Raman Spectra of Liquid Nitromethane under Singly Shocked Conditions
2016-09-13YapingWangFushengLiuQijunLiuNingchaoZhangSchoolofPhysicalScienceandTechnologyKeyLaboratoryofAdvancedTechnologiesofMaterialsMinistryofEducationofChinaSouthwestJiaotongUniversityChengdu610031ChinaDatedReceivedonMarch11
Ya-ping Wang,Fu-sheng Liu,Qi-jun Liu,Ning-chao ZhangSchool of Physical Science and Technology,Key Laboratory of Advanced Technologies of Materials,Ministry of Education of China,Southwest Jiaotong University,Chengdu 610031,China(Dated:Received on March 11,2015;Accepted on October 10,2015)
Raman Spectra of Liquid Nitromethane under Singly Shocked Conditions
Ya-ping Wang,Fu-sheng Liu∗,Qi-jun Liu,Ning-chao Zhang
School of Physical Science and Technology,Key Laboratory of Advanced Technologies of Materials,Ministry of Education of China,Southwest Jiaotong University,Chengdu 610031,China
(Dated:Received on March 11,2015;Accepted on October 10,2015)
Raman spectra of liquid nitromethane were measured in single-shock experiments using transient Raman scattering system with high sensitivity.The measurement system was combined with a two-stage light gas gun to interrogate the vibrational mode-dependent behaviors of shock-compressed nitromethane molecules.Up to 12 GPa,all Raman peaks were able to be clearly detected,and showed the shock-induced shifting and broadening,but no signs of chemical changes occurred in the sample.Thus,it is concluded that chemical reactions could not be initiated in singly-shocked nitromethane below 12 GPa.
Raman spectra,Shock wave,Energetic liquid explosives,Nitromethane
I.INTRODUCTION
The shock-induced reaction stages in energetic materials at high temperatures and high pressures have long been a significative issue of considerable interest in the field of chemistry and material science.When subjected to the shock loading,energetic materials such as the explosives commonly undergo a fast and highly exothermic chemical reaction process accompanied with the formation of gaseous products[1].At present,reaserchers have been devoting themselves to examine which chemical bond of energetic molecules firstly loses its structural stability in early reaction stages,and what type of the reaction mechanism occurs in the initial stages of the reactions when energetic materials experience extreme conditions under shock compression.Unfortunately,these basic questions remain largely unknown up to now.Therefore,a deep understanding of the reaction mechanisms for energetic materials under extreme shock conditions is of considerable importance,and it is also one of the fundamental problems in detonation science.However,the measurements of detonation processes in solid explosives under extreme shock-compressed conditions are quite complicated and difficult[2].Thus,it is reasonably inevitable to choose a model material for detailed analysis on shock-induced reactions at the molecular level. Nitromethane(CH3NO2)is often selected as a prototypical energetic material in studies of many complex explosives containing nitro groups,because(i)it is a liquid high explosive,which can avoid the complexities associated with solid materials;(ii)it is the simplest nitro-organic explosives;(iii)it has the nature of variable sensitivity in the presence of amines[3-5].
In shock wave experiments,in situ Raman spectroscopy is often used to monitor the kinetic responses of molecular materials under shock loading conditions. For the shock-compressed CH3NO2sample,some significant results on the reactions have been proposed[6-14]. Under the stepwise shock loading,the substantial reactions in neat CH3NO2were speculated to take place at about 17 GPa using time-resolved Raman spectroscopy technique,and a decomposition mechanism of the bimolecular reaction was proposed[6].This bimolecular mechanism was also favoured in static high pressure experiments[15]and theoretical studies of molecular dynamics simulations[16,17].In single shock experiments,however so far,the direct evidence on the reaction of molecular dissociation of CH3NO2has not been found,except that the strong background emissions were found below 8.5 GPa[10-14].The strong background emission is popularly thought of an indirect evidence that the reactions in CH3NO2occurred under compression,because the appearance of strong and featureless background was likely caused by emission from opaque or semitransparent reaction produces. Even more remarkable,there exist some different arguments on the onset of reactions in singly shocked CH3NO2.In particular,Renlund et al.suggested the initiation of chemical reactions at~6 GPa by in situ Raman measurement[10].But Von Holle and Moore et al.respectively suggested that the reactions occurred above 7 GPa from time-resolved infrared radiometry measurement[11],and above 7.6 GPa by in situ CARS measurement[12].In addition,Delpuech et al.reported all Raman bands for CH3NO2disappeared at 8.5 GPa but the initiation of chemical reactions was uncertain due to lack of the data between 5 and 8.5 GPa[14]. Based on these experimental studies mentioned above,one can reasonably draw conclusions that the reaction threshold pressure of shock-induced CH3NO2moleculesstill remains indeterminate.Thus,it is necessary to perform additional single-shock experiments for solving those arguments on shock-induced reactions in neat CH3NO2.
