Development of a panel of unigene-derived polymorphic EST-SSR markers in lentil using public database information
2016-10-24DbjyotSnGuptaPnCnGauravSablokDlTavarajaPusparajaTavarajaClarcCoynSvKumarMcalBaumRbccaMcG
Dbjyot Sn Gupta,Pn Cn,Gaurav Sablok,Dl Tavaraja, Pusparaja Tavaraja,Clarc J.Coyn,SvKumar,Mcal Baum,Rbcca J.McG*
aDepartment of Plant Sciences,North Dakota State University,Fargo,ND 58108,USA
bICAR-ARS,Indian Institute of Pulses Research,Kanpur,208024,UP,India
cDivision of Plant Sciences,University of Missouri,Columbia,MO 65211-7140,USA
dDepartment of Biodiversity and Molecular Ecology,Research and Innovation Centre,Fondazione Edmund Mach,Via E.Mach 1,38010 San Michele all'Adige,Trento,Italy
eAgricultural and Environmental Sciences,Clemson University,Clemson,SC 29634,USA
fInternational College Beijing,China Agricultural University,Beijing,China
gUSDA-ARS,Western Regional Plant Introduction Station,Washington State University,Pullman,WA 99164,USA
hBIGM Program,International Center for Agricultural Research in the Dry Areas(ICARDA),Rabat-Instituts,Rabat,Morocco
iBIGM Program,International Center for Agricultural Research in the Dry Areas(ICARDA),Beirut,Lebanon
jUSDA-ARS,Grain Legume Genetics and Physiology Research Unit,Washington State University,Pullman,WA 99164,USA
Development of a panel of unigene-derived polymorphic EST-SSR markers in lentil using public database information
Debjyoti Sen Guptaa,b,1,Peng Chengc,1,Gaurav Sablokd,Dil Thavarajahe, Pushparajah Thavarajahf,Clarice J.Coyneg,ShivKumarh,Michael Baumi,Rebecca J.McGeej,*
aDepartment of Plant Sciences,North Dakota State University,Fargo,ND 58108,USA
bICAR-ARS,Indian Institute of Pulses Research,Kanpur,208024,UP,India
cDivision of Plant Sciences,University of Missouri,Columbia,MO 65211-7140,USA
dDepartment of Biodiversity and Molecular Ecology,Research and Innovation Centre,Fondazione Edmund Mach,Via E.Mach 1,38010 San Michele all'Adige,Trento,Italy
eAgricultural and Environmental Sciences,Clemson University,Clemson,SC 29634,USA
fInternational College Beijing,China Agricultural University,Beijing,China
gUSDA-ARS,Western Regional Plant Introduction Station,Washington State University,Pullman,WA 99164,USA
hBIGM Program,International Center for Agricultural Research in the Dry Areas(ICARDA),Rabat-Instituts,Rabat,Morocco
iBIGM Program,International Center for Agricultural Research in the Dry Areas(ICARDA),Beirut,Lebanon
jUSDA-ARS,Grain Legume Genetics and Physiology Research Unit,Washington State University,Pullman,WA 99164,USA
A R T I C L E I N F O
Article history:
29 June 2016
Accepted 11 July 2016
Available online 26 July 2016
Lens culinaris
EST-SSRs
Functional annotation
Unigene sequences
EST database
Genetic resources
Lentil(Lens culinarisMedik.),a diploid(2n=14)witha genomesizegreaterthan4000 Mbp,isan important cool season food legume grown worldwide.The availabilityof genomic resources is limited inthiscropspecies.Theobjectiveof thisstudy wastodeveloppolymorphicmarkersin lentil using publicly available curated expressed sequence tag information(ESTs).In this study,9513 ESTs were downloaded from the National Center for Biotechnology Information(NCBI)database to develop unigene-based simple sequence repeat(SSR)markers.The ESTs were assembled into 4053 unigenes and then analyzed to identify 374 SSRs using the MISA microsatellite identification tool.Among the 374 SSRs,26 compound SSRs were observed. Primer pairs for these SSRs were designed using Primer3 version 1.14.To classify the functional annotation of ESTs and EST-SSRs,BLASTx searches(using E-value 1×10-5)against the public UniProt(http://www.uniprot.org/)and NCBI(http://www.ncbi.nlh.nih.gov/)databases were performed.Further functional annotation was performed using PLAZA(version 3.0)comparative genomics and GO annotation was summarized using the Plant GO slim category.Among the synthesized 312 primers,219 successfully amplified Lens DNA.A diverse panel of 24 Lens genotypes was used to identify polymorphic markers.A polymorphic set of 57 markers successfully discriminated the test genotypes.This set of polymorphic markers with functional annotation data could be used as molecular tools in lentil breeding.
Production and hosting by Elsevier B.V.on behalf of Crop Science Society of China and
Institute of Crop Science,CAAS.This is an open access article under the CC BY-NC-ND
license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
Lentil(Lens culinaris L.Medik.)is a nutritious food legume crop grown throughout the world.Primary production regions include Canada,Australia,northwestern USA,Turkey,Syria,and the Indian subcontinent(Nepal,India,and Bangladesh). World annual production is nearly five million tons[1].Lentil is classified into several market classes,including extra small red,small red,large red,small green,medium green,large green,Spanish brown,zero tannin,and Puy.
Lentil originated in the Fertile Crescent(southwest Asia and Mediterranean region)and is believed to be one of the earliest domesticated food crops[2].The cultivated lentil,L.culinaris,has two types,macrosperma and microsperma,based on seed and pod characteristics[3].Like other food legumes,lentil has a narrow genetic base.Realization of potential yield is limited by various biotic and abiotic stresses such as foliar and root diseases,highorlowtemperature,soilpH(<5.4),and waterlogging.Optimization of crop management is also important,as weed management,water availability,and soil fertility vary among growing environments.Breeding programs worldwide are working to breed high-yielding lentil cultivars with resistance to one or more of these stresses.Many breeding programs have implemented marker-assisted selection to speed up the selection process.
