Synthesis and Surface Activity of Heterogemini Imidazolium Surfactants
2016-09-13XiaohuiZhaoZhiwenYeSchoolofChemicalEngineeringNanjingUniversityofScienceandTechnologyNanjing210094ChinaDatedReceivedonJune222015AcceptedonAugust062015
Xiao-hui Zhao,Zhi-wen YeSchool of Chemical Engineering,Nanjing University of Science and Technology,Nanjing 210094,China(Dated:Received on June 22,2015;Accepted on August 06,2015)
Synthesis and Surface Activity of Heterogemini Imidazolium Surfactants
Xiao-hui Zhao,Zhi-wen Ye∗
School of Chemical Engineering,Nanjing University of Science and Technology,Nanjing 210094,China
(Dated:Received on June 22,2015;Accepted on August 06,2015)
A series of heterogemini imidazolium surfactants with two-methylene spacer groups([Cm-2-Cnim]Br2,m,n=8,10,12,14,16;m/=n)have been synthesized and characterized by1H NMR and ESI-MS spectroscopy.The effects of various reaction parameters,including stoichiometry,reaction temperature and time,were investigated.In addition,the surface activity study about heterogemini imidazolium surfactants was carried out and the influences of dissymmetric degree on the surface properties were also discussed.
Heterogemini imidazolium surfactants,Synthesis,Optimal conditions,Surface activity,Dissymmetric degree
I.INTRODUCTION
Gemini surfactants are composed of two amphiphilic moieties covalently connected by a rigid or flexible spacer at the level of the head groups.As a new generation of surfactants,gemini surfactants[1-3]demonstrate remarkable features in reducing surface tension and forming micelles.In addition,they have special physicochemical properties,such as high density,low Krafft point,and unique rheological properties[4].Consequently,gemini surfactants have promising applications in skin care,chemical separations,organic synthesis,emulsifier,medicine,electrochemistry,nanomaterial synthesis,and biocatalysis[5],etc.Compared with the conventional gemini surfactants,gemini imidazolium surfactants[6]have several advantages and potential applications in many areas.For example,owing to the charge density dispersion of imidazolium head groups,this kind of gemini imidazolium surfactants show the inherent ionic nature of ionic liquids[7].As cationic micelle systems,they display a significantly stronger tendency toward self-aggregation due to the distinct polarizability of imidazolium head groups and thus they can be used as supramolecular templates for the synthesis of functional materials[8].As the reverse-micelle systems,the reverse micelles with big imidazolium head groups show higher capacity for solutes than those of quaternary ammonium cationic gemini surfactants.Besides,they can form compact membranes owing to the strong attraction between imidazolium head groups and aromatic rings through π-π interaction[9],which extends their potential applications in biology[18].
Heterogemini surfactants are a relatively new class of gemini surfactants which have the dissymmetric molecular structures with two different hydrophobic chains or two different hydrophilic head groups,linked by a rigid or flexible spacer group.On the account of more molecular structure factors that can be regulated and governed,some novel results have been obtained,such as,enhanced driving force of enthalpy factor,constructed counterions-free systems by the heterogeminis containing cationic-anionic head groups and bound vesicles formed by heterogeminis making use of their different length alkyl chains,etc.[10-14].These gemini surfactants offer additional ways to control the shape of surfactant assemblies by varying the difference in the length of the hydrocarbon chains or the nature of the head groups.Oda and co-workers[15,16]studied dissymmetric cationic quaternary ammonium gemini surfactants with the general structure[CmH2m+1(CH3)2N-(CH2)s-N(CH3)2CnH2n+1]Br2,designated as CmCsCnBr2series(m/=n).Their results indicated that the hydrophobic chain length and dissymmetry of the surfactants have a strong influence on the micellization process. Sikiri´c et al.reported that C12C2C14Br2exhibited peculiar properties in aqueous solution[17],i.e.,high polydispersity and the coexistence of three populations of differently sized aggregates.Therefore,it is significant to study the synthesis and properties of heterogemini surfactants.
