Ion Product of Pure Water Characterized by Physics-Based Water Model
2016-09-13BinbinJieChihtangSahaDepartmentofPhysicsXiamenUniversityXiamen361005ChinaChineseAcademyofSciencesBeijing100864ChinaDatedReceivedonJuly162015AcceptedonSeptember212015
Bin-bin Jie,Chihtang Saha.Department of Physics,Xiamen University,Xiamen 361005,China b.Chinese Academy of Sciences,Beijing 100864,China(Dated:Received on July 16,2015;Accepted on September 21,2015)
Ion Product of Pure Water Characterized by Physics-Based Water Model
Bin-bin Jiea∗,Chihtang Saha,b∗
a.Department of Physics,Xiamen University,Xiamen 361005,China b.Chinese Academy of Sciences,Beijing 100864,China
(Dated:Received on July 16,2015;Accepted on September 21,2015)
Pure water has been characterized for nearly a century,by its dissociation into hydronium(H3O)1+and hydroxide(HO)1-ions.As a chemical equilibrium reaction,the equilibrium constant,known as the ion product or the product of the equilibrium concentration of the two ion species,has been extensively measured by chemists over the liquid water temperature and pressure range.The experimental data have been nonlinear least-squares fitted to chemical thermodynamic-based equilibrium equations,which have been accepted as the industrial standard for 35 years.In this study,a new and statistical-physics-based water ion product equation is presented,in which,the ions are the positively charged protons and the negatively charged proton-holes or prohols.Nonlinear least squares fits of our equation to the experimental data in the 0-100◦C pure liquid water range,give a factor of two better precision than the 35-year industrial standard.
Solid state physics,Liquid state chemistry,Pure water,Ion product,Positive proton,Negative proton-hole or prohol
I.INTRODUCTION
Chemical thermodynamics has accounted well for the energetics of equilibrium chemical reactions,such as the ion product of pure liquid water.However,when extrapolated to non-equilibrium,it does not account for the rates of ionic reaction by recombination and dissociation,and the rates of ionic transport by drift and diffusion.In this work,we report a study using statistical physics-based kinetic model to account for chemical reactions and transports in pure liquid water.The ion product of pure water,an equilibrium property,is modeled by the physics-based kinetic model which is set at thermodynamic equilibrium.A better agreement between physics-based theory and experiments than between the chemical thermodynamic model and experiments is obtained.
II.THEORETICAL ION-PRODUCT AND CONCENTRATION FORMULA
Results of our studies of physics-based water models have been described elsewhere[1-3].A brief review is given as follows.
(i)Our water model starts from the idealized crystal with a periodic lattice.The particle picture of electrical current flowing in water of our model consists of the movements of two species of mobile ions∶(a)the positively charged protons,to be represented by p+,representing the positively charged protons drifting from a(H3O)1+site to a(H2O)0site and(b)the negatively charged proton holes,to be represented by p-and named as prohols,representing the drifting of the positively charged proton from a(H2O)0site to a(HO)1-site.The p+and p-physical pathways are not identical,and the two proton species experience different amounts of scattering collisions with the vibrating oxygen core ions.In the energy band picture,this water model contains five charged particles∶two mobile charges(proton p+and prohol p-)and the three charge states of the immobile neutral protonic traps(V+,V0,and V-).This model may be further refined and generalized upon a more detailed knowledge of the protonic energy bands of pure water.
(ii)The idealized crystal in our water model is a periodic structure of double negatively charged oxygen cores,each with a+8 charged(8 protons and 8 neutrons)oxygen atomic nucleus(of about 1 fm diameter),two 1s core electrons,and eight(2s and 2p)valence electrons,all tightly bound to the oxygen nucleus.In this water model,an oxygen core is surrounded tetrahedrally by four adjacent oxygen cores,with two proton sites,located approximately at the two trisecting points on the line joining the two adjacent oxygen cores,known as“disordered”.The connections of the proton sites to the physical chemists’hydronium ion(H3O)1+,hydroxide ion(HO)1-,and the physical chemists’ignored water molecule(H2O)0are given by the following relations,where we use the capital letter V to designate the four proton sites closest to the oxy-gen core,as in vacancy,when the site is not occupied by a proton∶(H3O)1+=V+∶3 of the 4 proton sites surrounding an oxygen core are each occupied by a proton.(H2O)0=V0∶2 of the 4 proton sites surrounding an oxygen core are each occupied by a proton.(HO)1-=V-∶1 of the 4 proton sites surrounding an oxygen core is occupied by a proton.
(iii)In our water model described above,the ion product,which has been extensively measured by chemists,is actually the product of the equilibrium concentrations of the two mobile charge-carrying species in water,the protons and the prohols.This product is given by p+p-.
Similar to the theoretical formula for the product of electron and hole concentrations in semiconductors,such as silicon[4],the following theoretical formula of ion product of the proton and proton-hole or prohol of our water model is obtained.