Availability of molecular markers and their ease of use in large breeding programs is a priority for many crop species. However,because of the unavailability of a full genome sequence as well as the complexity of the large(4063 Mbp)genome[4],the number of available polymorphic markers is limited in lentil.Hamweigh et al.[5]developed 14microsatellite markers from a genomic library developed from the lentil cultivar“ILL5588”.The genetic diversity index calculated from the number of alleles amplified was high and markers could accurately discriminate cultivated from wild types.In another study,Kaur et al.[6]developed expressed sequence tag-simple sequence repeat(EST-SSR)markers by transcriptome sequencing of lentil and validated 79 polymorphic EST-SSRs among 13 lentil genotypes including one Lens nigricans accession. Verma et al.[7]developed EST-SSRs by transcriptome sequencing of the lentil genotype“Precoz”[8]and validated 54 polymorphic EST-SSRs among 22 lentil genotypes including one L.culinaris subsp.orientalis and two Lens lamottei genotypes.
The total number of lentil ESTs(9513)in the National Center for Biological Information (NCBI)database has remained constant.Development of genomic or transcriptome libraries is expensive and time-consuming.Researchers working in various crop species including rice[9],wheat[9],maize[10],chickpea[10],faba bean[11],pea[12],tea tree[13],and Medicago[14]have developed polymorphic markers using sequence information available in public databases.
TheuseofgenicSSRmarkersorEST-SSRsisimportantfroma breeding point of view.Despite recent advances in molecular markers such as single-nucleotide polymorphisms(SNPs)or DNA array-based markers,SSRs hold promise as breederfriendly markers involving limited technical or operating difficulties.SSR markers are reproducible and PCR-based,resulting in easy application in breeding programs for marker-assisted selection or prediction of breeding values.Public databases such as NCBI NR(http://www.ncbi.nlm.nih.gov/),UniProt(http:// www.uniprot.org/),and TAIR(http://www.arabidopsis.org/)can be used to functionally annotate the ESTs or EST-SSRs.This algorithm-based or alignment-based prediction of gene functions can be verified in trait-specific cases.The synchronization between functional annotation and wet-lab validation depends largely on the standard of draft sequence available.Functional annotation of the SSRs provides an opportunity for expression analysis of specific genes.
The objectives of this study were to(1)develop polymorphic SSR markers in lentil using EST sequences,(2)validate polymorphic EST-SSR markers in a diverse panel of Lens genotypes including wild lentil species,and(3)functionally annotate the EST-SSRs using public protein databases.

Table 1-Summary of data mining of unigene sequences of Lens culinaris.
2.Materials and methods
2.1.EST sequence assembly,SSR detection,and functional annotation
Microsatellite or SSRs were developed from 9513 expressed sequence tags(ESTs)downloaded from the NCBI database using the queries“Lens culinaris”[Organism]OR Lens culinaris[All Fields]AND “Lens culinaris”[porgn](Table 1).ESTs representing(“Lens culinaris/Colletotrichum truncatum mixed EST library”[porgn:__txid880151])were excluded and only L.culinaris-specific ESTs were used for the analysis.The downloaded ESTs were cleaned of contamination using UniVec(http://www.ncbi.nlm.nih.gov/tools/vecscreen/univec).Cleaned ESTs were assembled using the Overlap-Layout-Consensus assembler MIRA(parameters:job=denovo,est,accurate,454 using the-notraceinfo option)[15].Following the MIRA assembly,unigenes were created using CAP3[16]with parameters -p 95,-o 49,and-t 10,000 as previously described[17-19].In addition to the parameters described in Zheng et al.[18],the parameter-t was assigned a value of 10,000,a choice that can substantially improve the quality of the assembly using the maximumavailablememory.Thisdecisionavoidedthe misassembly of the ESTs and formation of counterfeit long assemblies,as previously suggested[18,19].The assembled unigenes were searched for SSRs using MISA[20](http://pgrc. ipk-gatersleben.de/misa/).SSRs were defined by a minimum repeat sequence of 10 nucleotides as mono-,a sequence of six consecutive repeat units as di-,and a sequence of five repeat units for tri-,tetra-,penta-and hexanucleotide sequences.To identify and classify compound repeats,theminimum distancebetween two repetitive units was kept at≤100 bp as suggested in MISA[20].Open reading frames were extracted from the assembled unigenes using the getorf function of the EMBOSS package(http://emboss.sourceforge.net/).
2.2.Database mining
2.2.1.Development of EST-SSRs and primer design
Following the identification of SSRs,primer pairs were designed using Primer3 version 1.1.4(http://primer3.sourceforge.net/)withminimumandmaximumampliconsize100-300 bp,primer size(minimum,optimum,and maximum)18-27 bp,primer Tm(minimum,optimum,and maximum)57-63°C,primer GC content 30-70%,CG clamp 0,maximum end stability 250,maximum Tmdifference 2,maximum self-complementarity 6,maximum 39 self-complementarity 3,maximum Ns accepted 0,and maximum poly-X 5.
2.2.2.Functional annotation of unigenes and EST-SSRs
Functional annotation and gene ontology of the ESTs and EST-SSRs were performed using BLASTx searches(E-value,1×10-3)againstGenBank (http://www.ncbi.nlm.nih.gov/),UniProt(http://www.uniprot.org/),and TAIR(https://www. arabidopsis.org/)databases.Additional functional annotation and gene ontology were obtained using FastAnnotator[21].GO annotations obtained were further analyzed using GO-SLIM(Plant)(http://www.geneontology.org/ontology/subsets/goslim_ plant.obo)and functional GO-SLIM categories were defined.