In this work,we focus on the examination of vibrational mode-dependent behavior of singly shockcompressed liquid CH3NO2using a new Raman spectroscopic system with high sensitivity.The information on the shock-induced reactions are provided by interrogating the vibrational spectra of molecular materials under shock compression.The changes in Raman bands(e.g.,frequency shift,band shape,spectral intensity,and so on)can reflect the detailed information on molecular changes,molecular interactions,the breaking of chemical bonds,and some new species.
II.EXPERIMENTS
The sample was neat liquid CH3NO2in our experiments,and it was considered to remain in the liquid state under single shock compression.Shock pressure was determined using the impedance-matching method with the measured projectile velocity and the initial density of the sample.Hugoniot equation of CH3NO2under shock conditions(Hugoniot equation relates the thermodynamic quantities of shocked state to those of initial state)was determined by the linear relation∶Us=1.65+1.64Up,where Usand Updenote the shock velocity and the particle velocity in unit of km/s.The schematic of the experimental setup in this work was very similar to that in previous work on shocked liquid benzene under shock compression[18,19],but with a significant improvement that an input optical fiber,instead of previous mirrors,was used to irradiate the laser into the central part of the sample in a direction perpendicular to the rear surface of the target. The experimental configuration consisted of four main systems∶a target assembly,the Nd∶YAG laser source(532 nm,10 ns),the spectrograph,and the time delay units.Other detailed descriptions could be found in the report of shocked benzene[19].Here a brief description of our experiments is given.
Shock wave was generated when a projectile impacted an aluminum base plate(2 mm thick)in front of the target.The projectile with an aluminum flyer(24 mm diameter,4 mm thick)was accelerated by a two-stage light-gas gun.The shock wave went through the base plate and the silica front window(2 mm thick),and then into the CH3NO2sample(3 mm thick).As soon as the shock wave arrived at the detected part of the sample which was located by assembling the target,the output of single-pulsed laser was Q-switch triggered from the laser system and irradiated into this specific region via an input optical fiber.The scattered light from the detected region of the sample was collected at an angle of 45◦with reference to the incident direction of laser,and coupled into a spectrometer via an output optical fiber. The Raman signal output from the spectrometer was amplified and recorded by a gated intensified chargecoupled device(ICCD)detector system.The spectral resolution of the detector system was about 10 cm-1. In the shock experiments,only the scattered light to be collected came mostly from the shock-compressed region of the sample.For this,it is the most important step to keep the synchronization between the laser pulse and the detection system,because it controlled the output time of the laser pulse to ensure that detected scattered light was from the shock-compressed region of the sample.The synchronization method used here was similar to that described by Knudson et al.[20].However,an improvement have been made in our experiments that the shock-induced emission from the interface between base plate and the silica front window,was converted to an electric signal to trigger Q-switch and ICCD,instead of the previous electric pin.The synchronization requirement of two trigger signals was ensured by the time delay unit.The Raman spectrum of shock-compressed CH3NO2was measured during period of single shock compression.
