24-表油菜素内酯对葡萄叶片抵御霜霉菌侵染的影响
2016-09-16栾雪涛徐世彦高江曼惠竹梅
刘 庆,栾雪涛,徐世彦,孟 莹,高江曼,惠竹梅
(西北农林科技大学葡萄酒学院/陕西省葡萄与葡萄酒工程中心/陕西省果树繁育中心,陕西杨凌 712100)
24-表油菜素内酯对葡萄叶片抵御霜霉菌侵染的影响
刘 庆,栾雪涛,徐世彦,孟 莹,高江曼,惠竹梅
(西北农林科技大学葡萄酒学院/陕西省葡萄与葡萄酒工程中心/陕西省果树繁育中心,陕西杨凌 712100)
【目的】研究外源 24-表油菜素内酯(24-epibrassinolide,EBR)处理对霜霉菌侵染葡萄叶片的影响,为探究葡萄霜霉病的致病机理提供参考。【方法】试验以欧亚种酿酒葡萄(Vitis vinifera. L)赤霞珠(Cabernet Sauvignon)叶片为材料,在霜霉菌侵染离体葡萄叶片的初期,研究不同浓度的EBR处理(0.1、0.5和1.0 mg·L-1EBR)对葡萄叶片霜霉病发病率和病情指数、霜霉菌菌丝生长、孢囊梗的形成、气孔周围孢子数量、葡萄叶片气孔开度和内源激素含量的影响及相互关系。【结果】EBR各处理均显著抑制了接种霜霉菌0.5 h后葡萄叶片气孔开度,以及接种后1 d和2 d病菌菌丝的发育。0.5和1.0 mg·L-1EBR处理均显著抑制了接种霜霉菌0.5 h后叶片气孔周围的游动孢子数量和3 d后菌丝体在侵染区域的覆盖面积;接种4 d后,EBR各处理均显著抑制了霜霉菌孢子囊数量以及叶片发病率与病情指数,且0.5 mg·L-1和1.0 mg·L-1EBR处理降低发病率和病情指数的幅度最大,发病率分别比CK降低51.4%和45.0%,病情指数分别降低71.2%和62.9%。总体而言,0.5 mg·L-1和1.0 mg·L-1EBR处理抑制霜霉菌生长发育较为显著,且二者之间无显著性差异。0.5 mg·L-1EBR处理的叶片脱落酸(ABA)、茉莉酸(JA)和水杨酸(SA)含量与CK之间均存在显著差异,气孔孔径与SA含量,ABA含量与JA含量呈显著正相关。【结论】EBR处理提高了葡萄叶片抵御霜霉菌侵染的能力,可能与其抑制病菌发育,改变寄主内源激素含量,从而促进气孔关闭等因素有关。
葡萄;霜霉病菌;24-表油菜素内酯;诱导抗病性;气孔开度
0 引言
【研究意义】葡萄霜霉病[Plasmopara viticola (Berk. & Curt.) Berl. & de Toni]是葡萄生产中最重要的病害之一。在温暖和湿润的地区,霜霉病在短时间内迅速蔓延,影响葡萄叶片和果实正常发育,造成巨大的经济损失[1]。油菜素内酯(Brassinosteroids,BRs),是植物中广泛存在的甾醇类植物激素。研究发现BRs能够调节一系列的植物生长与发育[2],而且越来越多的试验证明 BRs不仅能够缓解植物非生物胁迫,例如盐胁迫[3-4]、寒冷胁迫[5-6]、干旱胁迫[7-8]、重金属胁迫[9-10],还能够提高植物对生物胁迫的抗性[11-13]。NAKASHITA等[11]研究指出BRs能够激发烟草针对烟草花叶病毒(TMV)、烟草野火病菌(Pseudomonas syringae pv. Tabaci)和粉孢菌(Oidium sp.)的侵染产生防御反应。在冬枣与柑橘的贮藏试验中,用BRs与EBR分别处理均减轻了采后果实的发病[14-15]。因此,研究24-表油菜素内酯(EBR)提高葡萄叶片对霜霉病的抗性及其作用机理对霜霉病的防治具有重要意义。