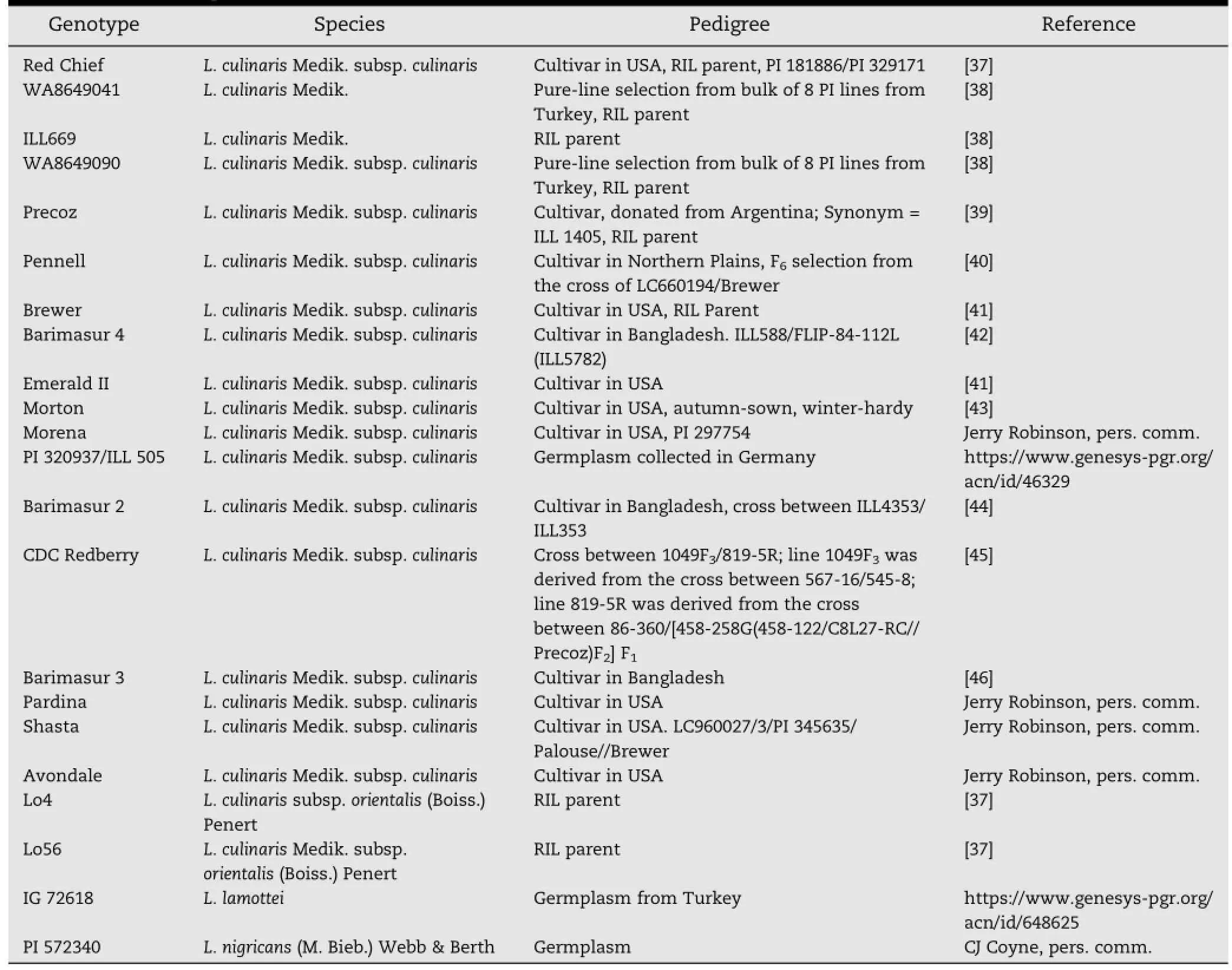
Table 2-Details of plant materials used.
2.3.Plant materials and DNA extraction
Four Lens genotypes were used for initial screening of 312 primers,including three L.culinaris(Red Chief,ILL669,and WA8649041)and one L.nigricans(PI 572340)genotype.A diverse panel of 22 Lens genotypes,consisting of L.culinaris advanced breeding lines,parents of mapping populations,wild taxa,and genotypes of L.nigricans,L.culinaris ssp.orientalis,and L.lamottei was used to identify polymorphic markers among primers amplifying Lens DNA(Table 2).DNA samples were extracted from individual plant leaf tissue(100 mg)when seedlings were twoweeks oldusing a DNeasy Plant MiniKit(QIAGEN,Valencia,CA,USA).The DNA concentrations of the extracted samples were recorded using a Nanodrop 2000c spectrophotometer(Nanodrop,Wilmington,DE USA).The extracted DNA samples were diluted to a uniform concentration of 20 μg μL-1for successful PCR amplification.
