Exercise-induced rhabdomyolysis mechanisms and prevention:A literature review
2016-10-24JooyoungKimJoohyungLeeSojungKimHoYoungRyuKwangSukChaDongJunSung
Jooyoung Kim,Joohyung Lee,Sojung Kim,Ho Young Ryu,Kwang Suk Cha,Dong Jun Sung,*
aHealth and Rehabilitation Major,College of Physical Education,Kookmin University,Seoul 136-702,Republic of Korea
bDepartment of Physical Education,Global Campus,Kyung Hee University,Suwon 446-701,Republic of Korea
cDivision of Sport Science,College of Science and Technology,Konkuk University,Choong-Ju 380-702,Republic of Korea
Exercise-induced rhabdomyolysis mechanisms and prevention:A literature review
Jooyoung Kima,†,Joohyung Leea,†,Sojung Kimb,#,Ho Young Ryuc,Kwang Suk Chac,Dong Jun Sungc,*
aHealth and Rehabilitation Major,College of Physical Education,Kookmin University,Seoul 136-702,Republic of Korea
bDepartment of Physical Education,Global Campus,Kyung Hee University,Suwon 446-701,Republic of Korea
cDivision of Sport Science,College of Science and Technology,Konkuk University,Choong-Ju 380-702,Republic of Korea
Exercise-induced rhabdomyolysis(exRML),a pathophysiological condition of skeletal muscle cell damage that may cause acute renal failure and in some cases death.Increased Ca2+level in cells along with functional degradation of cell signaling system and cell matrix have been suggested as the major pathological mechanisms associated with exRML.The onset of exRML may be exhibited in athletes as well as in general population.Previous studies have reported that possible causes of exRML were associated with excessive eccentric contractions in high temperature,abnormal electrolytes balance,and nutritional deficiencies possible genetic defects.However,the underlying mechanisms of exRML have not been clearly established among health professionals or sports medicine personnel.Therefore,we reviewed the possible mechanisms and correlated prevention of exRML,while providing useful and practical information for the athlete and general exercising population.
Acute renal failure;Calcium(Ca2+);Creatine kinase;Myoglobin(Mb);Rhabdomyolysis
1.Introduction
The number of people participating in regular as well as organized exercise programs has been continuously increasing. The increase in popularity of physical activity and exercise may be due to positive effects on physical and mental health. However,excessive or intense exercise beyond the extent of personal or physical limits may induce various types of musculoskeletal damage,including exercise-induced rhabdomyolysis(exRML),a pathophysiological condition of skeletal muscle cell damage.1exRML may be manifested by an increase in creatine kinase(CK)or myoglobin(Mb),seeping into the blood stream through damaged cell membrane as results of excessive or intense exercise.1exRML may lead to acute renal failure(ARF),liver dysfunction,compartment syndrome,heart failure,arrhythmias,electrolyte imbalance,and in severe cases also to death.2,3exRML can occur via highly intense and prolonged exercise or due to sudden and excessive contraction of skeletal muscles. Symptoms of exRML are similar to those of delayed onset muscle soreness that can be easily overlooked.4Despite its importance,people who participate in exercise may not be aware of exRML.Therefore,the purposes of this review are to provide exercising population with information about the possible mechanisms of exRML and offer preventive strategies to avoid exRML based on results of previously conducted studies.
2.Pathophysiology of exRML
2.1.Role of calcium in the pathogenesis of exRML
Ca2+hasbeensuggestedasanimportantfactorinthepathogenesisofexRML(Fig.1).Numerousstudies5,6haveshownincreased levels of Ca2+in cells of exRML patients.The concentration of Ca2+should remain at nano-molar levels under resting conditions. Ca2+would increase to mille-molar levels through cell activation and muscle contraction during exercise.7Ryanodine receptors in the sarcoplasmic reticulum,dihydropyridine receptors(i.e.,voltage-gated L-type Ca2+channels),and Ca2+pump are the 3 major pathways controlling the Ca2+in skeletal muscle cells.8-10Transientreceptorpotentialchannel(non-selectivecationchannel),11Ca2+-induced Ca2+release,12and Na+-Ca2+exchanger13contribute to the control of Ca2+.Increased Ca2+concentration has been reported in the sarcoplasm of exRML patients6with deficiency or depletion of adenosine triphosphate(ATP)due to intensity of exercise.ATP is continuously synthesized during exercise.When the amount of ATP is severely depleted,ATP-dependent ion transporter may be affected.14ATP-dependent transporters of skeletal muscle cells are Na+-K+ATPase15and Ca2+ATPase16ion transporters.When skeletal muscle cells are excited,Na+influx through the voltage-gated Na+channel creates an action potential,resulting in similar amounts of K+efflux through the K+channel.The movement of these ions strengthens the capacity of Na+-K+ATPase to readjust the distribution of ions in the sarcoplasm.15The efflux of Na+and the influx of K+becomeATP-dependent and move in the opposite direction of the concentration gradient.If the amount ofATP is deficient or insufficient,the activity of Na+-K+ATPase would be reduced. Decreased amount ofATP could cause dysfunction of the Na+-K+ATPase,14resulting in an increased level of Na+in the cells.17Normal function of Na+-K+would activate Na+-Ca2+exchanger in the forward mode(Ca2+extrusion).However,due to the dysfunction of Na+-K+ATPase,increased level of Na+in cells would activate the reverse mode of Na+-Ca2+exchanger(Ca2+influx),thereby increasing the level of Ca2+in the cells.18During the cycle of contraction and relaxation of skeletal muscle,Ca2+in the sarcoplasm repeatedly gain sentry through the Ca2+pump in the membrane of sarcoplasmic reticulum.7Normal function of the Ca2+pump requires the hydrolysis of ATP.19,20If the amount of ATP is insufficient,the Ca2+pump would result in abnormal function.Zhang21suggested that dysfunction of ion regulation proteins as,a Na+-K+ATPase,Na+-Ca2+exchanger,and Ca2+pumpinskeletalmusclemaybestronglyrelatedto rhabdomyolysis(RML).
Peer review under responsibility of Shanghai University of Sport.
#Current address:Department of Physical Therapy,College of Health Science,University of Massachusetts,Lowell,MA 01854,USA.
http://dx.doi.org/10.1016/j.jshs.2015.01.012
2095-2546/©2016 Production and hosting by Elsevier B.V.on behalf of Shanghai University of Sport.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
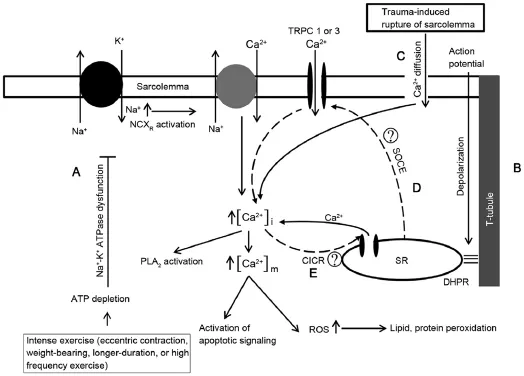
Fig.1.The pathophysiological mechanism of rhabdomyolysis focusing on the increase of Ca2+.A:Deficiency ofATP due to high intensity exercise and continuous muscle contraction could induce the dysfunction of Na+-K+ATPase,causing subsequent activation of reverse mode Na+-Ca2+exchanger;B:Depolarization of sarcolemma and T-tubule by an action potential could activate dihydropyridine receptor and promote the secretion of Ca2+via ryanodine receptor in sarcoplasmic reticulum;C:The increase of Ca2+due to Ca2+diffused by the rupture of sarcolemma from trauma;D:The entry of store-operated Ca2+through transient receptor potential Channel 1 or transient receptor potential Channel 3 with reduced levels of Ca2+in the sarcoplasmic reticulum;E:The secretion of Ca2+(Ca2+-induced Ca2+release)from sarcoplasmic reticulum in accordance with the increase of Ca2+in sarcoplasm.→represents activation;represents inhibition,represents candidate mechanisms in the regulation of Ca2+.ATP=adenosine triphosphate;CICR=Ca2+-induced Ca2+release;DHPR=dihydropyridine receptor;NCXR= Na+-Ca2+exchanger;PLA2=phospholipaseA2;ROS=reactive oxygen species;SOCE=store-operated Ca2+entry;SR=sarcoplasmic reticulum;TRPC=transient receptor potential cation channels.