III.RESULTS AND DISCUSSION
The measured Raman spectra of liquid CH3NO2at ambient conditions and from the shock experiment at 8 GPa are shown in Fig.1.At ambient conditions,only symmetric stretching modes are typically selected for detailed examination,i.e.,the CN stretching band(917 cm-1),the NO2symmetric stretching mode overlapped with the CH3symmetric bending mode(NO2/CH3,~1400 cm-1),and the CH3stretching band(2968 cm-1).This is due to the fact that the three bands are relatively strong and apparently separated from each other.In general,the vibration frequency of a stretching mode shifts to higher energy with the increase of shock pressure.As seen in Fig.1,all Raman modes are shifted towards higher frequency under shock compression.This is because that the bond lengths of molecules are inherently reduced with increasing pressure and their effective force constants become usually larger at the new equilibrium position than those at the original equilibrium position[21].Moreover,the magnitude of the pressure-induced shifting relies on vibration modes.In particular,the NO2/CH3mode shows significantly smaller blue shift than other modes due to the effect of increased intermolecular interactions under compression[9].In contrast to the behavior of the NO2/CH3mode,the CN and CH3modes exhibit larger shifting.However,the CH3mode appears to show larger shifting than the CN mode.This indicates that the CH3mode may be more affected by shock compression due to the low mass of the hydrogen atoms and the position of these atoms on the periphery of the CH3NO2molecule[6].Thus,it is plausible that the CH3mode is the most sensitive to increased intermolecular inter-actions under high shock compression.It is also seen in Fig.1 that all Raman bands show broadening.This is likely caused by the increased intermolecular interactions and the temperature increase due to hot bands.

FIG.1 Raman spectra of CH3NO2shocked to 8 GPa.
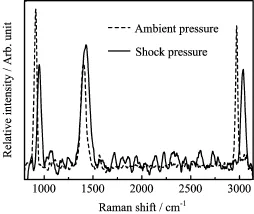
FIG.2 Raman spectra of CH3NO2shocked to 12 GPa.
In addition to the frequency shift and the band broadening,the Raman peaks also show intensity changes in Fig.1.The intensities of the CN and CH3stretching modes decrease under this conditions but that of the NO2/CH3mode(the NO2stretching mode is main)is oppositely enhanced.It is worth noting here that the NO2/CH3enhancement is mainly due to the contribution of the NO2mode because the CH3symmetric bending mode is much less sensitive to intermolecular interactions than the NO2stretching mode[6].The NO2/CH3enhancement may be likely caused by a resonance Raman effect.This result was also observed in previous reports and was discussed in terms of a resonance Raman effect[6,7].Under the shock-wave compression,the band edge of the n-π∗absorption band of CH3NO2molecules shows a substantial shift to longer wavelengths.This band edge red-shifting may cause a small amount of absorption at the 532 nm laser line,which could result in the resonance of the absorption band in CH3NO2molecules and the exciting irradiation.Since the enhancement in the Raman peak observed here is not large,the band edge itself would not have to be very close to the laser line to account for the effect.Thus,it is reasonable that the NO2/CH3enhancement here is due to a resonance Raman effect. In CH3NO2molecules,the highest occupied molecular orbital(HOMO)and the lowest unoccupied molecular orbital(LUMO)are both localized on the NO2group[22].It means that the NO2group is the chromophore for the HOMO-LUMO(n-π∗)transition.Since only vibrational modes of the chromophore which couple to the absorption band will be resonantly enhanced[23],the CN and CH3modes would not be expected to show significant enhancement.The fact that the CN and CH3modes are not enhanced also supports the explanation of the the NO2/CH3enhancement.
What is more interesting,it is to see that all Raman peaks were clearly visible at 8 GPa.Although changes in Raman shift,peak width,and intensity were observed,no chemical changes occurred under this condition because all Raman bands were clearly distinguished.It should be noted here that in most cases the initiation of shock-induced reactions is indicated by the appearance of a strong and featureless background signally attributed to emission from opaque or semitransparent reaction products,the disappearance of vibrational bands,and the appearance of new bands. Renlund et al.proposed chemical reactions in CH3NO2occurred at 6.8 GPa due to extensive band broadening[10].However,the clear Raman bands were detected at the higher pressure of 8 GPa than 6.8 GPa in our experiments.Thus,the results obtained here show a strong evidence that the shock-induced reactions in liquid CH3NO2would not occur below 8 GPa.