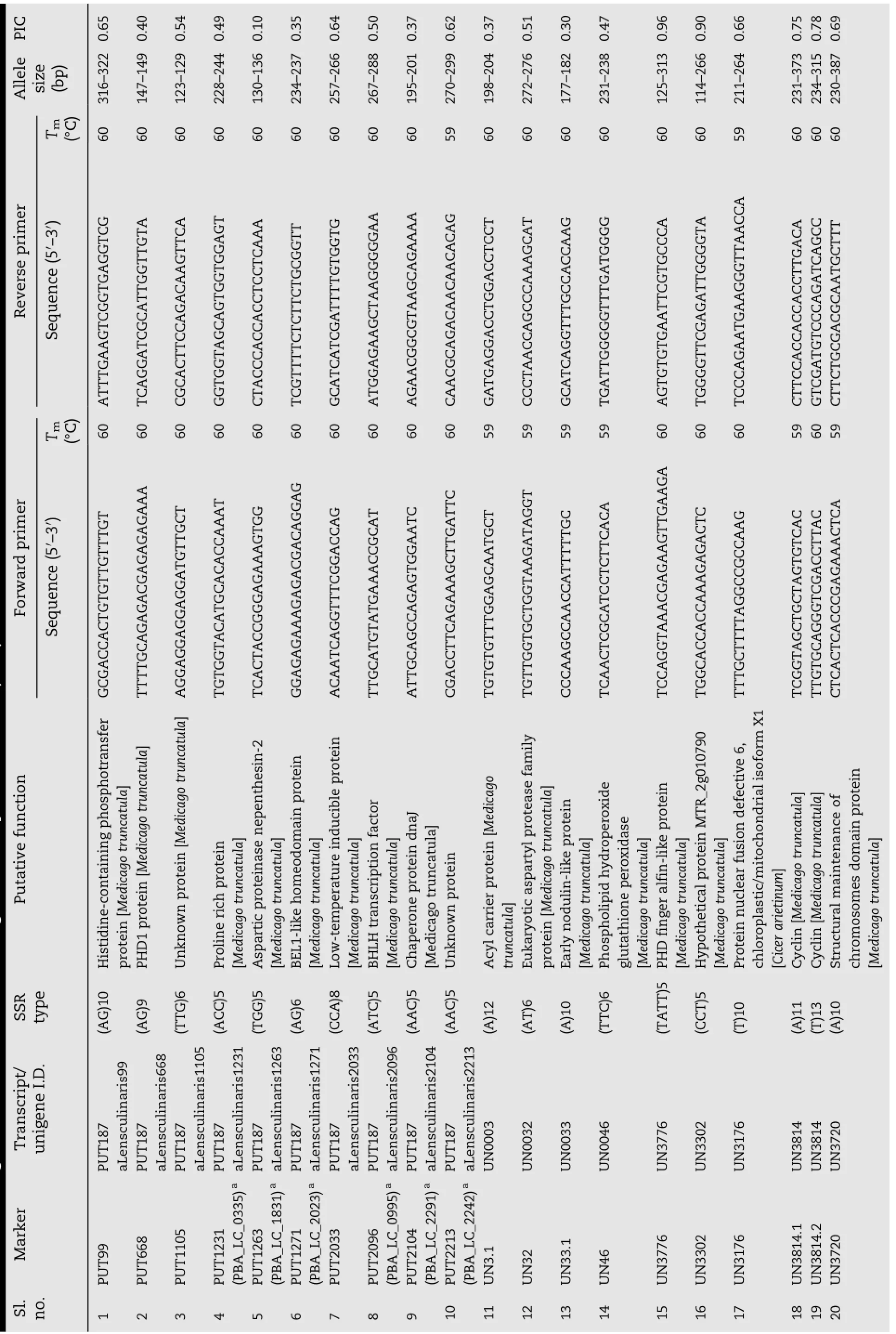
Table 3 - Tm, allele size, and polymorphism information content (PIC) of expressed sequenced tagged-simple sequence repeat (EST-SSRs) polymorphic primers; putative functions were assigned based on BLASTx search against the nr protein database (NCBI).

(continued on next page)
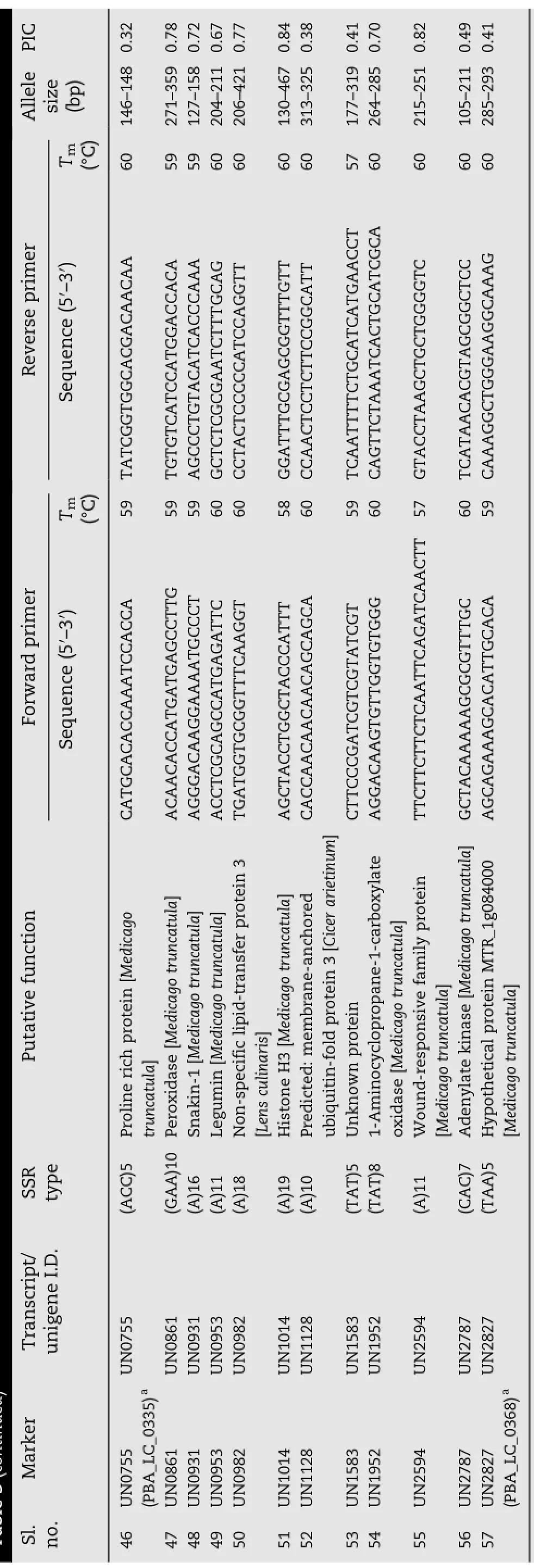
Table 3 (continued)
2.4.PCR amplification
Three hundred and twelve primer pairs were synthesized by Eurofins MWG Operon(Louisville,KY,USA)and used in this study.PCR reactions(25 μL volume)were conducted in an ABI 7500(Applied Biosystems,Foster,CA,USA)thermocycler,with each reaction containing 2.5 μL of Taq buffer,1.5 μL of MgCl2(25 mmol L-1),0.20 mmol L-1of each dNTP(all from Promega,Madison,WI,USA),0.50 mmol L-1of each primer(Eurofins MWG Operon),0.25 μL of Hot Start Taq polymerase(Promega)and 20 ng of template DNA.For initial screening of primers,touchdown PCRs were performed using DNA from four lentil genotypes and the following program:94°C for 3 min,followed by 18 cycles of 94°C for 50 s,65-55°C for 50 s,and 72°C for 50 s,followed by 20 cycles of 94°C for 50 s,55°C for 50 s,and 72°C for 50 s,and a final elongation of 72°C for 7 min.The PCR products were resolved in 2%agarose gels(molecular biology grade,Sigma,USA)and bands were scored using a gel documentation system.Primers amplifying Lens DNA were validated on a set of 22 diverse Lens genotypes in an ABI 7500 thermocycler using the following program:94°C for 5 min,followed by 42 cycles of 94°C for 1 min,50°C for 1 min,and 72°C for 1 min,followed by a final elongation step of 72°C for 5 min.Forward primers were tagged with M13 sequence(5′-CACGACGTTGTAAAACGAC-3′)at the 5′end. Four dyes were used to set up the multiplex PCR reactions. PCR products were separated using an ABI 3730xl(Applied Biosystems,Foster,CA,USA)according to the manufacturer's instructions with the addition of an ABI GeneScan LIZ500 size standard,and amplification product sizes were determined with GeneMapper v3.7 software(Applied Biosystems).
3.Results
3.1.Assembly and SSR detection
A set of 9513 ESTs were downloaded in FASTA file format from NCBI[22]and clustered by an identity of 0.95 into 4106 unigene sequences.Unigenes shorter than 100 bp were removed and the homopolymer ends of unigenes longer than 100 bp were trimmed.MIRA assembly of the 9513 EST sequences ultimately generated 4053 unigene sequences.The total length of the analyzed sequences was 2,574,487 bases. MIRA detected 374 SSR-bearing EST sequences among these unigenes.Of these EST-SSRs there were 36 sequences with more than one SSR.Also,26 compound SSRs were observed. For further analysis,348 EST-SSRs were