here PHis the product of the equilibrium concentrations of the protons and prohols;dW(T)is the dimensionless temperature dependent water mass density(per unit volume)normalized to the value at a specific temperature;the exponents,m,n,and g are our physics-based protonic energy band model parameters,which indicate our model and physics success,if the choice of their numerical value resulted in little or no temperature dependence in D0(T)and E0(T),since the major temperature dependencies are already factored out through the water mass density and represented by m,n,and g,based on our water physics and model.m comes from the temperature dependence of the effective density of states or the density of state effective mass of the protons and the proton-holes(prohols).n comes from statistical average over the exponential distributions∶Boltzmann(at thermodynamic equilibrium)or Maxwellian(far from thermodynamic equilibrium),with a physics-based value of 3.g comes from the temperature dependence of the energy gap between the particle-hole(proton-prohol)pairs.
III.THE BEST-FITTING PARAMETERS
Extensive experimental data of the ion product of pure water,reported in the literature over 100 years,since Noyce et al.and associates first reported in 1907 and 1910 which were cited in Ref.[5],have recently been nonlinear least squares fitted(NLSF)by Marshall and Franck[5]to an empirical Arrhenius equation for the ion product,which consists of two empirical polynomials of third and second order in the power of(1/T),given by the Marshall-Franck equation[5].This empirical equation was issued by the International Association for the Properties of Steam(IAPS)as an international standard for the ion product of pure water[5,6].
The Marshall-Franck equation actually contains two sets of data for two sets of experimental conditions,which are constant water mass density,dW(T)=1.0,and temperature dependent water mass density,dW(T). Both sets represent accurately the ion product in pure water,dW(T)=1.0 especially for the ideal experiments with data-taking temperature over 0-100◦C,in which there is no loss of water molecule from evaporation or there is an infinitely large number of water molecules,and also no change of the interatomic distances of the molecules in the water containing a sufficiently large number of water molecules.Strictly speaking,there is no translational motion of the water molecules in the volume of fluid or liquid water,at the supposedly thermodynamic equilibrium experimental condition when the ion products were measured.
The initial test is a determination of the effect of the loosened experimental condition due to thermal expansion or temperature variation of the water density. Three quantities are evaluated on their temperature dependence,suggested by the following Arrhenius equation.

where A=-4.098,B=-3245.2 K,C=+2.2362×105K2,D=-3.984×107K3,E=+13.957,F=-1262.3 K,G= +8.5641×105K2[5],NA=6.02214×1023mol-1which is the Avogadro constant.dWis equal to the reciprocal of the dimensionless specific volume of the water[7].K0(T)and K1(T)have their units in cm-6and K,respectively.
Figure 1(a)shows the actual values and percentage difference of the first two quantities[PH(T)]1/2,[K0(T)]1/2,Fig.1(b)shows those of the third quantity K1(T),when the water density,dW(T),is not kept constant(real water case)while taking data over the entire 0-100◦C,from that when the water density is kept constant,dW(T)=1.0(ideal water case).The%RMS deviation of the three quantities is computed over the entire temperature range,given by the labels in Fig.1. The values of these three quantities shown in Fig.1 are in the freshman physics units∶concentration in particle number per cubic centimeter,and energy in millielectron-volt.
As expected,the percentage difference and%RMS deviations are small,because water just does not expand and contract much in the liquid state,or basically,the interatomic distances among the atoms forming the water,H and O,do not change much with temperature in the liquid temperature range,0-100◦C.But still,the most surprising is the unexpected(physics-based)small variation of these quantities with temperature as indicated in Fig.1,which could come from the empirical engineering NLSF of Marshall and Franck,that could hide if not wipe out the details.

FIG.1(a)Actual values and percentage difference of the two quantities[PH(T)]1/2,[K0(T)]1/2,and(b)the third quantity K1(T),given by the Marshall-Franck equation,of real water(the water density is not kept constant)and ideal water(the water density is constant of 1).
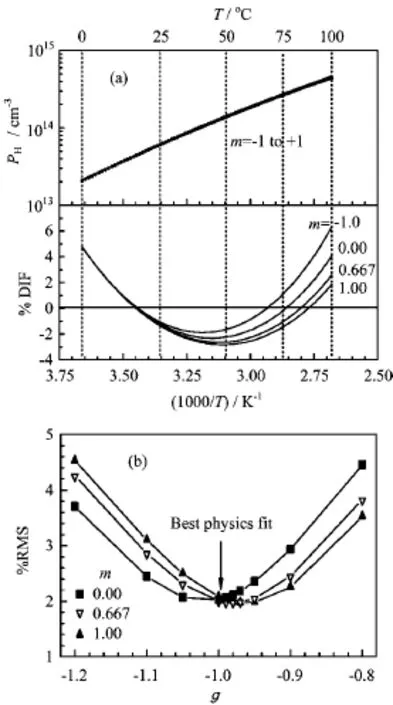
FIG.2(a)Theoretical and experimental ion product values of real water as a function of temperature over the entire liquid water range(0-100◦C)at the normal pressure,and their differences with parameters n=3,g=-1 and m=-1 to +1.(b)Their%RMS deviation over the entire liquid water range as a function of parameter g with different values of m with parameter n=3.