To explore the field of heterogemini imidazolium surfactants based on symmetric gemini imidazolium surfactants,the synthesis,characterization,and properties of symmetric gemini imidazolium surfactants have been investigated extensively[18-21].However,because of the difficulty for synthesis,the preparation of heterogemini imidazolium surfactants has not been focused on.Hence we made an attempt to synthesize and study the surface properties of heterogemini imidazolium surfactants with different long hydrocarbon groups.
In this work,a series of heterogemini imidazolium surfactants with two-methylene spacer groups are de-signed and synthesized([Cm-2-Cnim]Br2,m,n=8,10,12,14,16;m/=n)(Scheme 1).Their structures are clearly confirmed by1HNMR and ESI-MS spectroscopy.Besides,optimized reaction conditions and surface properties of[Cm-2-Cnim]Br2are researched. The effects of the hydrophobic chain length and dissymmetry on the surface adsorption and the formation of micelles are explored in detail.

Scheme 1 Synthetic procedure of[Cm-2-Cnim]Br2.
II.EXPERIMENTS
A.Materials and methods
All chemicals were of analytical grade and used without any further purifications.1H NMR were recorded on a Bruker 500 MHz spectrometer.Mass spectra were performed by a Finnigan TSQ Quantum ultra AM mass spectrometer.The surface tension and critical micelle concentration(cmc)of the products were determined by a BZY-2 fully automatic surface tensiometer using the Wilhelmy Type method at 25.0◦C.
B.Synthesis of heterogemini imidazolium surfactants([Cm-2-Cnim]Br2)
1.N-alkylimidazole
A mixture of imidazole(14.7 mmol),potassium hydroxide(14.7 mmol)and dimethyl sulfoxide(5 mL)was placed in a 50 mL three-necked flask.After vigorous shaking for 2 h at room temperature,1-bromoalkane(14.0 mmol)was dropped slowly into the above reaction mixture and the mixture was stirred for additional 4 h and monitored by thin layer chromatography(TLC)analysis.Upon completion,the distilled water(15 mL)was poured into the flask followed by extraction with chloroform(5×15 mL)in a separatory funnel.The combined organic layers were collected then dried over anhydrous magnesium sulfate,filtered,and evaporated. The residue was subjected to flash chromatography with ethyl acetate as eluant to give N-alkylimidazole. The yields of N-octyl imidazole,N-decyl imidazole,N-dodecyl imidazole,N-tetradecyl imidazole,and N-cetyl imidazole are 82.3%,81.2%,80.5%,80.4%and 79.8%,respectively.
N-octyl imidazole∶1H NMR(500 MHz,CDCl3)δ/ppm 7.44(s,1H,CH),7.03(s,1H,CH),6.88(s,1H,CH),3.90(t,2H,CH2),1.75(m,2H,CH2),1.24-1.27(m,10H,CH2),0.85(t,3H,CH3);MS(ESI)∶m/z 181.10([M+H]+).N-decyl imidazole∶1H NMR(500 MHz,CDCl3)δ/ppm 7.41(s,1H,CH),6.99(s,1H,CH),6.86(s,1H,CH),3.88(t,2H,CH2),1.74(m,2H,CH2),1.21-1.25(m,14H,CH2),0.85(t,3H,CH3);MS(ESI)∶m/z 209.07([M+H]+).N-dodecyl imidazole∶1H NMR(500 MHz,CDCl3)δ/ppm 7.44(s,1H,CH),7.03(s,1H,CH),6.88(s,1H,CH),3.90(t,2H,CH2),1.75(m,2H,CH2),1.23-1.27(m,18H,CH2),0.88(t,3H,CH3);MS(ESI)∶m/z 237.08([M+H]+).N-tetradecyl imidazole∶1H NMR(500 MHz,CDCl3)δ/ppm 7.43(s,1H,CH),7.01(s,1H,CH),6.88(s,1H,CH),3.89(t,2H,CH2),1.75(m,2H,CH2),1.22-1.26(m,22H,CH2),0.85(t,3H,CH3);MS(ESI)∶m/z 265.19([M+H]+). N-cetyl imidazole∶1H NMR(500 MHz,CDCl3)δ/ppm 7.45(s,1H,CH),7.05(s,1H,CH),6.90(s,1H,CH),3.91(t,2H,CH2),1.76(m,2H,CH2),1.25-1.32(m,26H,CH2),0.87(t,3H,CH3);MS(ESI)∶m/z 293.29([M+H]+).