chosen.Using Primer3,primer pairs were designed for these 348 EST-SSRs(Table S1).In addition,279 primer pairs(Table S2)were designed based on the plant GDB assembly(version 187a)of L.culinaris(http://www.plantgdb.org/download/download. php?dir=/Sequence/ESTcontig/Lens_culinaris/current_version). These were designed based on the detected EST-SSRs,and were further e-validated using iPCRessipcress software(http://www.ebi.ac.uk/about/vertebrate-genomics/software/ ipcress-manual).Primers co-amplifying products are listed in Table S2.In the validation experiment,312 primers were used: 48 primers from the e-validated list and 264 primers from Table S1.E-validated primers are coded with prefix“PUT”and other primers with“UN”(Table 3).
3.2.Structural and functional annotation of ESTs and EST-SSRs
Contig length ranged between 199 and 2599 bp(Fig.S1).The most prevalent contig length was 600-799 bp,followed by 400-599 and 800-999.Functional GO-SLIM analysis showed that the distribution of unigenes among GO,Domain,and Enzyme categories was 55.57%,39.91%,and 4.52%,respectively(Fig.S2).This distribution was consistent with the categorization of the EST-SSRs into functional GO,Domain,and Enzyme categories,which accounted for 54.25%,42.38%,and 3.70%,respectively(Fig.S3).In the GO category Biological Process,the first four processes were oxidation-reduction process,ribosome biogenesis,translation and regulation of transcription,and DNA dependent(Fig.S4).A similar trend was observed using GO Biological Process analysis of the ESTSSRs,in which the ranking of processes was as follows: oxidation-reduction process,regulation of transcription,DNA dependent,ribosome biogenesis,and translation(Fig.S5).The first four functions of the unigenes in the GO category Molecular Function were DNA binding,nutrient reservoir activity,structural constituent of ribosome,and zinc ion binding(Fig.S6).However,in the GO category Molecular Function,for the EST-SSRs,the first four functions were ATP binding,DNA binding,structural constituent of ribosome,and zinc ion binding(Fig.S7).In the GO category Cellular Process,the first four functions of the unigenes were nucleus,cytosol,plasma membrane and chloroplast(Fig.S8)and for the ESTSSRs the first four functions were cytosol,plasma membrane,nucleus,and integral to membrane(Fig.S9).
3.3.Frequency and distribution of EST-SSRs
The frequency of SSRs was 6.89 per kb of sequence analyzed(374 SSRs/2575 kb of sequence).There were 21 SSR repeat patterns(Fig.S10).The most prevalent were trinucleotide,followed by mono-,tetra-,andpentanucleotide repeatpatterns. The most frequently observed repeat was AG/CT,followed by AAG/CTT,AAC/GTT,ATC/ATG,and AT/AT(Fig.S10).