Figure 2 shows Raman spectra of liquid CH3NO2singly shocked up to 12 GPa.The Raman bands were still clearly observed,and they were expected to exhibit significant shifting and broadening at this shock pressure.It demonstrates that no chemical reactions occurred in the shock-compressed CH3NO2sample at this pressure,although the Raman bands showed clear changes in the intensity,width,and frequency shift. Thus,the spectral data detected here provide a cogent evidence that the initiation of chemical reactions do not occur in shocked CH3NO2up to 12 GPa.This shock pressure is considerably quite higher than previously reported upper limit pressures at which all Raman spectra disappeared,and it is near to the Chapman-Jouguet detonation pressure of liquid CH3NO2.The C-J detonation pressure and temperature of CH3NO2were respectively reported at about 13 GPa and 2000 K[24]. Moreover,this result is also indirectly supported by the Hugoniot data of shocked CH3NO2,because the Hugoniot of CH3NO2shocked up to about 10 GPa was represented by a linear line,which reveals there is no chemical reaction occurred in the sample at this pressure[25].The results in this work demonstrate the shockinduced chemical reactions do not occur at the shockpressure between 6 and 8.5 GPa,and it solves those disputes about chemical reactions occurring in singlyshocked liquid CH3NO2.

FIG.3 The shocked-to-ambient ratios for the CN and CH3stretching mode for the full-width at half-maximum in this work.Open squares:the C-N stretching mode(917 cm-1);Closed squares:the CH3stretching mode(2968 cm-1).

FIG.4Raman frequency shift vs.shock pressure for CH3NO2.(a)CN stretch at 917 cm-1,(b)CH3stretching made at 2968 cm-1.
Figure 3 shows the shocked-to-ambient ratio of the full-width at half-maximum(FWHM)for the CN and CH3stretching modes as a function of shock pressure. The data for the NO2/CH3mode were not plotted because it is not quite straightforward for the overlapped peaks to respectively show the dynamic pressure response of the NO2mode and the CH3bending mode. The broadening occurring in both the CN and CH3bands was likely caused by the temperature increase due to hot bands[13],and increased intermolecular interaction[6].The increase in the FWHM for the CH3bands was significantly larger than that for the CN stretching mode.This was likely due to the fact that the CH3bands are more affected by shock compression than the CN bands.Because of the low mass of hydrogen atoms and the relatively weak bond between C and H atoms,the CH3mode may be more sensitive to increased intermolecular interaction under shock compression[6].Increased intermolecular interaction can cause faster relaxation time,which would further contribute to the broadening of the Raman peaks[26].The formation of the hydrogen bonding can also produce a substantial amount of broadening in vibrational modes involving the proton donor[27].Therefore,it is plausible for the CH3mode involved in a hydrogen bond(C-H-O)that the large broadening in this mode may be due to the effect of the hydrogen bonding generated under shock compression,as is discussed in previous reports[6,28,29].
In Fig.4,Raman frequency shifts of the CN and CH3stretching modes of shocked CH3NO2in this work are plotted versus shock pressures,compared with the earlier data[7,12].The data for the NO2/CH3bands are not plotted because the peak position of this overlapped bands is not straightforward.The data reported by Moore et al.[12]were obtained in singleshock experiments,and those reported by Pangilinan et al.[7]were obtained in multiple-shock experiments. It is known from the figure that the frequency shift data of the CN and CH3stretching modes in this work show the monotonic increase trend towards higher vibrational frequency with increasing pressure.As noted above,these blue shifts result from the effect of both the changes of the bond lengths and the effective force constants of NM molecules under shock compression. The shift of the CH3stretching mode appears to be somewhat larger than that of the CN stretching mode,when compared with each other at the same pressure. This may be explained as a result that the CH3stretching mode is more sensitive to pressure than the CN stretching mode.Thus,this larger sensitivity of the CH3stretching mode to pressure leads to the result that its frequency shifts are larger than those of the CN stretching mode at the same temperature.