2.1-(2-Bromoethyl)-3-alkylimidazolium bromide
The N-alkyl imidazole(in which alkyl=octyl,decyl,dodecyl)was reacted with a large excess(more than 3-fold)of 1,2-dibromoethane in dry acetone at 50◦C under nitrogen atmosphere.The reaction was monitored by TLC analysis.At the end of the reaction,the solvent was removed by evaporation invacuo and the unreacted 1,2-dibromoethane was washed out thoroughly with hexane.The resulting viscous pale yellow oil was purified by silica column chromatography(acetone∶MeOH=10∶1)to afford 1-(2-bromoethyl)-3-alkylimidazolium bromide.The yields of 1-(2-bromoethyl)-3-octylimidazolium bromide,1-(2-bromoethyl)-3-decylimidazoliumbromide,1-(2-bromoethyl)-3-dodecylimidazolium bromide are 72.5%,74.1%,and 76.8%,respectively.
1-(2-Bromoethyl)-3-octylimidazolium bromide∶1H NMR(500 MHz,CDCl3)δ/ppm 10.23(s,1H,CH),7.81(s,1H,CH),7.37(s,1H,CH),4.94(t,2H,CH2),4.29(t,2H,CH2),3.93(t,2H,CH2),1.91(m,2H,CH2),1.23-1.31(m,10H,CH2),0.84(t,3H,CH3);MS(ESI)∶m/z 289.10([M-Br]+).1-(2-Bromoethyl)-3-decylimidazolium bromide∶1H NMR(500 MHz,CDCl3)δ/ppm 10.33(s,1H,CH),7.78(s,1H,CH),7.35(s,1H,CH),4.96(t,2H,CH2),4.29(t,2H,CH2),3.93(t,2H,CH2),1.91(m,2H,CH2),1.23-1.31(m,14H,CH2),0.86(t,3H,CH3);MS(ESI)∶m/z 317.07([M-Br]+).1-(2-Bromoethyl)-3-dodecylimidazolium bromide∶1H NMR(500 MHz,CDCl3δ/ppm 10.27(s,1H,CH),7.76(s,1H,CH),7.34(s,1H,CH),4.96(t,2H,CH2),4.30(t,2H,CH2),3.93(t,2H,CH2),1.92(m,2H,CH2),1.23-1.32(m,18H,CH2),0.87(t,3H,CH3);MS(ESI)∶m/z 345.26([M-Br]+).
3.[Cm-2-Cnim]Br2
A mixture of the intermediates 1-(2-bromoethyl)-3-alkylimidazolium bromide(alkyl=octyl,decyl,dodecyl)and two equiv of N-alkyl imidazole(in which the alkyl chains were different from the intermediates)in toluene was stirred at 80◦C for 48 h under nitrogen atmosphere.After the reaction completed,the solution was cooled down to the room temperature.A white precipitate was isolated,recrystallized in acetone for three times and dried under vacuum to yield[Cm-2-Cnim]Br2as a white powder.The yields of[C8-2-C12im]Br2,[C8-2-C14im]Br2,[C8-2-C16im]Br2,[C10-2-C12im]Br2,[C10-2-C14im]Br2,[C10-2-C16im]Br2,[C12-2-C14im]Br2and[C12-2-C16im]Br2are 85.5%,87.3%,88.2%,89.0%,88.6%,89.4%,88.7%and 88.3%,respectively.