【前人研究进展】在一个生长季节内,葡萄霜霉菌(P. viticola)的孢子囊和游动孢子可通过气孔对葡萄进行多次再侵染[16],而在霜霉菌侵染葡萄叶片的过程中,通过叶片的组织观察可以直观高效地观察到寄主与病原菌之间的相互作用,进而判断寄主的抗病性或霜霉菌的致病力[17]。通过荧光显微镜观察霜霉菌侵染葡萄叶片(接种后24、30、48和120 h),发现用硫胺素处理能提高葡萄叶片的抗病性[18]。在研究核黄素诱导葡萄对霜霉菌侵染产生防御反应的试验中,通过接种霜霉菌后24、48和72 h显微观察病菌从孢子囊长出初生菌丝到形成网状菌丝体,明确了核黄素处理提高了葡萄对霜霉病的抗性[19]。气孔是病菌侵染的潜在天然通道,植物激素在调节气孔关闭上发挥着重要的作用[20-22],能通过保卫细胞关闭气孔,阻止病菌的侵染、蔓延[20]。大量试验证明脱落酸(ABA)积极地参与调控气孔关闭过程,以此来抵御病原菌对寄主细胞侵染[14,23]。保卫细胞在感知病菌相关分子信号后,激发水杨酸(SA)通过ABA信号途径诱导气孔关闭[20];另外,研究还发现茉莉酸(JA)依靠Ca2+,NO、ROS信号,并激发K+通道和S型阴离子通道调节气孔开度[20-21]。【本研究切入点】EBR处理能够缓解葡萄非生物胁迫,例如寒冷胁迫,盐胁迫等。而EBR处理缓解能否缓解葡萄生物胁迫,如霜霉病菌侵染等还未见报道。【拟解决的关键问题】以欧亚种酿酒葡萄赤霞珠叶片为材料,通过离体叶片接种葡萄霜霉菌,采用荧光显微镜和扫描电镜观察 EBR处理对霜霉菌侵染葡萄叶片过程中形态结构和叶片气孔开度的影响;并通过测定激素水平变化与气孔开度的关系,探明 EBR处理提高葡萄叶片抵御霜霉菌侵染的作用机制,为全面揭示葡萄抗霜霉病的作用机理提供理论依据。
1 材料与方法
试验于 2015年在西北农林科技大学葡萄酒学院进行。
1.1试验材料
试验场所为葡萄冷棚温室。供试品种为欧亚种(Vitis vinifera L.)酿酒葡萄赤霞珠(Cabernet Sauvignon)。选择长势一致、健康状况良好的植株,带柄采集副梢幼叶(从上至下第3—6节叶片)。
在陕西杨凌地区葡萄霜霉病发病初期,采集发病的葡萄幼叶,用无菌水冲洗叶片发病区域,收集霜霉菌孢子囊悬浮液并摇匀,利用血球计数板统计霜霉菌孢子囊数量,调配孢子囊悬浮液至试验浓度(5×105个/mL)。
1.2试验设计
本试验采用的24-表油菜素内酯(24- epibrassinolide,EBR)购自美国Sigma公司,Ruibio分装。试验共设4个处理,分别是①EBR1:0.1 mg·L-1EBR;②EBR2:0.5 mg·L-1EBR;③EBR3:1.0 mg·L-1EBR;④CK:清水处理。EBR母液配置方法:称取1.0 mg EBR,用98%(v/v)乙醇将其溶解。将 EBR母液稀释到适宜浓度,乙醇最终含量为0.1%(v/v),用吐温-80作为
展开剂,最终含量为 0.1%(v/v)。清水对照中加入同样体积的98%乙醇和吐温-80。从温室中采集的葡萄叶片(带叶柄)经无菌水清洗2—3遍,自然晾干。将葡萄幼叶的叶柄斜切至一定长度,并将葡萄叶柄浸没在不同浓度的 EBR溶液中,然后将叶片放置在温度22℃、湿度95%、光照强度1 000 lx的人工气候箱中3 h。激素处理结束后,立即用打孔器(d=14 mm)在叶片上打孔,收集叶圆片并置于垫有湿润滤纸的培养皿中。EBR处理3.5 h后接种葡萄霜霉菌,每个叶圆片接种40 μL葡萄霜霉菌悬浮液,且浓度为5×105个孢子囊/mL。接种完成后,将培养皿放置于白天温度为22℃、湿度95%、光照强度1 000 lx和夜晚温度20℃、湿度90%的人工气候箱中。试验中每个处理设置3次重复,每个重复包含10个培养皿,每个培养皿中放置10枚葡萄叶圆片。分别在接种霜霉菌后0.5 h、1、2、3和4 d后采样,用于观察和测定霜霉菌侵染过程中病菌和叶片相关指标。