Ca2+-induced Ca2+release has been detected earlier than inositol 1,4,5-triphosphate-induced Ca2+release for the reservation or mobilization of Ca2+in the sarcoplasm.12Ca2+-induced Ca2+release is not the primary Ca2+control mechanism in the skeletal muscles.It is achieved via protein-protein interaction of voltage sensor dihydropyridine receptor of the T-tubule with the Ca2+release channel ryanodine receptor of the sarcoplasmic reticulum membrane.22,23According to the Ca2+-induced Ca2+release mechanism,membrane depolarization caused by the action potential increases the levels of Ca2+in the sarcoplasm,thus releasing Ca2+from the Ca2+store(sarcoplasmic reticulum).Ryanodine receptor and inositol 1,4,5-triphosphate are both associated with Ca2+-induced Ca2+release.12Due to consistent contraction of skeletal muscles,increased level of Ca2+in the sarcoplasm may activate Ca2+-induced Ca2+release,which may have asynergistic effect and subsequently increase the level of Ca2+even more.Transient receptor potential channels are non-selective cation channels permeable to Na+and Ca2+.7In skeletal muscles,transient receptor potential canonical(TRPC)Types 1 and 3 have been identified,with TRPC3 being reported to interact with ryanodine receptor.24,25The activation of store-operated Ca2+entry byTRPC1/3 with a Ca2+deficiency of the sarcoplasmic reticulum due to ryanodine receptor or inositol 1,4,5-triphosphate activation may increase the levels of Ca2+in the sarcoplasm.26,27The malignant hyperthermia is characterized by an increase in RML28and store operated cation channels involving TRPC3 accelerated activation by malignant hyperthermia.29This leads to increasing intracellular Ca2+and indicates that store operated cation channels and/or TRPC3 is contributing to the development of RML.
Increased level of Ca2+has been reported to have influence on the activation of proteases and phospholipase A2.30,31These responses are strongly associated with damage or decomposition of phospholipids of the cell membrane,32which could induce damage to the cell membrane and reveal toxicity caused by several types of molecular efflux.5In addition,the increase in Ca2+concentration in the mitochondria due to chemical gradient of Ca2+between the sarcoplasm and mitochondria may be another plausible mechanism of damage.20,33This reaction could promote the creation of reactive oxygen species(ROS)in the mitochondria,34which could damage proteins,lipids,and nucleic acids.35,36This type of damage could reduce the synthesis of cell membrane,proteins,and/or ATP.34The increase of Ca2+in the sarcoplasm and mitochondria may amplify the signaling of apoptosis and promoting cell death.37-39Furthermore,rupture of muscular cell membrane caused by injury,toxicity,or exercise may induce the influx of Ca2+into cells due to concentration gradient,contributing to the elevation of Ca2+concentration in the sarcoplasm.20,37Therefore,the increase in Ca2+in cells may induce exRML by creating energy,while controlling the cell signaling pathway system through interactions that may cause cell death.
2.2.Role of myoglobin in exRML-induced ARF
Among complications of exRML,ARF has shown the greatest incidence rate increase.40,41Park et al.42reported that 10%-30%of exRML patients may have accompanyingARF,making exRML a clinically important condition due to strong correlation betweenARF and death.ARF from exRML may be caused by the delay of treatment due to failure of recognizing severe muscle damage and the presence of renal diseases or agerelated biological decline.43While the pathogenesis of an ARF originating from exRML has not been clearly recognized,previous studies have suggested an association between increased Mb and K+ion and uric acid affecting the glomerulus of kidneys.40Particularly Mb could easily permeate the glomerular membrane and subsequently increase the amount of Mb as results of continued muscle damage.44
The mechanism of exRML-induced ARF may be referred to vasoconstriction.Necrosis in muscular tissues may create additional space for increased accumulation of intravascular fluid and generate hypovolemia,45,46that may activate sympathetic tone and renin angiotensin-aldosterone system,inducing vasoconstriction and activate additional vasoactivator(e.g.,endothelin1,vasopressin)thatareknowntosuppress vasodilation induced by protaglandins.47-49Damage to muscles promotes extrication of endotoxins and cytokines into systemic circulation and thus promoting vasoconstriction.50,51Mb also plays an important role in decreasing nitric oxide and vasodilation.52,53Under these conditions,the creation of ATP would be reduced resulting in vasoconstriction,renal ischemia,and reduction in oxygen.45
Cast formation is a contributor in the development of exRML-inducedARF.37,44Deficit inATP may cause necrosis of epithelial cells,accumulation of dead cells in the tubular lumen,resulting in the precipitation of Mb and formation of casts.47,54Mb is filtrated at the renal glomeruli.55The increase in Mb in pre-urine is accompanied by acidification,and thus,increasing the accumulation of Mb and Mb cast formation in the distal convoluted tubules.56The accumulation of Mb induces constriction of blood vessels and initiates ischemia,reducing the function of renal tubules in filtering metabolites and waste products.53The accumulation of Mb also creates ROS and induces lipid peroxidation that produces cell membrane and blood vessels in kidneys causing temporary or chronic impairment of normal kidney function.57,58
2.3.Primary factors
During exercise,factors that may cause exRML include the exercise experience of participants,level of physical fitness,the intensity,duration,and types of exercises.Line and Rust59reported that exRML tends to appear more often in people with little or no exercise experience or in athletes who are less trained than others.In addition,a positive relationship was found between exRML and soldiers performing sedentary duties compared to trained soldiers.60Paul et al.61reported that highly experienced weight-lifters exhibited relatively lower levels of CK and Mb than less experienced weight-lifters.
Other important factors in exRML are the intensity and duration of exercises.Clarkson62found that the typical onset of exRML was extreme muscle soreness and brown colored urine in 12-year-old boys who performed squat jumps 250-500 times.Moeckel-Cole and Clarkson63also reported the onset of exRML in college soccer players who conducted highly intense weight training and performed 300 squat jumps.In addition,Russo and Bass64reported exRML in a 17-year-old male basketball player who had CK level of 241,026 U/L after completing 800 sit-ups,400 push-ups,and a 3.2 km run.The determinations of exRML manifested from other sources are summarized in Table 1.