[C8-2-C12im]Br2∶1H NMR(500 MHz,DMSO-d6)δ/ppm 9.13(s,2H,CH),7.82(s,2H,CH),7.68(s,2H,CH),4.70(s,4H,CH2),4.13(t,4H,CH2),1.74(m,4H,CH2),1.20-1.24(m,28H,CH2),0.85(t,6H,CH3);MS(ESI)∶m/z 222.16([M-2Br]2+).[C10-2-C12im]Br2/[C8-2-C14im]Br2∶1H NMR(500 MHz,DMSO-d6)δ/ppm 9.16(s,2H,CH),7.81(s,2H,CH),7.68(s,2H,CH),4.70(s,4H,CH2),4.12(t,4H,CH2),1.73(m,4H,CH2),1.18-1.23(m,32H,CH2),0.85(t,6H,CH3);MS(ESI)∶m/z 236.22([M-2Br]2+).[C10-2-C14im]Br2/[C8-2-C16im]Br2∶δ/ppm 9.17(s,2H,CH),7.82(s,2H,CH),7.69(s,2H,CH),4.72(s,4H,CH2),4.13(t,4H,CH2),1.74(m,4H,CH2),1.18-1.24(m,36H,CH2),0.85(t,6H,CH3);MS(ESI)∶m/z 250.39([M-2Br]2+).[C10-2-C16im]Br2/[C12-2-C14im]Br2∶1H NMR(500 MHz,DMSO-d6)δ/ppm 9.17(s,2H,CH),7.80(s,2H,CH),7.68(s,2H,CH),4.69(s,4H,CH2),4.13(t,4H,CH2),1.71(m,4H,CH2),1.19-1.23(m,40H,CH2),0.84(t,6H,CH3);MS(ESI)∶m/z 264.21([M-2Br]2+).[C12-2-C16im]Br2∶1H NMR(500 MHz,DMSO-d6)δ/ppm 9.07(s,2H,CH),7.80(s,2H,CH),7.64(s,2H,CH),4.67(s,4H,CH2),4.11(t,4H,CH2),1.72(m,4H,CH2),1.18-1.23(m,44H,CH2),0.84(t,6H,CH3);MS(ESI)∶m/z 278.33([M-2Br]2+).
C.Synthesis of 1,2-bis(3-alkylimidazolium-1-yl)ethane bromide([Cm-2-Cmim]Br2)
A solution of the N-decyl imidazole,N-dodecyl imidazole or N-tetradecyl imidazole(5 mmol)and 1,2-dibromoethane(2 mmol)in isopropanol(10 mL)was refluxed for three days at 60◦C under nitrogen atmosphere.After removal of isopropanol,the residue was washed with ethyl acetate,further purified four times by recrystallization in acetone and then dried under vacuum for two days.The resulting white powder[Cm-2-Cmim]Br2was obtained.The yields of[C10-2-C10im]Br2,[C12-2-C12im]Br2and[C14-2-C14im]Br2are 92.4%,94.7%and 93.3%,respectively.
[C10-2-C10im]Br2∶1H NMR(500 MHz,DMSO-d6)δ/ppm 9.13(s,2H,CH),7.81(s,2H,CH),7.68(s,2H,CH),4.70(s,4H,CH2),4.11(t,4H,CH2),1.73(m,4H,CH2),1.19-1.24(m,28H,CH2),0.85(t,6H,CH3);MS(ESI)∶m/z 222.24([M-2Br]2+).[C12-2-C12im]Br2∶1H NMR(500 MHz,DMSO-d6)δ/ppm 9.16(s,2H,CH),7.81(s,2H,CH),7.68(s,2H,CH),4.70(s,4H,CH2),4.12(t,4H,CH2),1.73(m,4H,CH2),1.18-1.23(m,36H,CH2),0.84(t,6H,CH3);MS(ESI)∶m/z 250.23([M-2Br]2+).[C14-2-C14im]Br2∶1H NMR(500 MHz,DMSO-d6)δ/ppm 9.12(s,2H,CH),7.80(s,2H,CH),7.66(s,2H,CH),4.69(s,4H,CH2),4.11(t,4H,CH2),1.72(m,4H,CH2),1.20-1.25(m,44H,CH2),0.83(t,6H,CH3);MS(ESI)∶m/z 278.15([M-2Br]2+).