1.3测定指标与方法
1.3.1气孔孔径(stomatal aperture)的测定 接种霜霉菌0.5 h后,切取边长为5 mm的正方形叶片,浸没于2 mL 4%的戊二醛溶液中12 h,固定叶片组织。次日将葡萄叶片用磷酸缓冲液清洗3次,每次10 min。随后用30%、50%、70%、80%和90%的梯度酒精进行脱色处理,每个浓度处理一次,每次10—15 min,最后用100%的酒精脱色3次,每次30 min。脱色后的叶片用乙酸异戊酯置换30 min。叶片经零界点干燥和喷金处理后,置于场发射扫描电镜下观察[24]。
1.3.2气孔周围平均游动孢子数量(number of spores per stoma) 接种霜霉菌0.5 h后,将采集到的葡萄叶圆片样品浸没在盛有9.2 mmol·L-1三氯乙酸溶液的试管中进行温和脱色。配置1%(v/v)Blankophor (Maya,China)母液,并用蒸馏水稀释母液浓度至5%。将葡萄叶圆片放置在载玻片上,并向葡萄叶圆片背面上添加2 mL稀释后的荧光增白剂溶液,染色2 min后,盖上盖玻片。将制备好的样品放置在荧光显微镜下观察。荧光发射的激发波长为340 nm,在380 nm滤光停止[16]。
1.3.3苯胺蓝染色法 将葡萄叶圆片浸没在盛有 1 mol·L-1KOH溶液的试管中,并将试管放置在121℃的灭菌锅中10 min,进行高温脱色。脱色完成后,用无菌水清洗叶圆片,共清洗3次,每次15 min,然后用0.05%苯胺蓝染液(以 pH 9—10的 0.067 mol·L-1K2HPO4溶液为溶剂)进行组织结构染色[16]。苯胺蓝染色后在荧光显微镜下观察拍照[25],记录菌丝长度与霜霉菌第1—4天的形态特征变化。
1.3.4发病率与病情指数的调查 先针对叶圆片进行数码拍照,然后应用Photopshop CS5统计发病面积[25];依据霜霉病发病面积占叶圆片面积的百分比划分8个等级,其中0级:未发病,1级:0.1%—5.0%,2级:5.1%—15.0%,3级:15.1%—30.0%,4级:30.1%—45.0%,5级:45.1%—60.0%,6级:60.1%—85.0%,7级:85.1%—100%。

1.3.5叶片内源激素ABA、SA、JA含量的测定 采用高效液相色谱-质谱联用仪测定[26]。
1.4数据处理与分析
试验采用SPSS 18.0、Photoshop CS5和Excel软件进行数据分析,采用Origin 8.5软件进行作图分析。
2 结果
2.1EBR处理对叶片发病率与病情指数的影响
接种霜霉菌第4天,部分叶圆片上的发病症状肉眼即可观察,在病原菌侵染区域开始观察到孢囊梗。由图1中叶圆片发病照片可知,CK对应的叶圆片上附着大量孢囊梗,而EBR处理的叶圆片孢囊梗和菌丝体数量显著低于CK,其中EBR2与EBR3处理对应的病菌发育受到的抑制最明显。EBR各处理的葡萄叶圆片的发病率与病情指数均显著低于CK(图2),其中以EBR2和EBR3处理的抑制效果最显著,叶片发病率分别比CK降低了51.4%和45.0%。
2.2EBR处理对叶片气孔孔径及气孔周围游动孢子的影响
EBR处理显著影响寄主叶片的气孔张开程度(图3)。接种霜霉菌0.5 h后,CK的气孔孔径显著高于所有EBR处理;随着EBR浓度的增加,叶片气孔孔径降低,其中,EBR2和EBR3处理使气孔孔径分别比CK降低了63.7%和77.8%。接种0.5 h后,EBR2 和EBR3处理的葡萄叶片气孔周围孢子数量显著低于CK,但EBR1与CK之间无显著差异(图4)。