In Fig.4(a),it is shown that vibrational frequencies of the CN modes shift with increasing pressures.The data of the shifting for the CN stretching mode in this work show a linear increase trend with increasing pressures,which is in accord with the results from other studies shown in the figure.The shifts of the CN stretching mode in this work is in good agreement with those Moore et al.reported[12],and these data show a linear increase trend with shock pressures as a whole.Thus,the work at shock pressures between 8 and 12 GPa can be thought as the continuation of the earlier studies below 8 GPa performed by Moore et al.[12].It isalso known in the figure that the shifting data of the CN stretching mode reported by Pangilinan et al.under multiple shock compression[7]are somewhat larger than that obtained in this work at the same pressure. It is well known that the temperance is much higher in single-shock experiments than in multiple-shock experiments when up to the same pressure,and the vibrational mode always shifts towards lower vibrational frequency with increasing temperature.It means that the vibrational mode under higher temperature condition should show smaller shifting when up to the same pressure.Hence,the magnitude of the shift for vibrational mode under multiple-shock conditions is generally larger than that under single-shock conditions. The similar result is also shown in Fig.4(b)where it is shown that vibrational frequencies of the CH3modes shift with increasing pressures.The shifting data of the CH3stretching mode under multiple shock compression[7]are larger than that obtained in this work at the same pressure due to the difference of temperance in CH3NO2under the two different loading conditions.
The results about the shifting of vibrational modes noted above demonstrate the pressure response of molecular changes in liquid CH3NO2is temperature dependent under shock conditions.The magnitude of blue shift in vibrational bands results from the combined effects of high temperature and pressure,and a subtle balance between shock temperature and pressure might be causing unexpected behaviors of molecular stretching modes,as is shown in multiply shocked liquid benzene[26,30].Moreover,in the theory of the steady-state detonation[31]as well as molecular dynamics simulation[32],it is observed that the thermal decomposition rate of liquid CH3NO2accelerates obviously with the increase of pressure,and that its decomposition reaction has the obvious dependency on shock pressures.Therefore,we propose that molecular changes in shocked CH3NO2may be determined by the common effects of high pressure and temperature under shock conditions.
IV.CONCLUSION
Raman spectroscopy technique with high sensitivity has been successfully applied to investigate the dynamic responses of liquid CH3NO2in this work for solving the arguments on the onset of reactions in shocked CH3NO2.To our knowledge,this work is the first attempt to obtain the vibrational spectra of liquid CH3NO2at the high pressure of 12 GPa under single-shock compression.The results in this work show the detailed and comprehensive insights into molecular changes in the CH3NO2sample,and it also provide a strong evidence that the shock-induced reactions in liquid CH3NO2could not be initiated below 12 GPa,which solves those arguments on chemical reactions of singly-shocked CH3NO2in earlier studies[10-14].According to the theory of the steady-state detonation,the ignition shock pressure of dissociation of CH3NO2molecules should be higher than its C-J detonation pressure[31].Thus,it is reasonable that the shock-induced reactions in CH3NO2would not occur below 12 GPa.The results in this work also show the CH3stretching mode is more easily affected than the CN stretching mode under single-shock compression.To progress in the understanding of molecular mechanisms governing shock-induced chemical decomposition in liquid CH3NO2,it would be desirable and essential to be able to detect vibrational spectra of transient state and of reaction products at above 12 GPa. Hence,more experiments should be performed in the next work for obtaining information on chemical reactions in singly shocked liquid CH3NO2.Currently,we are trying to measure Raman spectra of liquid CH3NO2above 12 GPa in single-shock experiments by suppressing the background light as possible as we can.
V.ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China(No.11574254 and No.11072225),the Fundamental Research Fund for the Central Universities of China(No.2682014ZT31),and the National Basic Research Program of China(No.2011CB808201).
[1]M.R.Manaa,L.E.Fried,C.F.Melius,M.Elstner,and Th.Frauenheim,J.Phys.Chem.A 106,9024(2002).
[2]Y.A.Gruzdkov and Y.M.Gupta,J.Phys.Chem.A 102,8325(1998).
[3]R.Engelke,W.L.Earl,and C.M.Rohlfing,J.Chem. Phys.84,142(1986).
[4]C.P.Constantinou,T.Mukundan,andM.M. Chaudhri,Philos.Trans.R.Soc.Lond.A 339,403(1992).
[5]Y.A.Gruzdkov and Y.M.Gupta,J.Phys.Chem.A 102,2322(1998).
[6]J.M.Winey and Y.M.Gupta,J.Phys.Chem.B 101,10733(1997).
[7]G.I.Pangilinan and Y.M.Gupta,J.Phys.Chem.98,4522(1994).
[8]Y.M.Gupta,G.I.Pangilinan,J.M.Winey,and C.P. Constantinou,Chem.Phys.Lett.232,341(1995).