The type of exercise is also considered an important factor in the development of exRML.It has been found that eccentric contraction of muscles may cause exRML more often than concentric contraction.40,65,66Kinematic factors of tension,changesinthelengthofeccentriccontractionofthemuscle,and attenuation of the bonding between contractile proteins have been suggested to explain these findings.57,67According to a previous study,66muscle soreness along with the appearance of CKorMbinthebloodappearedofteninthebloodafterexercises containing an excessive component of eccentric contractions. Prolongedandhighintensityexercises(e.g.,marathon,triathlon,soccer,body-building,orCrossfit)havebeenreportedtoactivate exRML.45,58,59
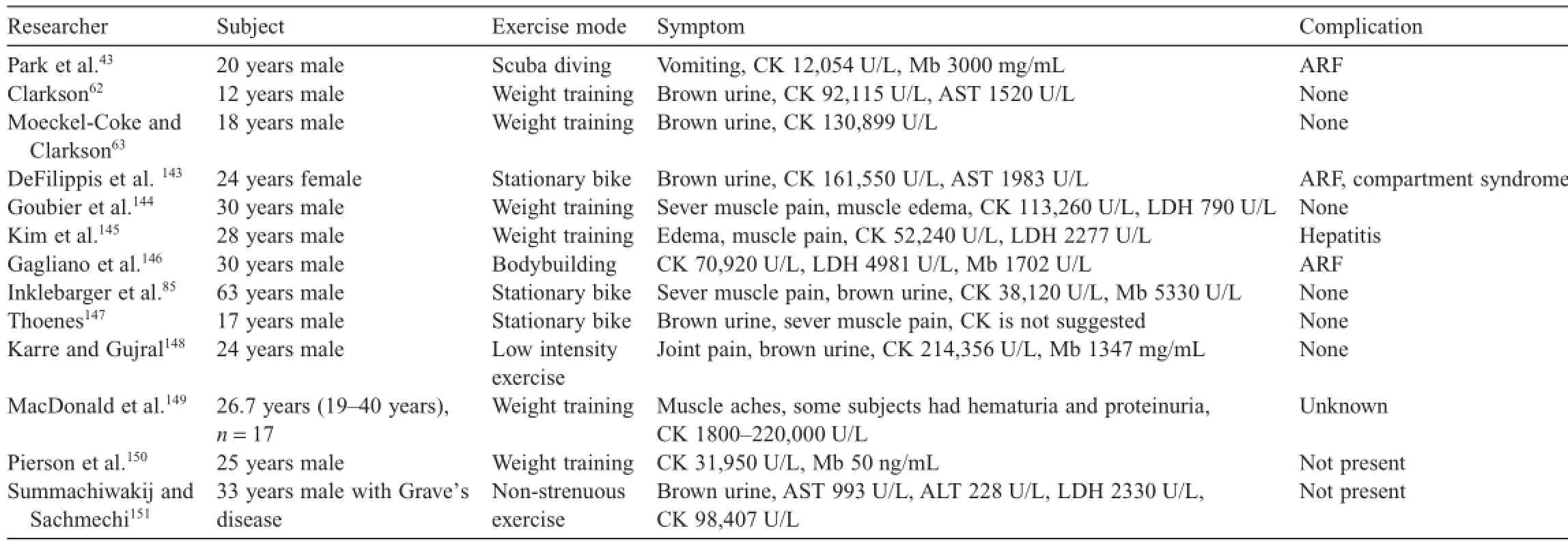
Table 1 Case reports of exercise-induced rhabdomyolysis.
2.4.Secondary factors
2.4.1.Hot environments
Exertional heat stroke syndrome induced fever and encephalopathy(delirium,seizures,and coma)as well as the muscle weakness could lead to exRML.37A hyperthermal environments may increase body temperature above 42°C accompanied by liquation of the lipids constituting the muscle cell membrane disturbance by suppressing the process of internal oxidative phosphorylation or inducing protein denaturation in the mitochondria,resulting in hemodynamic changes and subordinate activation of inflammatory cytokines that may be responsible for exRML.41Excessive exercise in high humidity and temperature has been reported to be the most severe condition that induces exRML.44Soldiers and athletes were reported to have more exRML compared to general population.68Soldiers who undergo special force physical training or ranger activities with long distance marching in hot outdoor environments69,70and athletes who are exposed to high heat in outdoor environments when participating in long activities for hours,such as marathon or triathlon,might be particularly susceptible to exRML.71,72
2.4.2.Electrolyte imbalance
Aizawa et al.73reported expression of exRML in a 22-yearold male soldier who presented with fever,retching,and fatigue after highly intense physical training for 3 days.They suggested that electrolyte imbalance(hypokalemia or hyponatremia)may have produced these symptoms.73K+ion generally would increase blood flow to the contracting muscles during exercise. However,in the case of excessive exercise in high temperatures,the body may compromise its homeostasis to control body heat. As a result,potential hypokalemia may be generated due to sweating,therefore reducing the blood flow to the muscles and induced exRML.74According to Park et al.42hypokalemia may lead to exRML by changing voltage of safety film on the cell membrane by impeding the synthesis of muscle energy substrates such as glycogen.Na+is closely associated with muscle contraction and Na+-K+ATPase may be markedly reduced in a high temperature environment,resulting in exRML.75It has been reported that exRML was induced in body builders who avoided Na+and water intake to generate a contrasting contour of muscles,which affected the electrolyte imbalance.76
2.4.3.Sex
The incidence of exRML in males has been reported to be higher compared to females.72,77,78A female group was reported to have less increase in CK level than the male group.79In menopausal women,the secretion of CK and lactate dehydrogenase(LDH)were diminished in the group taking estrogen hormone supplement.80The incidence of exRML was reported to be lower in female athletes than in male athletes.77A report from the U.S.Centers for Disease Control and Prevention also reported that exRML was observed in 32 men,but not in 84 women among 16,506 fire fighters who participated in a physical strength examination.81In an epidemiological investigation of exRML in high school students,male students were found to have more cases of exRML than female students.66At 24 h post marathon,the level of CK in the male group was 3322 IU/L that was significantly higher than in the female group constituting 946 IU/L.82Therefore,males are more vulnerable to exRML than females.It was reported that estrogen with similar structure as vitamin E may have suppressed oxidative stress due to exercise,thus squelching the activation of calpain,a protein with function of diminishing the infiltration of inflammatory cells such as neutrophil leukocyte and macrophages.83,84
2.4.4.Nutritional problems
Dietary composition of vegetarians with exRML has been discussed previously.85The amount of ingested protein has been reported to cause variation in the degree of exRML,86suggest-ing that exRML may be associated with deficiency of protein in the diet.Vegetarian athletes,who do not consume proper amount of protein with their meals may potentially develop exRML.87One young athlete manifested with exRML together with high levels of CK,muscle pains,discomfort,temporary tachycardia,and retching was found to have been on vegetarian diet.86Therefore,healthy diet containing proper amount of protein is required to prevent exRML.
Besides proteins,carbohydrate intake may also play a role in exRML.One male marathoner in his 30s who controlled carbohydrate intake through glycogen loading died from heat stroke accompanied with exRML and increased levels of CK after finishing the race.77According to Bank,88among track and field athletes who exhibited brown urine after glycogen loading,were reported to develop ARF.The manifestation of exRML appeared to originate from acidification and reduction of normal energy stores in muscles by increasing lactic acid as results of an increase in glycogen.77Although the exact mechanism has not been determined,it is possible that track and field athletes are more vulnerable to myoglobinuria attributed to glycogen loading.88Park et al.42suggested that hypokalemia induced by increased insulin from excessive intake of carbohydrate may be a possible reason for exRML in a body builder after finishing exercise.
2.4.5.Creatine supplements and alcohol
Creatine supplements have been used by athletes who require muscle power in a short time and by general public who may wish to increase the muscle mass.89Creatine is endogenous energysubstratesthatcanbetakenadditionallyassupplements.90The intake of 20-25 g/day of creatine for 5-7 days is recommended.However,over 80%of athletes appear to take much larger amount of the supplements than recommended.89Such excessive intake may cause imbalance in body water,triggering muscle cramps or dehydration,which may be the root cause of renal failure or exRML.A male weight-lifter was reported to have renal failure and compartment syndrome including exRML after taking high doses of creatine supplement.91A case of recurrence of steroid-responsive nephrotic syndrome along with reduced creatinine clearance rate caused by the intake of creatine supplement was also reported.92
The excessive use of medication for medical or entertainment purposes can also cause RML.Excessive exercise while taking drugs for medical reasons may lead to potentially adverse drug reactions.A rare case of induced RML by statin(medication administered for patients to control hyperlipidemia)was also reported.93It was found that statins may impede the activation ofATP and coenzyme Q10(antioxidant),making the muscle cell membrane susceptible to damage.44Similarly,steroids typically used by athletes may also induce RML and liver damage.94,95Recently,indication of RML was reported in a person who performed exercise after taking synephrine(similar to phenylpropanolamine or ephedrine)contained in supplements used for weight loss.96Compartment syndrome with RML was also reported in a soldier who took ephedrine after completion of physical training.97In addition,a woman in her 50s exhibited RML together with symptoms of extreme pain,muscle hyposthenia,and loss of weight and muscle power after taking oriental medicine containing Herba Ephedrae who later died.98
RML may be induced by ingestion of drugs such as heroin,cocaine,amphetamine,and cyclosporine(immunosuppressive agent after organ transplantation).44Alcohol may also cause RML by aggravating damage to muscles created by exercise.It was reported that alcohol ingestion after exercise may worsen edema,soreness,and dehydration.99Alcohol aggravates muscle damages by innate immunoreactions of the body influenced by differentiated activation of inflammatory cells during process of recovery from muscle damage.100
2.4.6.Other factors
Various diseases may also affect exRML.A young teenager who participated in a weight lifting training had exRML due to an influenza virus.101In addition,a young basketball player presented with exRML after taking medication to treat influenza.102Although the exact cause of exRML symptoms needs further clarification,it is possible that viral infection may play a role in the cases of exRML.