III.RESULTS AND DISCUSSION
A.Optimal reaction conditions
In the general synthesis of[Cm-2-Cnim]Br2,nucleophilic substitution of N-alkylimidazole and 1,2-dibromoethanewascritical.Takethesynthesis of 1-(2-bromoethyl)-3-dodecylimidazolium bromide for an example,the generation of symmetric gemini surfactants 1,2-bis(3-dodecylimidazolium-1-yl)ethane bromide([C12-2-C12]Br2)is unavoidable.Thus,to effectively reduce symmetric gemini surfactants,quaternization reaction of N-dodecylimidazole and 1,2-dibromoethane was investigated under different reaction conditions(Table I).We find that the increase of 1,2-dibromoethane/N-dodecylimidazole molar ratio is beneficial to the formation of 1-(2-bromoethyl)-3-dodecylimidazolium bromide.Considering the effect of 1,2-dibromoethane,temperature and time on yields,we select the molar ratio of 4∶1,reaction temperature of 50◦and time of 10 h as the optimal conditions. Higher temperature and longer time will lead to produce more symmetric gemini surfactant,resulting in diminished yield.Besides,dry acetone as the reaction solvent should be excessive,which creates the dilution reaction environment thus makes against the formation of[C12-2-C12]Br2.
B.Surface activity
It can also be found that[Cm-2-Cnim]Br2are immiscible with low polarity solvents,such as ethyl acetate,toluene,cyclohexane and petroleum ether,but miscible in methanol,chloroform and acetonitrile.The sur-face tension measurement is a classical method to study the surface properties of surfactants.The plots of surface tension versus log concentration(γ-lgc)for[Cm-2-Cnim]Br2by a surface tension apparatus using the Wilhelmy Type method at 25.0◦C are shown in Fig.1.A sharp break is shown in the surface tension plots,which are the indication of the critical micelle concentration(cmc)and the formation of micelles in the aqueous solutions.Here,it is worth mentioning that the purity of[Cm-2-Cnim]Br2was also confirmed from the observed sharp break points in the γ-lgc plots,that is to say,the absence of a minimum around the breakpoints suggests there are no higher active substances in these heterogemini imidazolium surfactants.The values of the cmc are listed in Table II.The cmc shows a monotonous decrease with the increasing overall hydrophobic chain length(m+n)regardless of the dissymmetry,implying that the longer overall hydrophobic chain length,the higher the aggregation ability is.Therefore,[C14-2-C14im]Br2or[C12-2-C16im]Br2has the higher aggregation ability than the other Gemini surfactants.Besides,the cmc of surfactants with equal m+n,such as C10-2-C10and C8-2-C12,C10-2-C12and C8-2-C14,C12-2-C12,C10-2-C14and C8-2-C16,C12-2-C14and C10-2-C16,or C14-2-C14and C12-2-C16,decrease linearly as the n/m ratio increases,as presented in the Table II.This observation indicates that higher structural dissymmetry results in a lower cmc value by employing n/m as the degree of dissymmetry and a similar relationship can also be observed from the other dissymmetric Gemini surfactants[11,22,23].Consequently,[C12-2-C16im]Br2possesses the highest aggregation ability in all the investigated heterogemini imidazolium surfactants.It is clear that the introduction of dissymmetry to the hydrophobic chains is more beneficial to the formation of micelles and adding two CH2groups to one longer chain is more efficient in lowering the cmc than adding each of them to two chains separately.The hydrophobic interactions have two contributions to the aggregation behavior,intermolecular and intramolecular.The latter will be the weaker interaction because the spacer group keeps the two hydrophobic chains apart.For the symmetric geminis,the number of hydrophobic units interacting will be the same for intramolecular and intermolecular interactions,and therefore the hydrophobic interaction will be minimized.However,for the dissymmetric geminis,as n/m ratio increases,the ratio of the number of hydrophobic units interacting intermolecularly to those interacting intramolecularly will increase[23].Thus,the overall hydrophobic interaction is gradually improved with the increasing n/m ratio,which makes a positive contribution to the formation of micelles.