3.4.Validation of EST-SSRs
Amongthesynthesized312primers,219successfully amplified Lens DNA.A diverse panel of 22 Lens genotypes,consisting of L.culinaris advanced breeding lines,parents of mapping populations,wild taxa,and genotypes of L.nigricans,L.culinaris ssp.orientalis,and L.lamottei was tested to identify polymorphic markers.A total of 57 polymorphic primers were found.The number of alleles amplified ranged from 2 to 17 for each primer and the polymorphic information content(PIC)ranged between 0.10 and 0.91.The average number of alleles produced per primer was seven.
4.Discussion
MIRA assembly is very flexible.Short reads,such as ESTs,can easily be assembled into contigs and specific trimming further improves the quality of the sequences(http://mira-assembler. sourceforge.net/docs/DefinitiveGuideToMIRA.html)[23].Thenumber of SSR-containing sequences detected was very high compared to those in other studies;however,they yielded fewer polymorphic markers.These polymorphic markers successfully discriminated the test genotypes and grouped genetically more closely related individuals.Simple sequencebased markers are the most robust and easy to use in marker systems.Moreover,sequencing-based gel separation technologies can now detect differences of a very few bases among alleles.It can be seen from the results of the present experiment that each polymorphic marker generated multiple alleles and that for several,up to 17 alleles were observed. Similar results were obtained with other crops for which EST databases were used to develop robust genic SSR markers[10-14,29].Recently,Kumar et al.[25]reviewed the recent development of genic SSR markers in lentil[6,7,26,27]and Andeden et al.[28]developed 78 polymorphic SSR markers in lentil.However,the number of polymorphic genic SSR markers is still limited.Kaur et al.[6]validated a subset of de novo discovered 192 EST-SSR markers across a panel of 12 cultivated lentil genotypes,observing 47.5%polymorphism using a set of 2393 EST-SSR markers.Kaur et al.[26]found 40 polymorphic markers after testing 516 EST-SSRs.Andeden et al.[28]developed(CA)n,(GA)n,(AAC)n,and(ATG)n repeatenriched libraries and by sequencing these libraries found 78 polymorphic SSR markers using a set of 15 Turkish lentil genotypes.They observed 21.6%polymorphism (of the 360 primers validated,78 were polymorphic).In the present study,a test of 219 markers on 22 cultivated and wild lentil genotypes yielded 26%polymorphism.Verma et al.[7]reported 42.59%polymorphism in 54 markers tested on 22 lentil,Medicago,Glycine,and Vigna genotypes.The inclusion of additional genera contributed to the high percentage of polymorphism that they reported.The use of SSR markers for diversity analysis or grouping of genotypes based on genetic relatedness in lentil or other closely related food legumes has been reported by several workers[29-32].Kaur et al.[6]and Verma et al.[7]also found comparable grouping ability of the test polymorphic markers.Verma et al.[7]and Andeden et al.[28]reported a lower number of alleles amplified per locus(2.3 and 5.1,respectively)than found in our study(7 alleles).Wong et al.[33]classified four gene pools in lentil using genotyping by sequencing(GBS)of 60 genotypes.These were primary,secondary,tertiary,and quaternary gene pools,formed by L.culinaris/Lens orientalis/ Lens tomentosus,L.lamottei/Lens odemensis,Lens ervoides,and L. nigricans,respectively.
Thedistributionsoffunctionalannotationcategories were dissimilar between the total ESTs and EST-SSRs.It is noteworthy that reducing the detail of the GO categories improved the authenticity of the annotation data.It was observed that most functional annotations remain the same between the unigenes and EST-SSRs.Functional annotations of the EST-SSR flanking regions indicated the involvement of the translated portion of the genome.This finding is important for the development of functional markers in lentil. The lentil genome sequencing project is under way.The most recent draft(version 0.7)has approximately 150×coverage,producing scaffolds covering about half of the genome. The initial assembly resulted in useful SNPs suitable for marker-assisted selection[34].
5.Conclusions
A polymorphic set of 57 markers was developed in lentil.Of these,14 amplified the same SSRs reported by Kaur et al.[6]. The markers were further validated among diverse lentil genotypes.They could be used by the lentil research community for molecular breeding.Development of dense genetic maps is a prerequisite for adoption of genomic tools in lentil[35]and recently EST-SSR and SNPs were mapped in lentil[26,36].Lists of unigene sequences for the polymorphic markers are available in the Cool Season Food Legume database(https://www.coolseasonfoodlegume.org/).
Supplementary data for this article can be found online at http://dx.doi.org/10.1016/j.cj.2016.06.012.
Acknowledgments
Financial assistance from ICARDA,Morocco,in the form of a brief project,and grant support from the Northern Pulse Growers Association and the USA Dry Pea and Lentil Council are gratefully acknowledged.Debjyoti Sen Gupta thanks the Indian Council of Agricultural Research,New Delhi,India for the ICAR International Fellowship for Doctoral Study.
R E F E R E N C E S
[1]FAOSTAT,Database,FAO,Rome,2013,Available at http://faostat.fao.org,(Accessed on November 12,2015).
[2]S.S.Yadav,A.Z.Rizvi,M.Manohar,A.K.Verma,R.Shrestha,C. Chen,G.Bejiga,W.Chen,M.Yadav,P.N.Bahl,Lentil growers and production systems around the world,in:S.S.Yadav,L. McNeil,P.C.Stevenson(Eds.),Lentil:An Ancient Crop of Modern Times,first ed.Springer,The Netherlands,2007.
[3]H.Barulina,Lentils of the USSR and Other Countries,in: Bulletin of Applied Botany,Genetics and Plant Breeding Supplement,USSR Institute of Plant Industry of the Lenin Academy of Agricultural Science,Leningrad,USSR,1930 265-304.
[4]K.Arumuganathan,E.D.Earle,Nuclear DNA content of some important plant species,Plant Mol.Biol.Report 93(1991)208-218.