Genetic deficiency of metabolic factors may also be implicated in RML.McArdle’s disease,a deficiency of myophosphorylase related to the metabolism of carbohydrate,may impede the supply of energy sources required for exercise due to the deficiency of enzymes essential for glycolysis and glycogenolysis.103Reduction or absence of glycolysis and glycogenolysis would have a negative influence on the synthesis of ATP as illustrated in Fig.1.Fatty acid oxidation disorders such as the disturbance of β-oxidation and other enzyme shave also been linked to RML.104,105Fatty acid oxidation is an important energy metabolism system in skeletal muscles,heart,liver,and kidneys.106
Deficiency of carnitine palmitoyltransferase II may cause RML via synthesis of ATP related to lipid metabolism during aerobic exercise.107Deficiency of carnitine palmitoyltransferase II is a common cause of myopathy,resulting in RML in adults.108
Mutations of LPIN 1 gene have been suggested as a novel factor in recurrent RML,109and are associated with the muscle specific phosphatidic acid phosphatase,a key regulator in triglyceride biosynthesis.110This gene,predominantly expressed in muscle and adipose tissues,111affected recurring RML in children.112The prognosis of LPIN 1 deficiency has been considered as a negative outcome,causing death in one-third of patients with RML.113
3.Symptoms and diagnoses
The symptoms of exRML may vary individually.However,changes in the color of urine and muscle soreness are common.114,115When RML occurs,excessive Mb contained in the urine may exhibit myoglobinuria with dark colors.Extreme muscle soreness is accompanied by cramps or muscular stiffness,nausea,headache,and fatigue.44,115
Blood tests and urinalysis have been adopted to diagnose for exRML.CK,Mb,LDH,aspartate aminotransferase(AST),troponin,and aldolase in blood are examined via various bloodtests that also include tests for CK and Mb.CK is the most sensitive indicator of RML.The normal level of CK is at 22-198 U/L.Depending on the degree of RML,the level of CK could increase up to 10,000-200,000 U/L.58CK level of 3,000,000 U/L was observed in 1 case report.115Thus,CK level in blood has been adopted as an indicator of RML.However,some studies have questioned the diagnosis employing CK.116It was reported that CK may be sensitive but not specific for RML.117The National Lipid Association’s Muscle Safety Expert Panel provided the level of CK to diagnose RML into 3 categories:1)levels less than 10 times of the upper limit of normal(ULN)was classified as mild;2)levels of 10-49 times of ULN was classified as moderate;and 3)levels exceeding 50 times of ULN have been classified as marked.116
Since Mb can be quickly removed from the serum,it has a relatively low reliability as an indicator for RML diagnosis.58,118In urinalysis,the ratio of nitrogen and creatinine has been determined to be positive when diagnosing for RML.The normal ratio of nitrogen and creatinine is 10:1.This ratio may decrease below 6:1 depending on the symptoms of RML.44In addition,electrolyte balance,arterial blood gas examination,muscle biopsy,and/or electrocardiogram are used for the diagnosis of RML.119Controversy exists that addresses possible and viable use of biomarkers for detection of RML.Thus,the determination of RML depends on symptoms recognized by exercise participants.Previous study has suggested to seek diagnosis and treatment when pain accompanied with dark urine color are observed 24-48 h after exercise.120
4.Rehabilitation protocol
Rehabilitation programs related to RML were introduced by Randall et al.68The initial rehabilitation program should be composed of exercise containing gradual resistive exercises to activate cell function and prevent energy deficiency.This would enable the exercise intensity of muscles to be placed below an aerobiosis.In general,the range of motion of joints should improve simultaneously.During the 1st stage of a rehabilitation program,manual efforts to secure a range of motions of joints may require some form of discomfort and perhaps some pain. Before recovering from complete joint mobilization,the 2nd stage of rehabilitation should increase gradually.The distal portion of the upper or lower part of the body should be manipulated gradually with very low intensity from 5 to 15 min using a non-weight bearing equipment.If no feeling of discomfort or pain is present within 24 h after the exercise,the 3rd stage of the rehabilitation program could be introduced.In the 3rd stage of the rehabilitation program,isotonic exercises such as stretching of the joints,modified flexion and extension of joints,or bench press should be gradually introduced.Modified flexion and extension of joints should start from forward tilted position with both hands touching the wall,and then proceed to a table,a footboard,or chairs,and finally to the floor to increase the joint mobility and exercise intensity.In the 4th stage of the rehabilitation program,one set of limited flexion and extension of the joints should be performed together with the normal exercise program.The limits of flexion and extension of joints is to restore performance capability before determination of RML without loss of range of motion of joints or pain.68
Guidelines of O’Connor et al.121divide the rehabilitation program in 3 phases for athletes at low risk for RML.In Phase 1,CK and urinalysis are monitored during moderate resting.In Phase 2,the guidelines suggest the initiation of physical activity.In Phase 3,the guidelines suggest a gradual comeback to sports activity.They recommend 72 h of rest and ample water intake after the onset of RML in Phase 1.Eight hours of sleep has been recommended together with remaining in a heatcontrolled environment in the presence of RML when accompanied with heat injury.Monitoring of CK and urinalysis every 72 h has also been recommended.Light physical activities in Phase 2 could be initiated after urinalysis results reveal CK levels below 5 times of the normal level.In cases where CK or the results of urinalysis are not normalized within 2 weeks,medical consultation was recommended.For Phase 2,physical activities considering self-paced distance should be practiced. The Phase 3 could be initiated along with necessary follow-ups when no clinical symptoms a represent during a 1-week follow-up in Phase 2.The rehabilitation program after the onset of RML should be advanced gradually while carefully monitoring symptoms(CK,pains,etc.).
5.Prevention guidelines
5.1.Consideration of exercise program components
It has been suggested that warm-up may be the best approach to improve exercise adherence,as it provides the participant with pre-practice of the actions required for corresponding exercises or games.Warm-ups could also reduce the chance or occurrence of musculoskeletal damage.122It may also be useful to utilize the same amount of time for warm-up and cool-down as demanded factual exercise or game.
Several studies have reported that periodic repetition of eccentric exercises could reduce the level of muscle damage,inducing positive changes in blood markers such as CK or LDH as well as diminished pains of the muscles.123-125To make these changes,several factors should be considered,including the interval time between each exercise,the number of repeated eccentric contractions,length of muscles,and the types of exercise.Various mechanisms related to repeated-bout effect120have been reported.Changes to muscle fibers and the nervous system would require additional motor units for successful eccentric contractions.Thus,muscles should be adapted by considering not only dynamic factors such as length-tension changes,but also reduction in intracellular events such as inflammatory reaction generated from damage or function of excitationcontraction coupling(E-C coupling)to prevent the onset of exRML.126,127
What type of exercise could prevent exRML?The answer to this question is not clear.However,it may be easy to identify the types of exercises that may promote exRML.CK is a crucial indicator for the diagnosis of exRML.High-intensity,longerduration,and weight-bearing exercise(eccentric contraction and downhill running)have been found to be responsible for the increase in CK concentration,128especially in men who arelacking physical strength.129Thus,the type and intensity of exercise must be considered prudently before participating in exercises training program.