TABLE I Optimization of reaction conditions on the synthesis of 1-(2-bromoethyl)-3-dodecylimidazolium bromide.

FIG.1 Surface tension γ of heterogemini imidazolium surfactants[Cm-2-Cnim]Br2vs.concentration c at 25◦C.
From the surface tension data,several surface property parameters,such as adsorption efficiency pc20,the surface pressure at the cmc(πcmc),the value of cmc/c20,the maximum surface excess(Γmax)and the minimum area occupied by a surfactant molecule at the air/solution interface(Amin)can be obtained.It is recognized that the pc20value can measure the efficiency of surfactant adsorbing at the air/solution interface.

where c20is defined as the surfactant concentration at which the surface tension of pure solvent is reduced by 20 mN/m.The larger the value of pc20,the higher the adsorption efficiency of the surfactant is.
The πcmcis defined as∶

where γ0is the surface tension of pure solvent and γcmcis the surface tension of surfactant solution at the cmc.This parameter reflects the maximum reduction of surface tension caused by the dissolution of surfactant molecules and thus becomes an indication of the ability of the surfactant to lower the surface tension of the solvent.
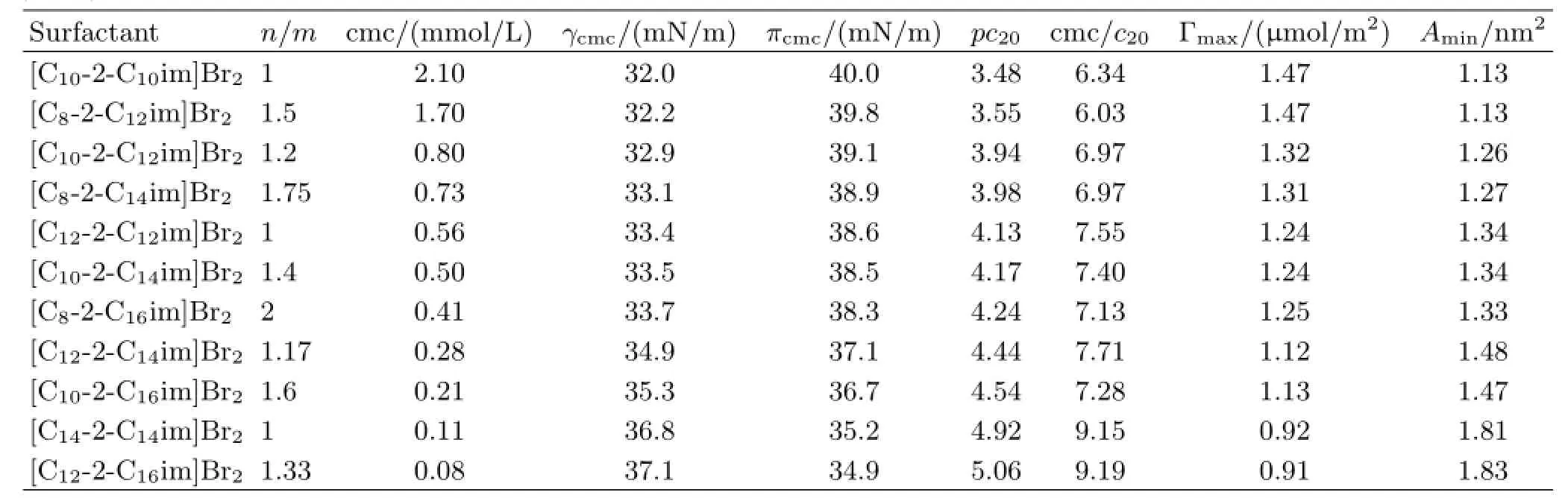
TABLE II Values of the cmc,surface tension at the cmc(γcmc),surface pressure at the cmc(πcmc),adsorption efficiency pc20,cmc/c20with c20being the surfactant concentration at which the surface tension of pure solvent is reduced by 20 mN/m,the maximum surface excess(Γmax),and the minimum area occupied by a surfactant molecule at the air/solution interface(Amin)for heterogemini imidazolium surfactants.