2.3EBR处理对霜霉菌侵染叶片过程中形态特征的影响
接种霜霉菌后第1天,气孔下囊泡已经长出初生菌丝(图5),EBR处理的霜霉菌菌丝长度均显著低于CK。其中,EBR2和EBR3处理的菌丝长度分别只有CK的48.0%和55.3%,且均显著低于EBR1处理,二者间无显著差异(图6-A)。
霜霉菌菌丝长出初生菌丝后,菌丝进一步伸长,陆续形成吸器(图5)。接种病菌后第2天,CK的菌丝长度显著高于所有EBR处理,EBR1、EBR2和EBR3的菌丝长度分别比CK降低了32.2%、58.0%和55.1%。EBR2和EBR3处理下的菌丝发育受到的抑制作用最为明显,且二者之间无显著性差异(图6-B)。

图1 EBR处理对抵御葡萄霜霉菌侵染叶片的影响Fig. 1 Effect of EBR treatments on the resistance against P. viticola
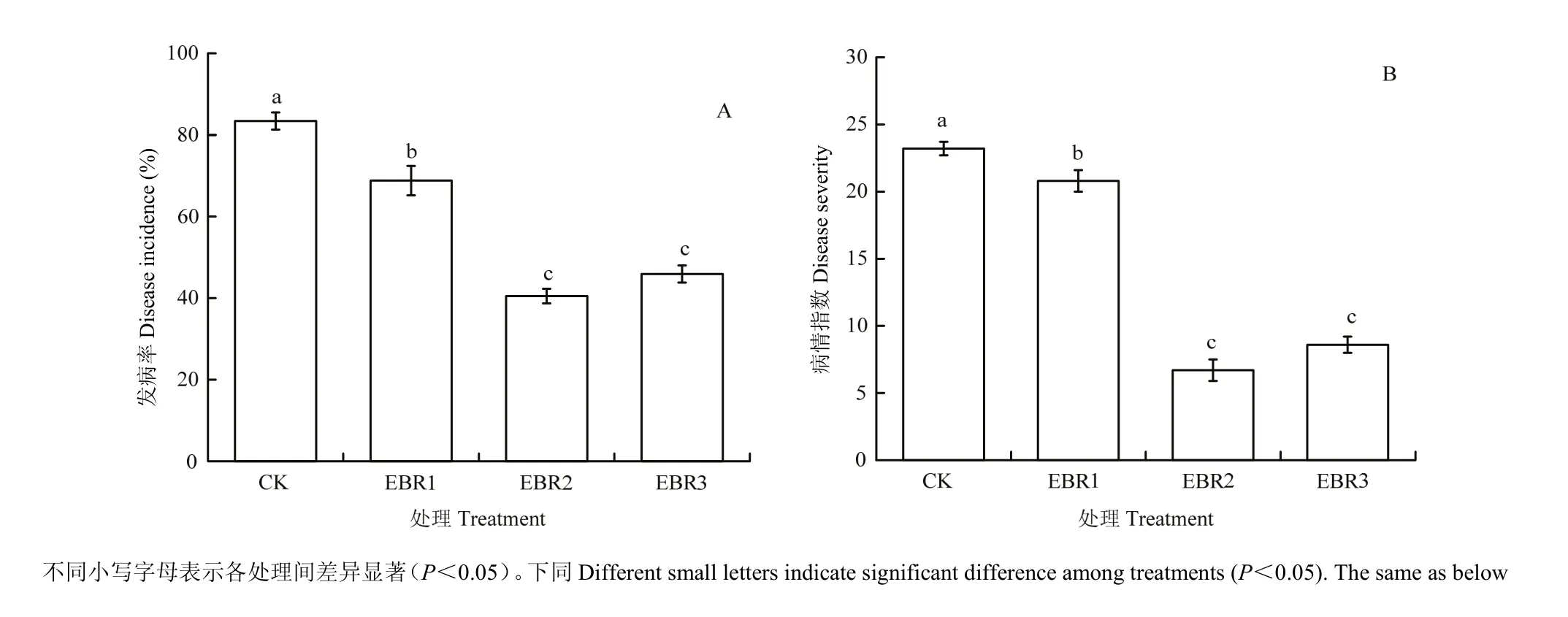
图2 EBR处理对葡萄叶片霜霉病的影响Fig. 2 Effect of EBR treatment on the downy mildew of grapevine leaf
接种霜霉菌后第3天,侵染区域的叶肉细胞周围出现网状的菌丝体,尤其是 CK,叶圆片上菌丝体基本已布满了侵染区域的叶肉细胞(图5)。相比之下,EBR2与EBR3的菌丝体生长发育受到强烈的抑制。
接种霜霉菌后第4天,叶片病菌侵染区域开始出现孢囊梗,CK处理的叶片上病菌侵染区域出现大量的孢囊梗,EBR1处理的叶圆片上可以观察到少量的孢囊梗,而EBR2与EBR3处理的侵染区域则未发现孢囊梗(图5)。
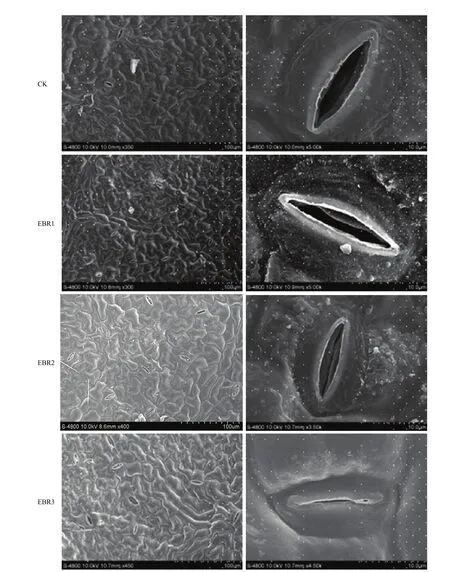
图3 葡萄叶圆片上气孔与表皮细胞Fig. 3 Stomata and epidermal cells on the grape leaf discs
2.4EBR处理对葡萄叶片内源激素的影响
接种霜霉菌0.5 h后,霜霉菌孢子向叶片气孔周围聚集。在受到外源EBR处理与病菌侵染而引起的寄主自身防御反应的作用下,葡萄叶片的内源激素发生变化,EBR2处理的叶片ABA、JA和SA含量与CK之间均存在显著差异(表1)。SA与气孔孔径相关系数是0.966,随着SA含量的增加,气孔孔径呈下降趋势。ABA与JA之间的相关系数为0.985,二者呈显著的正相关(表2)。
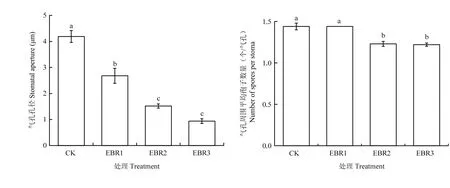
图4 EBR处理对葡萄叶片气孔孔径与气孔周围平均游动孢子数量的影响Fig. 4 Effect of EBR treatment on stomatal aperture and number of spores per stoma of grapevine leaf
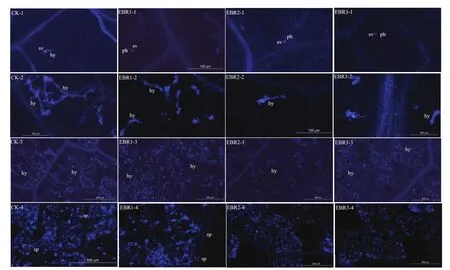
图5 EBR处理对霜霉菌侵染葡萄叶片过程中形态特征的影响Fig. 5 Effect of EBR treatment on the morphology of P. viticola invading the grapevine leaf
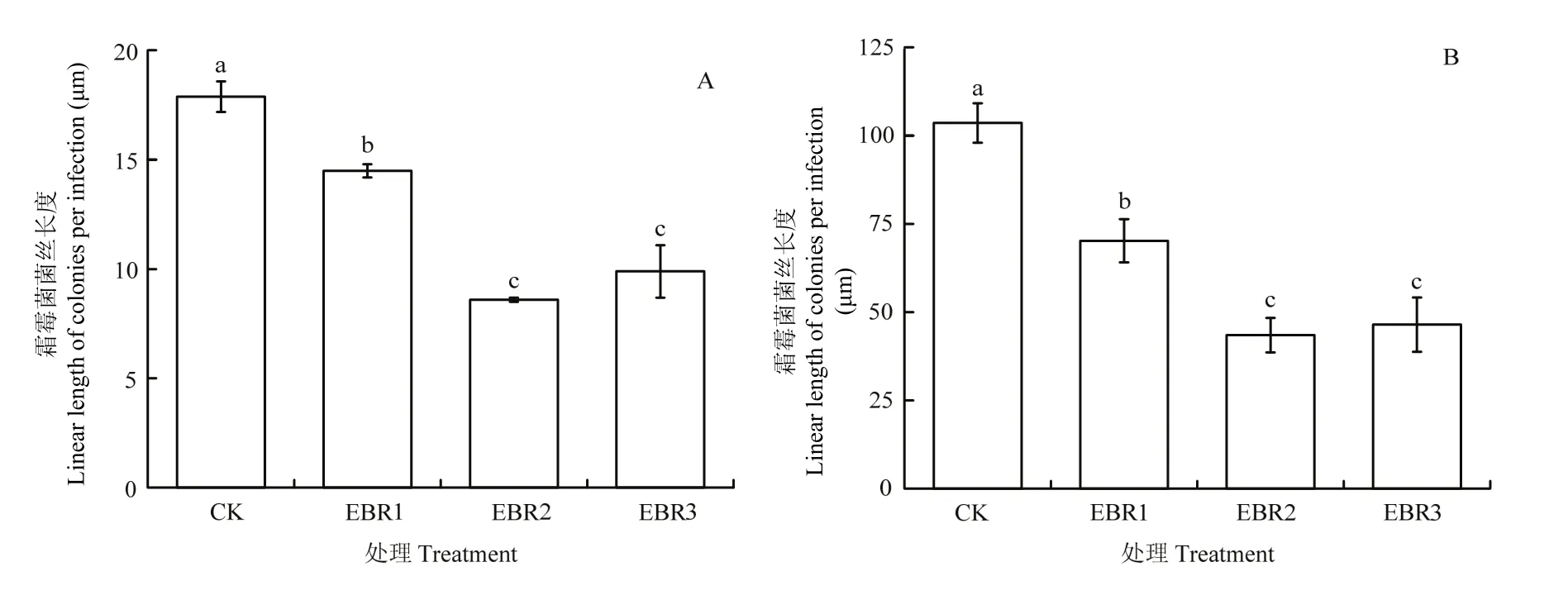
图6 EBR处理对平均每个感染区的霜霉菌菌丝长度的影响Fig. 6 Effect of EBR treatment on the linear length of P. viticola colonies per infection