[9]T.Kobayashi,T.Sekine,and H.He,J.Chem.Phys. 115,10753(2001).
[10]A.M.Renlund and W.M.Trott,in Shock Compression of Condensed Matter-1989,New York:Elsevier,875(1990).
[11]W.G.Von Holle,in Shock Waves in Condensed Matter-1981,New York:AIP Conf.Proc.,287(1982).
[12]D.S.Moore,S.C.Schmidt,J.W.Shaner,D.L. Shampine,and W.T.Holt,in Shock Waves in Condensed Matter-1985,New York:Plenum,207(1986).
[13]D.S.Moore,J.Phys.Chem.A 105,4660((2001).
[14]A.Delpuech and A.Menil,in Shock Waves in Condensed Matter-1983,New York:Elsevier Science,309(1984).
[15]M.Citroni,R.Bini,M.Pagliai,G.Cardini,and V. Schettino,J.Phys.Chem.B 114,9420(2010).
[16]L.E.Fried,M.R.Manaa,and E.J.Reed,Lecture Ser. Comput.Comput.Sci.3,1(2005).
[17]M.R.Manaa,E.J.Reed,L.E.Fried,G.Galli,and F. Gygi,J.Chem.Phys.120,10146(2004).
[18]Y.F.Chen,F.S.Liu,N.C.Zhang,B.J.Zhao,J. G.Wang,M.J.Zhang,and X.D.Xue,Chin.J.High Pressure Phys.27,505(2013).
[19]B.J.Zhao,F.S.Liu,N.C.Zhang,L.P.Feng,W.P. Wang,and M.J.Zhang,Chin.Phys.Lett.30,0307011(2013).
[20]M.D.Knudson,Y.M.Gupta,and A.B.Kunz,Phys. Rev.B 59,11704(1999).
[21]M.R.Zakin and D.R.Herschbach,J.Chem.Phys.85,2376(1986).
[22]H.H.Jaffe and M.Orchin,Theory and Applications of Ultra Violet Spectroscopy,New York:John Wiley and Sons,182(1962).
[23]T.G.Spiro and T.M.Loehr,In Advances in Infrared and Raman Spectroscopy,New York:Heyden,100(1975).
[24]S.Courtecuisse,F.Cansell,D.Fabre,and J.P.Petitet,J.Chem.Phys.108,7350(1998).
[25]T.R.Gibbs and A.Popolato,LASL Explosive Property Data,Berkeley:University of California Press,302(1980).
[26]S.Root and Y.M.Gupta,J.Phys.Chem.A 113,1268(2009).
[27]C.S.Yoo and N.C.Holmes,in High-pressure Science and Technology-1993,New York:AIP,1567(1994).
[28]S.N.Vinogradov and R.H.Linnell,Hydrogen Bonding,New York:Van Nostrand Reinhold Co.,53(1971).
[29]M.D.Joesten and L.J.Schaad,Hydrogen Bonding,New York:Marcel Dekker Inc.,(1974).
[30]B.J.Zhao,F.S.Liu,W.P.Wang,N.C.Zhang,L.P. Feng,M.J.Zhang,and X.D.Xue,Spectro.Spectral Anal.33,2603(2013).
[31]C.L.Mader,Numerical Modeling of Explosives and Propellants,Florida:CRC Press,1(1998).
[32]J.C.Xu and J.J.Zhao,Acta Phys.Sin.6,4144(2009).
∗Author to whom correspondence should be addressed.E-mail:fusheng-l@163.com,Tel.:+86-28-87601758
杂志排行
CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Tunneling Electron Induced Fluorescence from Single Porphyrin Molecules Decoupled by Striped-Phase Octanethiol Self-assembled Monolayer
- Ion Product of Pure Water Characterized by Physics-Based Water Model
- Laser Linewidth and Spectral Resolution in Infrared Scanning Sum Frequency Generation Vibrational Spectroscopy System
- Performances of Five Representative Force Fields on Gaseous Amino Acids with Different Termini
- Dynamics of Tripartite Entanglement and Intramolecular Energy in Symmetric Trimer Molecule
- Ab initio Study on Formation Mechanism of Spiro-Si-Heterocyclic Ring Compound Involving Ge from H2Ge=Si:and Formaldehyde