The value of cmc/c20ratio is correlated with structural factors in the micellization and adsorption processes.The surfactant with larger cmc/c20ratio has the greater tendency to adsorb at the interface than the tendency to form micelles.As can be shown in Table II,both pc20and cmc/c20ratio increase with the increase of the total carbon number of hydrophobic chains,which suggests that the adsorption at the interface is much easier than the micellization process for[C14-2-C14im]Br2or[C12-2-C16im]Br2.This is probably because that the greater repulsion action of longer hydrophobic chains makes them easily stretch into the external environment.[C10-2-C10im]Br2has the lower γcmcand higher πcmcvalue than other Gemini surfactants,indicating the higher effectiveness of surface tension reduction.However,the values of γcmc,pc20,cmc/c20and πcmcchange a little upon raising the n/m ratio for the surfactants with the same overall hydrophobic chains,which means the degree of dissymmetry has no significant effect on the surface activity of heterogemini imidazolium surfactants.

Thepackingdensitiesofsurfactantsatthe air/aqueous solution interface are important for the interpretation of the surface activity.Γmaxis a measure of how much of the air/solution interface changed by the surfactant adsorption and depends on the molecular structure of a surfactant molecule and its orientation at the interface.Aminreflects the packing densities. The higher the effectiveness of adsorption,the smaller the interfacial area occupied by a surfactant molecule. Γmaxwas calculated by applying the Gibbs adsorption isotherm Eq.(3).Amincan be estimated from the relation Eq.(4). where n represents the number of species at the interface;Γmaxis inµmol/m2,R is the gas constant(8.314 J/(mol·K),T is the temperature in Kelvin,and(dγ/dlgc)is the slope in the surface tension isotherm when the concentration is near the cmc.In aqueous solutions of[Cm-2-Cnim]Br2surfactants,n is taken as 3 due to the fact that the solute molecule dissolves into three ions-one divalent and two monovalent[24].NAis Avogadros number and Aminis in nm2/molecule.Both the Γmaxand Aminvalues are listed in Table II.The Aminvalues increase as the overall hydrophobic chain length is increased,which suggests that[C10-2-C10im]Br2has higher molecular compactness at the air/aqueous solution interface than those surfactants with longer overall hydrophobic chains.A reasonable explanation is that the longer hydrophobic chains are more prone to bend and thus make the surface area per molecule larger[25].On the other hand,surfactants with the same total carbon number of hydrophobic chains have similar Aminvalues.The surface adsorption properties,Γmaxand Amin,do not depend on the symmetry degree but only on the overall hydrophobicity of the surfactants.
IV.CONCLUSION
In summary,we have successfully developed a facile and efficient method to prepare[Cm-2-Cnim]Br2(m,n=10,12,14,16;m/=n).The surface properties of heterogemini imidazolium surfactants are investigated. Compared with their symmetric imidazolium gemini surfactants,the degree of dissymmetry(n/m)of heterogemini imidazolium surfactants shows a marked effect on the cmc values.For those surfactants with the same overall hydrophobic chains,as the n/m ratio increases,the cmc decreases linearly,suggesting greater ability to form micelles.However,the effects of n/m on the pc20,cmc/c20,πcmcand Aminare very small.This synthetic method also provides an opportunity to prepare more derivatives by changing the spacer chains and further study other properties of heterogemini imidazolium surfactants,such as aggregation behavior,thermodynamic parameters of micellization(∆Go-m,∆So-m,∆Ho-m),etc.
[1]D.Q.Xu and Z.W.Pan,Chin.Chem.Lett.25,1169(2014).