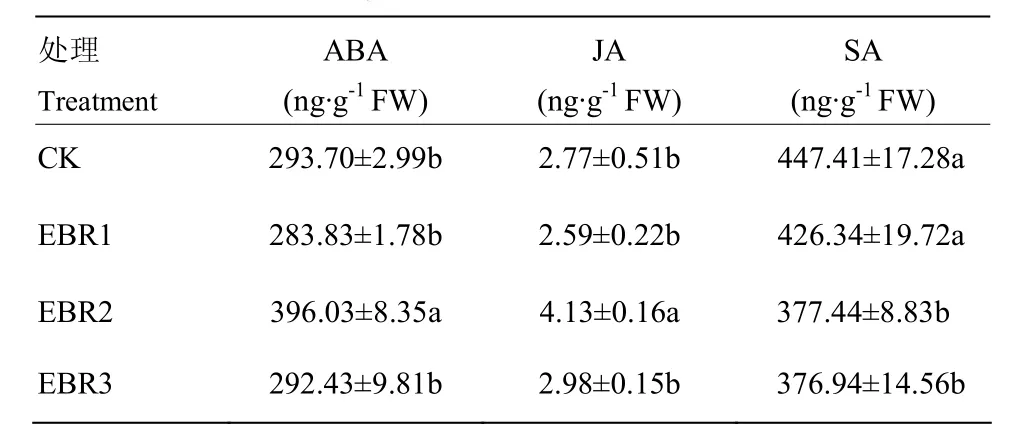
表1 EBR处理对葡萄叶片内源激素含量的影响Table 1 Effect of EBR treatment on the content of endogenous hormone in grapevine leaf
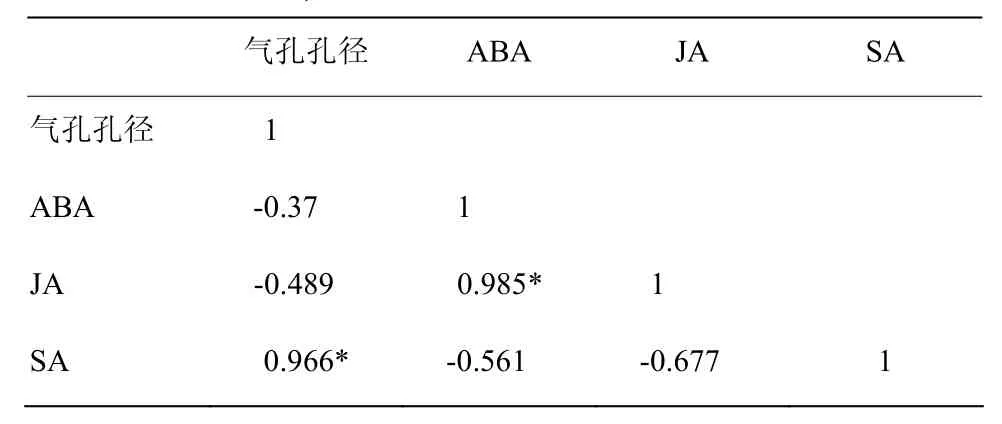
表2 气孔孔径、ABA、SA和JA含量之间的相关性Table 2 Correlations between stomatal aperture and contents of ABA, JA and SA
3 讨论
油菜素内酯作为一种天然的植物激素,既能促进植物生长发育,还能缓解一系列非生物与生物对植物的胁迫[27]。本研究结果表明,24-表油菜素内酯处理抑制了霜霉菌的菌丝伸长、菌丝体蔓延和孢子囊的产生,从而抑制了霜霉菌的初期生长发育。
植物的气孔既是调节气体交换与蒸腾作用的重要途径,也是病原菌侵染植物的潜在天然通道[20]。番茄细菌性斑点病菌(Pseudomonas syringae pv. Tomato DC3000)选择性地朝气孔方向移动,并从开放的气孔处侵入[28]。小麦条锈菌(Puccinia striiformis f.sp. tritici)侵染寄主的方式也是通过气孔侵入[29]。为了防止病原菌的侵染,保卫细胞在感知到病菌相关分子模型后,通过调节气孔孔径防止病原菌的入侵。本试验中,EBR处理接种霜霉菌后的葡萄叶片气孔孔径显著低于CK,葡萄霜霉菌生长发育受到抑制可能与叶片气孔孔径减小而不利于病原菌的侵入有关。
番茄属(Lycopersicon spp.)中的一些植物在ABA或黑暗环境的诱导下气孔关闭,降低了野油菜黄单胞菌(Xanthomonas campestris pv. vesicatoria)的发病率与病情指数[30]。KIEFER等[16]的研究也发现,ABA能诱导气孔关闭,在一定程度上抑制霜霉菌孢子朝气孔方向的游动。另外,两种SA缺陷植株(SA羟化酶过量表达的nahG植株和SA感应缺陷突变体sid2)均能破坏病菌相关分子模型(MAMP)或病菌诱导气孔关闭[27],说明SA在气孔运动中发挥着重要作用。ZENG等[31]的研究表明SA调节气孔关闭需要通过ABA信号途径发挥作用。JA迟钝突变体,jasmonate resistance 1 和coronatine insensitive 1均表现出ABA诱导气孔关闭效应减弱。相反地,ABA迟钝突变体aba intensitive 2和ABA低敏感突变体ost1、cpk6均表现为JA诱导气孔关闭的效应减弱[20]。由此可见,JA与ABA相互作用,共同参与了气孔运动的调节。本试验结果显示,EBR2处理的葡萄叶片ABA和JA含量显著高于CK,其气孔孔径的开张程度相比CK也受到显著抑制,且ABA与JA之间存在显著正相关。另外,随着处理的EBR浓度增加,SA含量逐渐下降,气孔孔径逐渐降低,且SA与气孔孔径之间存在显著正相关。EBR处理调节气孔关闭可能与内源激素ABA、SA和JA相互作用,共同调节气孔运动有关,但对ABA、SA和JA如何相互作用并调节气孔运动的作用机理还有待进一步研究。
4 结论