5.2.Education of exercise-induced rhabdomyolysis
Generalized guidelines for identification of exRML have not been established.Individuals participating in exercise requiring greater or more intense eccentric contraction of muscles should understand the danger and potential exRML to prevent this condition.66It is also important to educate coaches and others who are involved in training of athletes about the symptoms and signs of exRML.Lack of descriptions regarding the exRML in exercise physiology books and books addressing physical training are warranted.63Knowledge of exRML would enhance coaches and other professional of athletes in each field to recognize quickly when exRML may occur.130
5.3.Prudence in participating in exercise when having communicable diseases
Regular exercise may become a risk factor in individuals prone and susceptible to disease.Individuals with mild disease including communicable diseases should refrain from exercise,or at least limit the scope of the exercise.In cases of viral diseases including diarrhea or vomiting,exercise and training should be modified or abstain from training to prevent possible development of exRML.130Symptoms that are similar to those of influenza or communicable diseases should be considered to avoid further complications.4
5.4.Environmental factors to be considered in outdoor exercises
Many previous studies have reported that sufficient intake of water is effective in preventing heat induced disorders.Normal hydrationcouldensurethehomeostasisofbody temperature.131,132These relatively simple and common sense measures may prevent heat induced disorders and subsequently reduce the risk and onset of exRML.Since the degree of water loss in a high temperature environment is usually higher,coaches and other professional in the field should continually observe athletes to prevent exRML.In addition,clothing heat production needs to be considered as well.American football players often presented with exRML attributed to their heavy and thick uniforms.133When participating in exercise in high temperature,wearing clothing and uniforms that would assist and aid in heat dissipation and provide a cooling mechanism should be considered.134
5.5.Consideration of alimentation
Excessive exercise consumes large amounts of body energy. Thus,supplying the body with major nutrients after completing exercise,including protein,carbohydrates,and fat,is preferable and prudent practice.When muscles are damaged,catabolic state may aggravate the damage.These changes could be diminished by proper intake of balanced nutrients.135To promote the recovery and regeneration of damaged muscles,ingesting protein together with carbohydrate is more effective since car-bohydrate improves the rate of glycogen synthesis.136,137Sweating and muscular contraction may induce excessive loss of electrolytes as results of intense exercise or training.Thus,drinking fluids containing electrolytes during and after exercise is desirable.138Proper maintenance of fluids and nutrients after completing exercise could provide damaged muscles with necessary fuel for recovery and regeneration and prevent the potential to develop RML.
RML is also known to be associated with oxidative stress. Proper intake of exogenous antioxidants could be another way to prevent the onset of RML.Intake of antioxidants,oxidative stress caused by ROS may be reduced and prevent the damage or failure of kidneys.139Since RML is related to oxidative stress,the uptake of coenzyme Q10 may improve the activation of endogenous antioxidants such as glutathione,superoxide dismutase,and catalase and consequently reduce the levels of CK and LDH in blood.140Water soluble antioxidant vitamin C may contribute at least partially in preventing renal failure and morphological damage to kidneys due to vitamin C’s action that may prevent ARF induced by RML.141In another study,it was suggested that RML could be prevented via exogenous intake of antioxidants vitamin C by also reducing CK.142
6.Conclusion
When accompanied by various complications,exRML can lead to severe medical conditions.Therefore,it is important to know the related information about exRML as well as various kinds of exercise induced risk factors associated with exRML. Swift and timely measures indicative of symptoms could prevent medical and clinical complications.Return to the training and exercise through a basic rehabilitation protocol after suffering from exRML should be encouraged to prevent the exRML condition.The intent of this review is to provide athletes,coaches,training and medical professional,as well as general population with information necessary to identify various conditions that may lead to exRML as well as how to prevent it.Further studies on the mechanism of exRML are warranted to establish prudent or better guidelines to prevent future cases of exRML.
DJS,JK,and JL searched the related studies and contributed to the writing of the manuscript.SK,HYR,and KSC helped to draft the manuscript.All authors have read and approved the final version of the manuscript,and agree with the order of presentation of the authors.
Competing interests
None of the authors declare competing financial interests.
1.Clarkson PM,Hubal MJ.Exercise-induced muscle damage in humans. Am J Phys Med Rehabil 2002;81:52-69.
2.Baxter RE,Moore JH.Diagnosis and treatment of acute exertional rhabdomyolysis.J Orthop Sports Phys Ther 2003;33:104-8.
3.Patel DR,Gyamfi R,Torres A.Exertional rhabdomyolysis and acute kidney injury.Phys Sports Med 2009;37:71-9.
4.Clap F.Exertional rhabdomyolysis.Strength Cond J 2005;27:73-4.
5.Zager RA.Rhabdomyolysis and myohemoglobinuric acute renal failure. Kidney Int 1996;49:314-26.
6.GiannoglouGD,ChatzizisisYS,MisirliG.Thesyndromeof rhabdomyolysis:pathophysiology and diagnosis.Eur J Intern Med 2007;18:90-100.
7.Lee EH.Ca2+channels and skeletal muscle diseases.Prog Biophys Mol Biol 2010;103:35-43.
8.Zalk R,Lennart SE,Marks AR.Modulation of the ryanodine receptor and intracellular calcium.Annu Rev Biochem 2007;76:367-85.
9.Brandi CJ,deLeon S,Martin DR,MacLennan DH.Adult forms of the Ca2+-ATPase of sarcoplasmic reticulum.Expression in developing skeletal muscle.J Biol Chem 1987;262:3768-74.
10.Rossi AE,Dirksen RT.Sarcoplasmic reticulum:the dynamic calcium governor of muscle.Muscle Nerve 2006;33:715-31.
11.Nilius B,Voets T.TRP channels:a TR(I)P through a world of multifunctional cation channels.Pflugers Arch Eur J Phy 2005;451:1-10.
12.Endo K.Calcium-induced calcium release in skeletal muscle.Physiol Rev 2009;80:1153-76.
13.Knochel JP.Mechanism of rhabdomyolysis.Curr Opin Rheumatol 1993;5:725-31.
14.Green HJ.Cation pumps in skeletal muscle:potential role in muscle fatigue.Acta Physiol Scand 1998;162:201-13.
15.Clausen T.Na+-K+pump regulation and skeletal muscle contractility. Physiol Rev 2003;83:1269-324.
16.Yoshida M,Minamisawa S,Shimura M,Komazaki S,Kume H,Zhang M,et al.Impaired Ca2+store function in skeletal and cardiac muscle cells from sarcalumenin-deficient mice.J Biol Chem 2005;280:3500-6.
17.Knochel JP.Neuromuscular manifestations of electrolyte disorders.Am J Med 1982;72:521-35.
18.Tanaka H,Shimada H,Namekata I,Kawanishi T,Iida-Tanaka N,Shigenobu K.Involvement of the Na+/Ca2+exchanger in ouabain-induced inotropy and arrhythogenesis in guinea-pig myocardium as revealed by SEA0400.J Pharmacol Sci 2007;103:241-6.
19.Pfeiffer DR,Gunter TE,Eliseev R,Broekemeier KM,Gunter KK. Release of Ca2+from mitochondria via the saturable mechanism and the permeability transition.IUBMB Life 2001;52:205-12.
20.Campanella M,Pinton P,Rizzuto R.Mitochondrial Ca2+homeostasis in health and disease.Biol Res 2004;37:653-60.
21.Zhang M.Rhabdomyolysis and its pathogenesis.World J Emerg Med 2012;3:11-5.
22.Rios E,Pizarro G.Voltage sensor of excitation-contraction coupling in skeletal muscle.Physiol Rev 1991;71:849-908.
23.Schneider MF.Control of calcium release in functioning skeletal muscle fibers.Annu Rev Physiol 1994;56:463-84.
24.Formigli L,Sassoli C,Squecco R,Bini F,Martinesi M,Chellini F,et al. Regulation of transient receptor potential canonical channel 1(TRPC1)by sphingosine 1-phosphate in C2C1 myoblasts and its relevance for a role of mechanotransduction in skeletal muscle differentiation.J Cell Sci 2009;122:1322-33.
25.Woo JS,Kim DH,Allen PD,Lee EH.TRPC3-interacting triadic proteins in skeletal muscle.Biochem J 2008;411:399-405.
26.Sampieri A,Diaz-Munoz M,Antaramian A,Vaca L.The foot structure from the type 1 ryanodine receptor is required for functional coupling to store-operated channels.J Biol Chem 2005;280:24804-15.
27.Kiselyov KI,Shin DM,Wang Y,Pessah IN,Allen PD,Muallem S.Gating of store-operated channels by conformational coupling to ryanodine receptors.Mol Cell 2000;6:421-31.
