU of Escherichia coli K-12,Mol.Gen.Genet.246(1995)783-786.
[24]V.H.Lokhande,T.D.Nikam,S.Penna,Biochemical,physiological and growth changes in response to salinity in callus cultures of Sesuvium portulacastrum L,Plant Cell Tissue Organ Cult.102(2010)17-25.
[25]N.Verbruggen,X.J.Hua,M.May,M.Van,Environmental and developmental signals modulate proline homeostasis: evidence for a negative transcriptional regulator,Proc.Natl. Acad.Sci.U.S.A.93(1996)8787-8791.
[26]T.Fujiwara,S.Mitsuya,H.Miyake,T.Hattori,T.Takabe,Characterization of a novel glycinebetaine/proline transporter gene expressed in the mestome sheath and lateral root cap cells in barley,Planta 232(2010)133-143.
[27]M.J.Raymond,N.Smirnoff,Proline metabolism and transport in maize seedlings at low water potential,Ann.Bot.89(2002)813-823.
[28]S.Lehmann,C.Gumy,E.Blatter,S.Boeffel,W.Fricke,D. Rentsch,In planta function of compatible solute transporters of the AtProT family,J.Exp.Bot.62(2011)787-796.
[29]A.Ueda,W.M.Shi,K.Sanmiya,M.Shono,T.Takabe,Functional analysis of salt-inducible proline transporter of barley roots,Plant Cell Physiol.42(2001)1282-1289.
9 March 2016
in revised form 15 May 2016 Accepted 20 June 2016
.Tel.:+86 10 62175628.
E-mail address:shuminwang@caas.cn(S.Wang).
Peer review under responsibility of Crop Science Society of China and Institute of Crop Science,CAAS.1Jibao Chen and Jing Wu contributed equally to this article.
http://dx.doi.org/10.1016/j.cj.2016.05.009
2214-5141/©2016 Crop Science Society of China and Institute of Crop Science,CAAS.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
杂志排行
The Crop Journal的其它文章
- Development of a panel of unigene-derived polymorphic EST-SSR markers in lentil using public database information
- Characterization of chickpea germplasm conserved in the Indian National Genebank and development of a core set using qualitative and quantitative trait data
- Achievements and prospects of grass pea(Lathyrus sativus L.)improvement for sustainable food production
- Nutritional composition and antioxidant activity of twenty mung bean cultivars in China
- Genetics of seed flavonoid content and antioxidant activity in cowpea(Vigna unguiculata L.Walp.)
- Large-scale evaluation of pea(Pisum sativum L.)germplasm for cold tolerance in the field during winter in Qingdao