0.1、0.5和1.0 mg·L-1浓度的24-表油菜素内酯处理均能抑制葡萄霜霉菌侵染赤霞珠葡萄叶片,缓解葡萄霜霉病发生,其中0.5和1.0 mg·L-124-表油菜素内酯抑制效果较为显著。其缓解作用可能与 24-表油菜素内酯减小气孔孔径,降低气孔周围孢子囊数量,促进叶片气孔关闭有关。24-表油菜素内酯处理后,叶片ABA、JA和SA含量与CK之间均存在显著差异,且SA与气孔孔径呈显著相关,ABA与JA含量呈显著相关;推测气孔的关闭与EBR处理后内源激素ABA、SA和JA的相互作用有关,三者共同诱导了气孔运动。
References
[1] MÜLLER K, SLEUMER H. Biologische untersuchungen über die peronosporakrankheit des weinstocks mit besonderer berücksichtigung ihrer bekӓmpfung nach inkubationsmethode. Z Wiss Landwirtsch,1934, 79: 509-576.
[2] CLOUSE S D, SASSE J M. Brassinosteroids: Essential regulators of plant growth and development. Annual Review of Plant Physiology and Plant Molecular Biology, 1998, 49: 427-451.
[3] YUAN L, ZHU S, LI S, SHU S, SUN J, GUO S. 24-Epibrassinolide regulates carbohydrate metabolism and increases polyamine content in cucumber exposed to Ca(NO3)2stress. Acta Physiologiae Plantarum,2014, 36: 2845-2852.
[4] SHAHID M A, BALAL R M, PERVEZ M A, ABBAS T, AQEEL M A, RIAZ A, MATTSON N S. Exogenous 24-epibrassinolide elevates the salt tolerance potential of pea (Pisum sativum L.) by improving osmotic adjustment capacity and leaf water relations. Journal of Plant Nutrition, 2015, 38: 1050-1072.
[5] XI Z M, WANG Z Z, FANG Y L, HU Z Y, HU Y, DENG M M,ZHANG Z W. Effects of 24-epibrassinolide on antioxidation defense and osmoregulation systems of young grapevines (V. vinifera L.)under chilling stress. Plant Growth Regulation, 2013, 71: 57-65.
[6] WU X X, DING H D, CHEN J L, ZHU Z W, ZHA D S. Amelioration of oxidative damage in Solanum melongena seedlings by 24-epibrassinolide during chilling stress and recovery. Biologia Plantarum, 2015, 59: 350-356.