28.Rosenberg H,Sambuughin N,Dirksen R.Malignant hyperthermia susceptibility.In:GeneReviewsatGeneTests:medicalgenetics informationresource[databaseonline].Copyright.Seattle,WA:University of Washingtone;2012.p.1997-2011.Available at:http://www .genetests.org;[accessed 23.02.2014].
29.Yarotskyy V,Protasi F,Dirkesen RT.Accelerated activation of SOCE current in myotubesfrom two mouse models of anesthetic-and heat-induced sudden death.PloS One 2013;8(10):e77633.doi:10.1371/ journal.pone.0077633
30.AllenDG.Skeletalmusclefunction:roleofionicchangesin fatigue,damage and disease.Clin Exp Pharmacol Physiol 2004;31:485-93.
31.Moopanar TR,Allen DG.Reactive oxygen species reduce myofibrillar Ca2+sensitivity in fatiguing mouse skeletal muscle at 37°C.J Physiol 2005;564:189-99.
32.Nigam S,Schewe T.PhospholipaseA(2)s and lipid peroxidation.Biochim Biophys Acta 2000;1488:167-81.
33.Carafoli HJ.Intracellular calcium homeostasis.Ann Rev Biochem 1987;56:395-433.
34.Brookes PS,Yoon Y,Robotham JL,Anders MW,Sheu SS.Calcium,ATP,and ROS:a mitochondrial love-hate triangle.Am J Physiol Cell Physiol 2004;287:C817-33.
35.Scheffler IE.A century of mitochondrial research:achievements and perspectives.Mitochondrion 2001;1:3-31.
36.Nakahara K,Yada T,Kuriyama M,Osame M.Cytosolic Ca2+increase and cell damage in L6 rat myoblasts by HMG-CoA reductase inhibitors. Biochem Biophys Res Commun 1994;202:1579-85.
37.Warren JD,Blumbergs PC,Thompson PD.Rhabdomyolysis:a review. Muscle Nerve 2002;25:332-47.
38.Kantrow SP,Piantadosi CA.Release of cytochrome c from liver mitochondria during permeability transition.Biochem Biophys Res Commun 1997;232:669-71.
39.Zamzami N,Hirsch T,Dallaporta B,Petit PX,Kroemer G.Mitochondrial implication in accidental and programmed cell death:apoptosis and necrosis.J Bioenerg Biomembr 1997;29:185-93.
40.Elsayed EF,Reilly RF.Rhabdomyolysis:a review,with emphasis on the pediatric population.Pediatr Nephrol 2009;25:7-18.
41.Khan FY.Rhabdomyolysis:a review of the literature.Neth J Med 2009;67:272-83.
42.Park HS,Jang SI,Lee YK,An HR,Park HC,Ha SK,et al.A case of rhabdomyolysis in a body-builder.Korean J Nephrol 2009;28:335-8.
43.Park CW,Ok TG,Cho JH,Lee HY,Lee SW,Chung HH,et al. Rhabdomyolysis after scuba diving.A case report.J Korean Soc Emerg Med 2004;15:622-5.
44.Huerta-Alardin AL,Varon J,Marik PE.Bench-to-bedside review:rhabdomyolysis—anoverviewforclinicians.CritCare2005;9:158-69.
45.Chatzizisis YS,Misirli G,Hatzitolios AI,Giannoglou GD.The syndrome of rhabdomyolysis:complications and treatment.Eur J Intern Med 2008;19:568-74.
46.Holt SG,Moore KP.Pathogenesis and treatment of renal dysfunction in rhabdomyolysis.Intensive Care Med 2001;27:803-11.
47.Gonzalez D.Crush syndrome.Crit Care Med 2005;33(Suppl.1):S34-41.
48.Lameire N,Vanholder R.New perspectives for prevention/treatment of acute renal failure.Curr Opin Anaesth 2000;13:105-12.
49.Sheridan AM,Bonventre JV.Cell biology and molecular mechanisms of injury in ischemic acute renal failure.Curr Opin Nephrol Hypertens 2000;9:427-34.
50.Devarajan P.Cellular and molecular derangements in acute tubular necrosis.Curr Opin Pediatr 2005;17:193-9.
51.Zager RA,Prior RB.Gentamycin and gram negative bacteremia:a synergism for the development of experimental nephrotoxic acute renal failure.J Clin Invest 1986;78:196-204.
52.Furchgott RF,Jothianandan D.Endothelial-dependent and-independent vasodilation involving cGMP:relaxation induced by nitric oxide,carbon oxide and light.Blood Vessels 1991;28:52-61.
53.Sharma VS,Traylor TG,Gardiner R,Mizukami H.Reaction of nitric oxide with hemeproteins and model compounds of hemoglobin. Biochemistry 1987;26:3837-43.
54.Molitoris BA,Sandoval R,Sutton TA.Endothelial injury and dysfunction in ischemic acute renal failure.Crit Care Med 2004;30(Suppl.5):S235-40.
55.Akimau P,Yoshiya K,Hosotsubo H,Takakuwa T,Tanaka H,Sugimoto H. New experimental model of crush injury of the hindlimbs in rats.J Trauma 2005;58:51-8.
56.Salter MS,Mullins RJ.Rhabdomyolysis and myoglobinuric renal failure in trauma and surgical patients:a review.J Am Coll Surg 1998;186:693-716.
57.Sayer SP,Clarkson PM.Exercise-induced rhabdomyolysis.Curr Sports Med Rep 2002;1:59-60.
58.CriddleLM.Rhabdomyolysis,pathophysiology,recognitionand management.Crit Care Nurse 2003;23:14-22.
59.Line RL,Rust GS.Acute exertional rhabdomyolysis.Am Fam Physician 1995;52:502-6.
60.Brown JA,Elliot MJ,Sray WA.Exercise-induced upper extremity rhabdomyolysis and myoglobinuria in shipboard military personnel.Mil Med 1994;159:473-5.
61.Paul GL,DeLany JP,Snook JT,Seifert JG,Kirby TE.Serum and urinary markersofskeletalmuscletissuedamageafterweightliftingexercise.Eur J Appl Physiol Occup Physiol 1989;58:786-90.
62.Clarkson PM.Case report of exertional rhabdomyolysis in a 12-year-old boy.Med Sci Sports Exerc 2006;38:197-200.
63.Moeckel-Cole SA,Clarkson PM.Rhabdomyolysis in a collegiate football player.J Strength Cond Res 2009;23:1055-9.
64.Russo C,Bass E.African American adolescent male basketball player with recurrent rhabdomyolysis.Med Sci Sports Exerc 2007;39:115.doi:10.1249/01.mss.0000273380.58805.99
65.Hamer R.When exercise goes awry:exertional rhabdomyolysis.South Med J 1997;90:548-51.
66.Lin H,Chie W,Lien H.Epidemiological analysis of factors influencing an episode of exertional rhabdomyolysis in high school students.Am J Sports Med 2006;34:481-6.
67.Springer BL,Clarkson PM.Two cases of exertional rhabdomyolysis precipitated by personal trainers.Med Sci Sports Exerc 2003;35:1499-502.
68.Randall T,Butler N,Vance AM.Rehabilitation of ten soldiers with exertional rhabdomyolysis.Mil Med 1996;161:564-6.
69.Phinney LT,Gardner JW,Kark JA,Wenger CB.Long-term follow-up after exertional heat illness during recruit training.Med Sci Sports Exerc 2001;33:1443-8.
70.Wallace RF,Kriebel D,Punnett L,Wegman DH,Wenger CB,Gardner JW,et al.The effects of continuous hot weather training on risk of exertional heat illness.Med Sci Sports Exerc 2005;37:84-90.
71.Skenderi KP,Kavouras SA,Anastasiou CA,Yiannakouris N,Matalas AL.Exertional rhabdomyolysis during a 246-km continuous running race. Med Sci Sports Exerc 2006;38:1054-7.
72.Clarkson PM,Hubal MJ.Are women less susceptible to exercise-induced muscle damage?Curr Opin Clin Nutr Metab Care 2001;4:527-31.