[5]A.Hamwieh,S.M.Udupa,A.Sarkar,C.Jung,M.Baum,Development of new microsatellite markers and their application in the analysis of genetic diversity in lentils,Breed.Sci.59(2009)77-86.
[6]S.Kaur,N.O.Cogan,L.W.Pembleton,M.Shinozuka,K.W. Savin,M.Materne,J.W.Forster,Transcriptome sequencing of lentil based on second-generation technology permits large-scale unigene assembly and SSR marker discovery,BMC Genomics 12(2011)265.
[7]P.Verma,N.Shah,S.Bhatia,Development of an expressed gene catalogue and molecular markers from the de novo assembly of short sequence reads of the lentil(Lens culinaris Medik.)transcriptome,Plant Biotechnol.J 11(2013)894-905.
[8]L.Buchwalt,K.L.Anderson,R.A.A.Morrall,B.D.Gossen,C.C. Bernier,Identification of lentil germplasm resistant to Colletotrichum truncatum and characterization of two pathogen races,Phytopathology 94(2004)236-243.
[9]S.Gupta,R.Bharalee,R.Das,D.Thakur,Bioinformatics tools for development of fast and cost effective simple sequencerepeat(SSR),and single nucleotide polymorphisms(SNP)markers from expressed sequence tags(ESTs),Afr.J. Biotechnol 12(2013)4713-4721.
[10]S.Choudhary,N.K.Sethy,B.Shokeen,S.Bhatia,Development of chickpea EST-SSR markers and analysis of allelic variation across related species,Theor.Appl.Genet 118(2009)591-608.
[11]M.W.Akash,G.O.Myers,The development of faba bean expressed sequence tag-simple sequence repeats(EST-SSRs)and their validity in diversity analysis,Plant Breed 131(2012)522-530.
[12]Y.M.Gong,S.C.Xu,W.H.Mao,Q.Z.Hu,G.W.Zhang,J.Ding,Y.D.Li,Developing new SSR markers from ESTs of pea(Pisum sativum L.),J.Zhejiang Univ Sci.B(Biomed.Biotechnol.)11(2010)702-707.
[13]P.C.Sharma,A.Grover,G.Kahl,Mining microsatellites in eukaryotic genomes,Trends Biotechnol 25(2007)490-498.
[14]S.Gupta,M.Prasad,Development and characterization of eSSR markers in Medicago truncatula and their transferability in leguminous and non-leguminous species,Genome 52(2009)761-771.
[15]B.Chevreux,T.Pfisterer,B.Drescher,A.J.Driesel,W.E.G.Müller,T.Wetter,S.Suhai,Using themiraEST assembler for reliableand automated mRNA transcript assembly and SNP detection in sequenced ESTs,Genome Res 14(2004)1147-1159.
[16]X.Huang,A.Madan,CAP3:a DNA sequence assembly program,Genome Res 9(1999)868-877.
[17]A.Dubey,A.Farmer,J.Schlueter,S.B.Cannon,B.Abernathy,R.Tuteja,J.Woodward,T.Shah,B.Mulasmanovic,H.Kudapa,N.L.Raju,R.Gothalwal,S.Pande,Y.Xiao,C.D.Town,N.K. Singh,G.D.May,S.Jackson,R.K.Varshney,Defining the transcriptome assembly and its use for genome dynamics and transcriptome profiling studies in pigeonpea(Cajanus cajan L.),DNA Res 18(2011)153-164.
[18]Y.Zheng,L.J.Zhao,J.P.Gao,Z.J.Fei,iAssembler:a package for de novo assembly of Roche-454/Sanger transcriptome sequences,BMC Bioinformatics 12(2011)453.
[19]J.Duvick,A.Fu,U.Muppirala,M.Sabharwal,M.D.Wilkerson,C.J.Lawrence,C.Lushbough,V.Brendel,PlantGDB:a resource for comparative plant genomics,Nucleic Acids Res 36(2008)D959-D965.
[20]T.Thiel,W.Michalek,R.K.Varshney,A.Graner,Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley(Hordeum vulgare L.),Theor.Appl.Genet 106(2003)411-422.
[21]T.W.Chen,R.C.R.Gan,T.H.Wu,P.J.Huang,C.Y.Lee,Y.Y.M. Chen,C.C.Chen,P.Tang,FastAnnotator-an efficient transcript annotation web tool,BMC Genomics 13(2012)S9.
[22]NCBI,http://www.ncbi.nlm.nih.gov/nucest/?term=lentil(Accessed on June 2,2015).
[23]MIRA,http://mira-assembler.sourceforge.net/docs/ DefinitiveGuideToMIRA.html(Accessed on October 26,2015).
[25]S.Kumar,K.Rajendran,J.Kumar,A.Hamwieh,M.Baum,Current knowledge in lentil genomics and its application for crop improvement,Front.Plant Sci 6(2015)78.
[26]S.Kaur,N.O.Cogan,A.Stephens,D.Noy,M.Butsch,J.W. Forster,M.Materne,EST-SNP discovery and dense genetic mapping in lentil(Lens culinaris Medik.)enable candidate gene selection for boron tolerance,Theor.Appl.Genet 127(2014)703-713.
[27]P.Verma,T.R.Sharma,P.S.Srivastava,M.Z.Abdin,S.Bhatia,Exploring genetic variability within lentil(Lens culinaris Medik.)and across related legumes using a newly developed set of microsatellite markers,Mol.Biol.Rep 41(9)(2014)5607-5625.
[28]E.E.Andeden,S.B.Faheem,Ç.Esra,T.Faruk,Ö.Hakan,Development,characterization and mapping of microsatellite markers for lentil(Lens culinaris Medik.),Plant Breed 134(2015)589-598.