[7] TALAAT N B, SHAWKY B T, IBRAHIM A S. Alleviation of drought-induced oxidative stress in maize (Zea mays L.) plants by dual application of 24-epibrassinolide and spermine. Environmental and Experimental Botany, 2015, 113: 47-58.
[8] YUAN G F, JIA C G, LI Z, SUN B, ZHANG L P, LIU N, WANG Q M. Effect of brassinosteroids on drought resistance and abscisic acid concentration in tomato under water stress. Scientia Horticulturae,2010, 126: 103-108.
[9] Ali B, Hasan S A, Hayat S, Hayat Q, Yadav S, Fariduddin Q, Ahmad A. A role for brassinosteroids in the amelioration of aluminium stress through antioxidant system in mung bean (Vigna radiata L. Wilczek). Environmental and Experimental Botany, 2008, 62: 153-159.
[10] KANWAR M K, BHARDWAJ R, ARORA P, CHOWDHARY S,SHARMA P, KUMAR S. Plant steroid hormones produced under Ni stress are involved in the regulation of metal uptake and oxidative stress in Brassica juncea L. Chemosphere, 2012, 86: 41-49.
[11] NAKASHITA H, YASUDA M, NITTA T, ASAMI T, FUJIOKA S,ARAI Y, SEKIMATA K., TAKATSUTO S, YAMAGUCHI I,YOSHIDA S. Brassinosteroid functions in a broad range of disease resistance in tobacco and rice. Plant Journal, 2003, 33: 887-898.
[12] 屈淑平, 王力莉, 崔崇士. 表油菜素内酯诱导南瓜幼苗抗疫病研究. 中国蔬菜, 2008(5): 13-16. Qu S P, Wang L L, Cui C S. Studies on resistance of pumpkin seedlings to phytophthora capsici by exogenous 2,4-epibrassinolide treatment. China Vegetables, 2008(5): 13-16. (in Chinese)
[13] 尚庆茂, 张志刚, 董涛, 宋士清, 李晓芬. 油菜素内酯诱导黄瓜幼苗抗灰霉病研究. 应用与环境生物学报, 2007, 13(5): 630-634. SHANG Q M, ZHANG Z G, DONG T, SONG S Q, LI X F. Resistance of cucumber seedings to Botrytis cinerea induced by exogenous brassinolide treatment. Chinese Journal of Applied and Environmental Biology, 2007, 13(5): 630-634. (in Chinese)
[14] ZHU Z, ZHANG Z, QIN G, TIAN S. Effects of brassinosteroids on postharvest disease and senescence of jujube fruit in storage. Postharvest Biology and Technology, 2010, 56: 50-55.
[15] ZHU F, YUN Z, MA Q, GONG Q, ZENG Y, XU J, CHENG Y,DENG X. Effects of exogenous 24-epibrassinolide treatment on postharvest quality and resistance of Satsuma mandarin (Citrusunshiu). Postharvest Biology and Technology, 2015, 100: 8-15.
[16] KIEFER B, RIEMANN M, BUCHE C, KASSEMEYER H H, NICK P. The host guides morphogenesis and stomatal targeting in the grapevine pathogen Plasmopara viticola. Planta, 2002, 215: 387-393.
[17] DIEZ-NAVAJAS A M, GREIF C, POUTARAUD A, MERDINOGLU D. Two simplified fluorescent staining techniques to observe infection structures of the oomycete Plasmopara viticola in grapevine leaf tissues. Micron, 2007, 38: 680-683.
[18] BOUBAKRI H, WAHAB M A, CHONG J, BERTSCH C, MLIKI A,SOUSTRE-GACOUGNOLLE I. Thiamine induced resistance to Plasmopara viticola in grapevine and elicited host-defense responses,including HR like-cell death. Plant Physiology and Biochemistry,2012, 57: 120-133.
[19] BOUBAKRI H, CHONG J, POUTARAUD A, SCHMITT C,BERTSCH C, MLIKI A, MASSON J E, OUSTRE-GACOUGNOLLE I. Riboflavin (Vitamin B-2) induces defence responses and resistance to Plasmopara viticola in grapevine. European Journal of Plant Pathology, 2013, 136: 837-855.
[20] SAWINSKI K, MERSMANN S, ROBATZEK S, BOHMER M. Guarding the green: pathways to stomatal immunity. Molecular Plant-microbe Interactions, 2013, 26: 626-632.
[21] 陈德龙, 叶映微, 刘丽红, 张敏, 刘天宇, 汪俏梅. 植物保卫细胞的激素信号转导网络研究进展. 核农学报, 2016, 30(1): 65-71. CHEN D L, YE Y W, LIU L H, ZHANG M, LIU T Y, WANG Q M. Phytohormone signaling network in plant guard cells. Journal of Nuclear Agricultural Sciences, 2016, 30(1): 65-71. (in Chinese)
[22] 田露, 杨波, 田甜, 王兰兰. 植物激素对气孔运动的调节. 沈阳师范大学学报(自然科学版), 2015, 33(3): 442-446. TIAN L, YANG B, TIAN T, WANG L L. Regulation of stomatal movement by plant hormones. Journal of ShenYang Normal University (Natural Science Edition), 2015, 33(3), 442-446. (in Chinese)
[23] ESPINO R R C, NESBITT W B. Infection and development of Plasmopara viticola (B. et C.) Berl. et de T. on resistant and susceptible gravepines (Vitis sp.). Philippine Journal of Crop Science,1982, 7: 114-116.