73.Aizawa H,Morita K,Minami H,Sasaki N,Tobise K.Exertional rhabdomyolysis as a result of strenuous military training.J Neurol Sci 1995;132:239-40.
74.Singhal PC,Abramovici M,Venkatesan J,Mattana J.Hypokalemia and rhabdomyolysis.Miner Electrolyte Metab 1991;17:335-9.
75.Bruso JR,Hoffman MD,Rogers IR,Lee L,Towle G,Hew-Butler T. Rhabdomyolysis and hyponatremia:a cluster of five cases at the 161-km 2009 western states endurance run.Wilderness Environ Med 2010;21:303-8.
76.Britschgi F,Zünd G.Bodybuilding:hypokalemia and hypophosphatemia. Schweiz Med Wochenschr 1991;121:1163-5.
77.Knochel JP.Catastrophic medical events with exhaustive exercise:“white collar rhabdomyolysis”.Kidney Int 1990;38:709-19.
78.Tiidus PM,Deller M,Bombardier E,Gül M,Liu XL.Estrogen supplementation failed to attenuate biochemical indices of neutrophil infiltration or damage in rat skeletal muscles following ischemia.Biol Res 2005;38:213-23.
79.Enns DL,Tiidus PM.The influence of estrogen on skeletal muscle:sex matters.Sports Med 2010;40:41-58.
80.Dieli-Conwright CM,Spektor TM,Rice JC,Sattler FR,Schroeder ET. Hormone therapy attenuates exercise-induced skeletal muscle damage in postmenopausal women.J Appl Physiol 2009;107:853-8.
81.Centers for Disease Control(CDC).External rhabdomyolysis and acute renal impairment-New York city and Massachusetts,1988.Morb Mortal Wkly Rep 1990;26:751-6.
82.Craig S.Rhabdomyolysis.2007 Available at:www.emedicine.com/ emerg/topic508.htm;[accessed 15.02.2014].
83.Shumate JB,Brooke MH,Carroll JE,Davis JE.Increased serum creatine kinase after exercise:a sex-linked phenomenon.Neurology 1979;29:902-4.
84.Pizza FX,Clark BC,De Meersman RE,Phillips SM,Stupka N,Sipila S,et al.Comments on point:counterpoint:estrogenand sex do/do not influence post-exercise indexes of muscle damage,inflammation,and repair.J Appl Physiol 2009;106:1016-20.
85.Inklebarger J,Galanis N,Kirkos J,Kapetanos G.Exercise-induced rhabdomyolysis from stationary biking:a case report.Hippokratia 2010;14:279-80.
86.Borrione P,Spaccamiglio A,Salvo RA,Mastrone A,Fagnani F,Pigozzi F.Rhabdomyolysis in a young vegetarian athlete.Am J Phys Med Rehabil 2009;88:951-4.
87.Fernandez G,Spatz ES,Jablecki C,Phillips PS.Static myopathy:a common dilemma not reflected in clinical trials.Cleve Clin J Med 2011;78:393-403.
88.Bank WJ.Myoglobinuria in marathon runners:possible relationship to carbohydrate and lipid metabolism.Ann N Y Acad Sci 1977;301:942-8.
89.Juhn MS,O’Kane JW,Vinci DM.Oral creatine supplementation in male collegiate athletes:a survey of dosing habits and side effects.J Am Diet Assoc 1999;99:593-5.
90.Sandhu RS,Como JJ,Scalea TS,Betts JM.Renal failure and exerciseinduced rhabdomyolysis in patients taking performance-enhancing compounds.J Trauma 2002;53:761-3.
91.RobinsonSJ.Acutequadricepscompartmentsyndromeand rhabdomyolysis in a weight lifter using high-dose creatine supplementation.J Am Board Fam Pract 2000;13:134-7.
92.Pritchard NR,Kalra PA.Renal dysfunction accompanying oral creatine supplements.Lancet 1998;351:1252-3.
93.Phillips PS,Haas RH.Statin myopathy as a metabolic muscle disease. Expert Rev Cardiovasc Ther 2008;6:971-8.
94.PertusiR,DickermanRD, McConathyWJ.Evaluationof aminotransferase elevations in a bodybuilder using anabolic steroids:hepatitis or rhabdomyolysis?J Am Osteopath Assoc 2001;101:391-4.
95.Bolgiano EB.Acute rhabdomyolysis due to body building exercise. Report of a case.J Sports Med Phys Fitness 1994;34:76-8.
96.Burke J,Seda G,Allen D,Knee TS.A case of severe exercise-induced rhabdomyolysis associated with a weight-loss dietary supplement.Mil Med 2007;172:656-8.
97.Kuklo TR,Tis JE,Moores LK,Schaefer RA.Fatal rhabdomyolysis with bilateral gluteal,thigh,and leg compartment syndrome after the Army Physical Fitness Test.A case report.Am J Sports Med 2000;28:112-6.
98.Baek JH,Suh BC,Kim YB,Chung PW,Moon HS,Jin DK,et al. Myopathy following ingestion of Ma-Huang(ephedra)-based herbal remedy.Korean J Neurosci 2009;27:424-7.
99.Jung MK,Callaci JJ,Lauing KL,Otis JS,Radek KA,Jones MK,et al. Alcohol exposure and mechanisms of tissue injury and repair.Alcohol Clin Exp Res 2011;35:392-9.
100.Barnes MJ,Mündel T,Stannard SR.A low dose of alcohol does not impact skeletal muscle performance after exercise-induced muscle damage.Eur J Appl Physiol 2011;111:725-9.
101.Keverline JP.Recurrent rhabdomyolysis associated with influenza-like illness in a weight-lifter.J Sports Med Phys Fitness 1998;38:177-9.
102.Sevketoglu E,Kural B,Beskardes AE,Hatipoglu S.Exertional rhabdomyolysis after influenza A(H3N2)infection in a basketball player boy.Ann Trop Paediatr 2011;31:93-6.
103.Vissing J,Haller RG.The effect of oral sucrose on exercise tolerance in patients with McArdle’s disease.N Engl J Med 2003;349:2503-9.
104.Brumback RA,Feeback DL,Leech RW.Rhabdomyolysis in childhood.A primer on normal muscle function and selected metabolic myopathies characterized by disordered energy production.Pediatr Clin North Am 1992;39:821-58.
105.Stanley CA.New genetic defects in mitochondrial fatty acid oxidation and carnitine deficiency.Adv Pediatr 1987;34:59-88.
106.Felig P,Wahren J.Fuel homeostasis in exercise.N Engl J Med 1975;258:1078-84.
107.Tonin P,Lewis P,Servidei S,DiMauro S.Metabolic causes of myoglobinuria.Ann Neurol 1990;27:181-5.
108.Saudubray JM,Charpentier C.The online metabolic and molecular bases of inherited disease.Columbus,OH:McGraw-Hill;Available at:www .ommbid.com/OMMBID/the_online_metabolic_and_molecular_bases _of_inherited_disease/b/abstract/Part6/ch66;[accessed 04.03.2014].
109.Zeharia A,Shaag A,Houtkooper RH,Hindi T,de Lonlay P,Erez G,et al. Mutations in LPIN1 cause recurrent acute myoglobinuria in childhood. Am J Hum Genet 2008;83:489-94.
110.Quinlivan R,Jungbluth H.Myophathic causes of exercise intolerance with rhabdomyolysis.Dev Med Child Neurol 2012;54:886-91.
111.Reue K,Zhang P.The lipin protein family:dual roles in lipid biosynthesis and gene expression.FEBS Lett 2008;582:90-6.
112.Zutt R,van der Kooi AJ,Linthorst GE,Wanders RJ,deVisser M. Rhabdomyolysis:reviewoftheliterature.NeuromusculDisord 2014;24:651-9.
113.Michot C,Hubert L,Brivet M,De Meirleir L,Valayannopoulos V,Müller-Felber WV,et al.LPIN1 gene mutations:a major cause of severrhabdomyolysisinearlychildhood.HumMutat2010;31:1564-73.
114.Pearcey GE,Bradbury-Squires DJ,Power KE,Behm DG,Button DC. Exertional rhabdomyolysis in an acutely detrained athlete/exercise physiology professor.Clin J Sport Med 2013;23:496-8.