[2]X.J.Xu,J.W.Guo,and X.Zhong,Chin.Chem.Lett. 25,367(2014).
[3]X.Zhong,J.W.Guo,and L.J.Feng,Colloids Surfaces A 441,572(2014).
[4]R.Zana and J.D.Xia,Geminal Surfactants:Synthesis,Interfacial and Solution Phase Behavior,and Application,New York:CRC Press,(2003).
[5]P.Martin and P.Maja,J.Colloid Interface Sci.329,153(2009).
[6]Y.S.Ding,M.Zha,J.Zhang,and S.S.Wang,Chin. Chem.Lett.18,48(2007).
[7]Y.Q.Cai,G.Q.Yu,and C.D.Liu,Chin.Chem.Lett. 23,1(2012).
[8]M.Antonietti,D.B.Kuang,and B.Smarsly,Chem. Int.Ed.43,4988(2004).
[9]A.J.Fry,J.Electroanal Chem.546,35(2003).
[10]F.M.Menger and A.V.Peresypkin,J.Am.Chem.Soc. 123,5614(2001).
[11]G.Y.Bai,J.B.Wang,Y.J.Wang,and H.K.Yan,J. Phys.Chem.B 106,6614(2002).
[12]F.M.Menger and A.V.Peresypkin,J.Am.Chem.Soc. 125,5340(2003).
[13]M.Lukac,L.Timko,M.Mrva,F.Ondriska,J. Karlovska,J.Valentova,and I.Lacko,Heteroatom Chem.21,203(2010).
[14]Y.Takamatsu,N.Iwata,and K.Tsubone,J.Colloid Interface Sci.338,229(2009).
[15]R.Oda,I.Huc,and S.Candau,J.Chem.Commun. 2105(1997).
[16]R.Oda,I.Huc,J.C.Homo,B.Heinrich,M.Schmutz,and S.Candau,Langmuir 15,2384(1999).
[17]M.Sikiri´c,I.Primoˇziˇc,and N.Filipovi´c-Vincekoviczcu,J.Colloid Interface Sci.250,221(2002).
[18]M.Q.Ao,G.Y.Xu,Y.Y.Zhu,and Y.Bai,J.Colloid Interface Sci.326,490(2008).
[19]G.Y.Liu,D.M.Gu,H.Y.Liu,W.Ding,and Z.Li,J. Colloid Interface Sci.358,521(2011).
[20]M.Q.Ao,P.P.Huang,G.Y.Xu,X.D.Yang,and Y. J.Wang,Colloid Polym.Sci.287,395(2009).
[21]Y.S.Ding,M.Zha,J.Zhang,and S.S.Wang,Colloids Surf.A 298,201(2007).
[22]M.Zou,J.F.Dong,G.F.Yang,and X.F.Li,Phys. Chem.Chem.Phys.17,10267(2015).
[23]X.Y.Wang,J.B.Wang,and Y.L.Wang,J.Phys. Chem.B 107,11428(2003).
[24]S.D.Wettig and R.E.Verrall,J.Colloid Interface Sci. 235,310(2001).
[25]C.C.Ren,F.Wang,Z.Q.Zhang,H.H.Nie,N.Li,and M.Cui,Colloids Surf.A 467,4(2015).
∗Author to whom correspondence should be addressed.E-mail:yezw@mail.njust.edu.cn
杂志排行
CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Raman Spectra of Liquid Nitromethane under Singly Shocked Conditions
- Tunneling Electron Induced Fluorescence from Single Porphyrin Molecules Decoupled by Striped-Phase Octanethiol Self-assembled Monolayer
- Ion Product of Pure Water Characterized by Physics-Based Water Model
- Laser Linewidth and Spectral Resolution in Infrared Scanning Sum Frequency Generation Vibrational Spectroscopy System
- Performances of Five Representative Force Fields on Gaseous Amino Acids with Different Termini
- Dynamics of Tripartite Entanglement and Intramolecular Energy in Symmetric Trimer Molecule