[24] TROUVELOT S, VARNIER,A L, ALLEGRE M, MERCIER L,BAILLIEUL F, ARNOULD C. GIANINAZZI-PEARSON V,KLARZYNSKI O, JOUBERT J M, PUGIN A, DAIRE X. A beta-1,3 glucan sulfate induces resistance in grapevine against Plasmopara viticola through priming of defense responses, including HR-like cell death. Molecular Plant-Microbe Interactions, 2008, 21: 232-243.
[25] LIU R, WANG L, ZHU J, CHEN T, WANG Y, XU Y. Histological responses to downy mildew in resistant and susceptible grapevines. Protoplasma, 2015, 252: 259-270.
[26] PAN X, WELTI R, WANG X. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nature Protocols, 2010, 5:986-992.
[27] 惠竹梅, 王智真, 胡勇, 邓敏敏, 张振文. 24-表油菜素内酯对低温胁迫下葡萄幼苗抗氧化系统及渗透调节物质的影响. 中国农业科学, 2013, 46(5): 1005-1013. XI Z M, WANG Z Z, HU Y, DENG M M, ZHANG Z W. Effects of 24-epibrassinolide on the antioxidant system and osmotic adjustment substance in grape seedlings (V. vinifera L.) under chilling stress. Scientia Agricultura Sinica, 2013, 46(5): 1005-1013. (in Chinese)
[28] MELOTTO M, UNDERWOOD W, KOCZAN J, NOMURA K, HE S Y. Plant stomata function in innate immunity against bacterial invasion. Cell, 2010, 126: 969-980.
[29] MOLDENHAUER J, MOERSCHBACHER B M, VAN DER WESTHUIZEN A J. Histological investigation of stripe rust (Puccinia striiformis f.sp tritici) development in resistant and susceptible wheat cultivars. Plant Pathology, 2006, 55: 469-474.
[30] RAMOS L J, VOLIN R B. Role of stomatal opening and frequency on infection of Lycopersicon spp. by Xanthomonas campestris pv. vesicatoria. Phytopathology, 1987, 77: 1311-1317.
[31] ZENG W, HE S Y. A prominent role of the flagellin receptor FLAGELLIN-SENSING2 in mediating stomatal response to Pseudomonas syringae pv. tomato DC3000 in Arabidopsis. Plant Physiology, 2010, 153: 1188-1198.
(责任编辑 赵伶俐)
Effect of 24-epibrassinolide Treatment on Grapevine Leaf Against Plasmopara viticola
LIU Qing, LUAN Xue-tao, XU Shi-yan , MENG Ying, GAO Jiang-man, XI Zhu-mei
(College of Enology, Northwest A&F University/Shaanxi Engineering Research Center for Viti-Viniculture/Shaanxi Provincial Fruit Trees Propagation Center, Yangling 712100, Shaanxi)
【Objective】 The study researched the effect of 24-epibrassinolide (EBR) treatment on the resistance in grapevine leaf against Plasmopara viticola and the mechanism of the induced resistance. 【Method】 Cabernet Sauvignon (Vitis vinifera L.)grapevine leaves were used for experimental materials. At the early development of P. viticola invading grapevine leaf, the effect of different concentration of exogenous EBR (0.1, 0.5 and 1.0 mg·L-1EBR) on the disease incidence and severity of downy mildew of grapevine leaves, linear length of colonies per infection, the development of P. viticola sporangiophores while this pathogen invading the grapevine leaf, the number of spores per stoma, the stomatal aperture and the content of endogenous hormone and the relation between hormone content and stomatal aperture were investigated. 【Result】 EBR-treated leaves had a significantly lower stomatal aperture at 0.5 h after inoculation. Both on 1 d and 2 d, a significantly lower linear length of P. viticola colonies per infection was observed in all EBR treatments. The spread of mycelium almost covered most of the infection area in control while 0.5 and 1.0 mg·L-1EBR treated leaf resulted in a markedly restricted number of spores per stoma and growth of P. viticola after 3 days of inoculation. After 4 days of inoculation, EBR treatment significantly controlled the development of P. viticola sporangiophores,disease incidence and disease severity, and 0.5 mg·L-1and 1.0 mg·L-1EBR treatment resulted in higher resistance. The leaves treatedwith 0.5 mg·L-1and 1.0 mg·L-1had a lower disease incidence and severity of downy mildew, while disease incidence was decreased by 51.4% and 45.0%,and disease severity drop by 71.2% and 62.9%, and there is no significance between the two treatment. There was a significant difference in ABA, JA and SA contents in grape leaves between CK and 0.5 mg·L-1EBR treatment. Stomatal aperture has significantly positive correlation with SA content while ABA content does with JA content. 【Conclusion】 The increased resistance against P. viticola invasion was possibly related with the suppression of pathogen development and the stomata closure which plant hormone crosstalk involved in.
grapevine; Plasmopara viticola; 24-epibrassinolide; induced resistance; stomata aperture
2015-12-01;接受日期:2016-05-25
国家现代农业产业技术体系建设专项(CARS-30-zp-9)、陕西省自然科学基金(2011JM3004)、西北农林科技大学基本科研业务费专项(QN2009059)
联系方式:刘庆,E-mail:lq0418nwafu@sina.com。通信作者惠竹梅,E-mail:xizhumei@nwsuaf.edu.cn