115.RussellTA.Acuterenalfailurerelatedtorhabdomyolysis:pathophysiology,diagnosis,and collaborative management.Nephrol Nurs J 2000;32:409-17.
116.VisweswaranP,GuntupalliJ.Rhabdomyolysis.CritCareClin 1999;15:415-28.
117.Latham J,Campbell D,Nichols W,Mott T.Clinical inquiries.How much can exercise raise creatine kinase level-and does it matter?J Fam Pract 2008;57:545-7.
118.Vanholder R,Sever MS,Erek E,Lameire N.Rhabdomyolysis.J Am Soc Nepthrol 2000;11:1553-61.
119.Brudvig TJ,Fitzgerald PI.Identification of signs and symptoms of acute exertional rhabdomyolysis in athletes:a guide for the practitioner. Strength Cond J 2007;29:10-4.
120.Haskins N.Rhabdomyolysis and acute renal failure in intensive care.Nurs Crit Care 1998;3:283-8.
121.O’Connor FG,Brennan Jr FH,Campbell W,Heled Y,Deuster P.Return to physical activity after exertional rhabdomyolysis.Curr Sports Med Rep 2008;7:328-31.
122.Szymanski DJ.Recommendations for the avoidance of delayed-onset muscle soreness.Strength Cond J 2001;23:7-13.
123.Nosaka K,Sakamoto K,Newton M,Sacco P.The repeated bout effect of reduced-load eccentric exercise on elbow flexor muscle damage.Eur J Appl Physiol 2001;85:34-40.
124.Howatson G,van Someren KA.Repeated bout effect after maximal eccentric exercise.Int J Sports Med 2007;28:557-63.
125.Starbuck C,Eston RG.Exercise-induced muscle damage and the repeated bout effect:evidence for cross transfer.Eur J Appl Physiol 2012;112:1005-13.
126.McHugh MP.Recent advances in the understanding of the repeated bout effect:the protective effect against muscle damage from a single bout of eccentric exercise.Scand J Med Sci Sports 2003;13:88-97.
127.Brentano MA,Martins Kruel LF.A review on strength exercise-induced muscle damage:application,adaptation mechanism and limitations. J Sports Med Phys Fitness 2011;51:1-10.
128.RA McPhersonMR Pincus editors.Henry's clinical diagnosis and management by laboratory methods.21st ed.Philadelphia,PA:Saunders Elsevier;2007.p.489.
129.Noakes TD.Effect of exercise on serum enzyme activities in human. Sports Med 1987;5:245-67.
130.HarrelsonGL, FincherAL,RobinsonJB.Acuteexertional rhabdomyolysis and its relationship to sickle cell trait.J Athl Train 1995;30:309-12.
131.MontainSJ, LatzkaWA, SawkaMN.Fluidreplacement recommendations for training in hot weather.Mil Med 1999;164:502-8.
132.Sawka MN,Cheuvront SN,Carter 3rd R.Human water needs.Nutr Rev 2005;63(6 pt 2):S30-9.
133.Fowkes GS,Bartolozzi AR,Burkolter R,Sugarman E.Core temperature in a symptomatic NFL running back during a full padded pre-season practice with post practice urine indices of rhabdomyolysis.Med Sci Sports Exerc 2006;38(Suppl.5):S159.
134.Miners AL.The diagnosis and emergency care of heat related illness and sunburn in athletes:a retrospective case series.J Can Chiropr Assoc 2010;54:107-17.
135.Kerksick C,Harvey T,Stout J,Campbell B,Wilborn C,Kreider R,et al. International Society of Sports Nutrition position stand:nutrient timing.J Int Soc Sports Nutr 2008;5:17.doi:10.1186/1550-2783-5-18
136.Howarth KR,Moreau NA,Phillips SM,Gibala MJ.Coingestion of protein with carbohydrate during recovery from endurance exercise stimulates skeletal muscle protein synthesis in humans.J Appl Physiol 2009;106:1394-402.
137.Jentjens RL,van Loon LJ,Mann CH,Wagenmakers AJ,Jeukendrup AE. Addition of protein and amino acids to carbohydrates does not enhance post exercise muscle glycogen synthesis.JAppl Physiol 2001;91:839-46.
138.Millard-Stafford M,Childers WL,Conger SA,Kampfer AJ,Rahnert JA. Recovery nutrition:timing and composition after endurance exercise. Curr Sports Med Rep 2008;7:193-201.
139.Singh D,Kaur R,Chander V,Chopra K.Antioxidants in the prevention of renal disease.J Med Food 2006;9:443-50.
140.Ustundag S,Yalcin O,Sen S,Cukur Z,Ciftci S,Demirkan B. Experimental myoglobinuric acute renal failure:the effect of vitamin C. Ren Fail 2008;30:727-35.
141.Farswan M,Rathod SP,Upaganlawar AB,Semwal A.Protective effect of coenzyme Q10 in simvastatin and gemfibrozil induced rhabdomyolysis in rats.Indian J Exp Biol 2005;43:845-8.
142.Nakhostin-Roohi B,Babaei P,Rahmani-Nia F,Bohlooli S.Effect of vitamin C supplementation on lipid peroxidation,muscle damage and inflammation after 30-min exercise at 75%VO2max.J Sports Med Phys Fitness 2008;48:217-24.
143.Defilippis EM,Kleiman DA,Derman PB,DiFelice GS,Eachempati SR. Spinning-induced rhabdomyolysis and the risk of compartment syndrome and acute kidney injury:two cases and review of the literature.Sports Health 2014;6:333-5.
144.Goubier JN,Hoffman OS,Oberlin C.Exertion induced rhabdomyolysis of the long head of the triceps.Br J Sports Med 2002;36:150-1.
145.Kim SA,Jung SJ,Lee CY,Ha BG,Park KS.A case of exercise-induced rhabdomyolysiswithhepatitis.KoreanJOccupEnvironMed 2006;18:67-72.
146.Gagliano M,Corona D,Giuffrida G,Giaquinta A,Tallarita T,Zerbo D,et al.Low-intensity body building exercise induced rhabdomyolysis:a case report.Cases J 2009;2:7.doi:10.1186/1757-1626-2-7
147.Thoenes M.Rhabdomyolysis:when exercise becomes a risk.J Pediatr Health Care 2010;24:183-93.
148.Karre RP,Gujral J.Recurrent exercise-induced rhabdomyolysis due to low intensity fitness exercise in a healthy young patient.BMJ Case Rep 2011;pii:bcr0120113699.doi:10.1136/bcr.01.2011.3699
149.MacDonald R,Rosner Z,Venters H.Case series of exercise-induced rhabdomyolysis in the New York City jail system.Am J Emerg Med 2014;32:466-7.
150.Pierson EH,Bantum BM,Schaefer MP.Exertional rhabdomyolysis of the elbow flexor muscles from weight lifting.PM R 2014;6:556-9.
151.Summachiwakij S,Sachmechi I.Rhabdomyolysis induced by non strenuous exercise in a patient with graves’disease.Case Rep Endocrinol 2014;286450.doi:10.1155/2014/286450
.
E-mail address:sls98@kku.ac.kr(D.J.Sung).†These two authors contributed equally to this work.
s’contributions
28 June 2014;revised 26 October 2014;accepted 26 January 2015 Available online 3 June 2015
©2016 Production and hosting by Elsevier B.V.on behalf of Shanghai University of Sport.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
杂志排行
Journal of Sport and Health Science的其它文章
- Physical activity and health in the presence of China’s economic growth:Meeting the public health challenges of the aging population
- Physical activity and cognitive function among older adults in China:A systematic review
- Effects of Tai Ji Quan training on gait kinematics in older Chinese women with knee osteoarthritis:A randomized controlled trial
- Recruitment of older adults into randomized controlled trials:Issues and lessons learned from two community-based exercise interventions in Shanghai
- Associations between individual and environmental factors and habitual physical activity among older Chinese adults:A social-ecological perspective
- Scientific evidence is just the starting point:A generalizable process for developing sports injury prevention interventions