[29]K.Wu,M.M.Yang,H.Y.Liu,Y.Tao,J.Mei,Y.Z.Zhao,Genetic analysis and molecular characterization of Chinese sesame(Sesamum indicum L.)cultivars using Insertion-Deletion(InDel)and Simple Sequence Repeat(SSR)markers,BMC Genet 15(2014)35.
[30]S.J.Kwon,A.F.Brown,J.G.Hu,R.McGee,C.Watt,T.Kisha,G. Timmerman-Vaughan,M.Grusak,K.E.McPhee,C.J.Coyne,Genetic diversity,population structure and genome-wide marker-trait association analysis emphasizing seed nutrients of the USDA pea(Pisum sativum L.)core collection,Genes Genomics 34(2012)305-320.
[31]M.R.K.Reddy,R.Rathour,N.Kumar,P.Katoch,T.R.Sharma,Cross-genera legume SSR markers for analysis of genetic diversity in Lens species,Plant Breed 129(2010)514-518.
[32]J.Liu,J.P.Guan,D.X.Xu,X.Y.Zhang,J.Gu,X.X.Zong,Genetic diversity and population structure in lentil(Lens culinaris Medik.)germplasm detected by SSR Markers,Acta Agron.Sin 34(2008)1901-1909 in Chinese with English abstract.
[33]M.M.Wong,N.Gujaria-Verma,L.Ramsay,H.Y.Yuan,C. Caron,M.Diapari,A.Vandenberg,K.E.Bett,Classification and characterization of species within the genus Lens using genotyping-by-sequencing(GBS),PLoS One 10(2015),e0122025.
[34]K.Bett,L.Ramsay,C.Crystal,A.G.Sharpe,D.R.Cook,P.R. Varma,P.Chang,C.J.Coyne,R.McGee,D.Main,A. Vandenberg,Plant and Animal Genome XXIII Conference,LenGen:The International Lentil Genome Sequencing Project,2015,https://pag.confex.com/pag/xxiii/webprogram/ Paper14074.html,Accessed on March 12,2016.
[35]A.G.Sharpe,L.Ramsay,L.A.Sanderson,M.J.Fedoruk,W.E. Clarke,R.Li,S.Kagale,P.Vijayan,A.Vandenberg,K.E.Bett,Ancient orphan crop joins modern era:gene-based SNP discovery and mapping in lentil,BMC Genomics 14(2013)192.
[36]D.Gupta,P.W.J.Taylor,P.Inder,H.T.T.Phan,S.R.Ellwood,P.N.Mathur,A.Sarker,R.Ford,Integration of EST-SSR markers of Medicago truncatula into intraspecific linkage map of lentil and identification of QTL conferring resistance to Ascochyta blight at seedling and pod stages,Mol.Breed 30(2012)429-439.
[37]M.L.Havey,F.J.Muehlbauer,Linkages between restriction fragment length,isozyme,and morphological markers in lentil,Theor.Appl.Genet 77(1989)395-401.
[38]A.Kahraman,I.Kusmenoglu,N.Aydin,A.Aydogan,W.Erskine,F.J.Muehlbauer,Genetics of winter hardiness in 10 lentil recombinant inbred line populations,Crop Sci 44(2004)5-12.
[39]A.Kahraman,I.Kusmenoglu,N.Aydin,A.Aydogan,W. Erskine,F.J.Muehlbauer,QTL mapping of winter hardiness genes in lentil,Crop Sci 44(2004)13-22.
[40]F.J.Muehlbauer,K.E.McPhee,Registration of‘Pennell'lentil,Crop Sci 44(2004)1488.
[41]F.J.Muehlbauer,Registration of‘Brewer'and‘Emerald'lentil,Crop Sci 27(1987)1088-1089.
[42]A.Sarker,W.Erskine,M.S.Hassan,M.A.Afzal,A.N.M.M. Murshed,Registration of‘Barimasur-4'lentil,Crop Sci 39(1999)876.
[43]F.J.Muehlbauer,K.E.McPhee,Registration of‘Morton' winter-hardy lentil,Crop Sci 47(2007)438-439.
[44]A.Sarker,W.Erskine,M.S.Hassan,W.Mian,N.Debnath,Registration of‘Barimasur-2'lentil,Crop Sci 39(1999)875.
[45]A.Vandenberg,S.Banniza,T.D.Warkentin,S.Ife,B.Barlow,S.McHale,B.Brolley,Y.Gan,C.McDonald,M.Bandara,S. Dueck,CDC Redberry lentil,Can.J.Plant Sci 86(2006)497-498.
[46]A.Sarker,J.Kumar,M.M.Rahman,M.S.Hassan,W.Zaman,M.A.Afzal,A.N.M.M.Murshed,Registration of‘Barimasur-3' lentil,Crop Sci 39(1999)1536.
7 April 2016
in revised form
.
E-mail address:rjmcgee@wsu.edu(R.J.McGee).
Peer review under responsibility of Crop Science Society of China and Institute of Crop Science,CAAS.
1Debjyoti Sen Gupta and Peng Cheng contributed equally to this work.
http://dx.doi.org/10.1016/j.cj.2016.06.012
2214-5141/Production and hosting by Elsevier B.V.on behalf of Crop Science Society of China and Institute of Crop Science,CAAS.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
杂志排行
The Crop Journal的其它文章
- Editorial:Food Legume Diversity and Legume Research Policies
- QTL and candidate genes associated with common bacterial blight resistance in the common bean cultivar Longyundou 5 from China
- Two major er1 alleles confer powdery mildew resistance in three pea cultivars bred in Yunnan Province,China
- Construction of an integrated map and location of a bruchid resistance gene in mung bean
- Evaluation of common bean(Phaseolus vulgaris L.)genotypes for drought stress adaptation in Ethiopia
- Large-scale evaluation of pea(Pisum sativum L.)germplasm for cold tolerance in the field during winter in Qingdao