We now turn to the test to detect any proton energy band parameter information that might be hidden in the experimental data and that could be mined from the vast amount of temperature dependency data of the ion product of pure water.For this purpose,we employ the theoretical concentration formula Eq.(1)to provide the calculated experimental data since Marshall and Franck had already done,in great detail,the NLSF of the raw experimental data.
With the theoretical value 3 of parameter n,for the ideal water,the theoretical concentration formula is reduced into the following∶

We used the above theoretical formula to fit the ideal water ion product data given by the Marshall-Frank equation,and obtained the values of the two constants D0and E0.Then,for the real water,the theoretical concentration formula can be written as follows∶
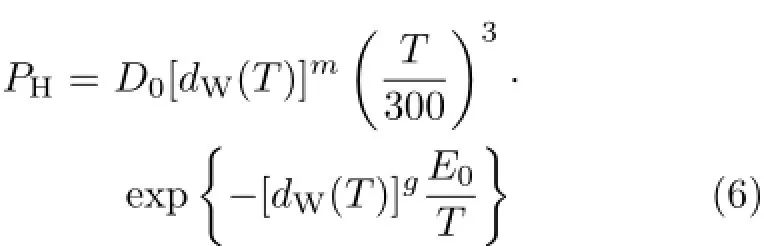
With the above formula,the theoretical values of the ion product as a function of temperature can be computed with the given(assumed)values of the parameters m and g.
Figure 2(a)shows the theoretical ion product of the real water as a function of temperature from 0◦C to100◦C with g=-1 and m=-1 to+1,and their percentage difference from the experimental ion product of the real water given by the Marshall-Frank equation,Eq.(2).The upper curves show the actual values of the ion product,which does not show a visual difference between the theoretical values and the experimental value on this compressed log scale.To observe the difference visually,we plotted the percentage difference,in expanded linear scale given by the lower curves,which show variations from+6%at the two temperature limits,0 and 100◦C,reducing down to zero and then to -3%in the middle temperature range around 50◦C.
Figure 2(b)gives the main test of the goodness of our physics-based model.It shows the%RMS deviation of our theoretical concentration formula,Eq.(6),from the experimental Marshall-Frank equation,over the temperature from 0◦C to 100◦C,with the parameters g and m varied to seek the least RMS deviation. As shown,with the fixed values for the parameter m,the%RMS deviation has a minimum value of about 2% near g=-1.00,for all values of m in the physics-based range of 0 to 1.The best physics fit of the parameter pair(g,m)is(-1,0).The physics meaning of g=-1 is that the dependence of energy gap on the temperature comes from the three dimensional thermal expansion of the water or the increasing oxygen interatomic distance in three spatial directions,that is,the volume expansion,not linearly expansion.The physics meaning of m=0 is that temperature dependence of the effective density of states of the protons and the proton-holes does not come from the thermal expansion of water.
IV.CONCLUSION
In this work,we showed that the thermodynamic equilibrium property of pure water,characterized by the fundamental quantity,the ion product,is better represented by our statistical-physics-based formula(~2%RMS deviation over the liquid water range,0-100◦C),than the world-wide standard since 1981,the Marshall-Franck chemical-thermodynamics-based formula(~5%)[8].The experimental-theoretical corroboration study of the electrical conductivity and ion mobilities in pure and impure water,are being published elsewhere.
[1]B.Jie and C.Sah,J.Semiconduc.34,121001(2013).
[2]B.Jie and C.Sah,J.Semiconduc.35,021001(2014).
[3]B.Jie and C.Sah,J.Semiconduc.35,041001(2014).
[4]Chih-Tang Sah,Fundamentals of Solid-State Electronics,World Scientific:Singapore,175(1991).
[5]W.L.Marshall and E.U.Franck,J.Phys.Chem.Ref. Data 10,295(1981).
[6]A.V.Bandura and S.N.Lvov,J.Phys.Chem.Ref.Data 35,15(2006).
[7]E.Schmidt,Properties of Water and Steam in SI-Units(0-800◦C and 0-1000 bar),New York:Springer-Verlag,192(1969).
[8]B.Jie and C.Sah,Bull.Am.Phys.Soc.March Meeting,(2015).
∗Authors to whom correspondence should be addressed.E-mail:bb-jie@msn.com,tom-sah@msn.com
杂志排行
CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Incorporation of Reactive Corrosion Inhibitor in Waterborne Acrylic Polyurethane Coatings and Evaluation of its Corrosion Performance
- Response Ability to External Signal Enhanced by Biological Spatial Configuration in Coupled Hindmarsh-Rose Neural System
- Synthesis and Surface Activity of Heterogemini Imidazolium Surfactants
- Substituent Effects on Reduction Potentials of Meta-substituted and Para-substituted Benzylideneanilines
- Preparation of Nitrogen-Doped Carbon Catalyst to Oxygen Reduction Reaction and Influence of Protective Gas Flowing on Its Activity
- Structural and Magnetic Properties of Chemically Synthesized Pd-Modified NiFe2O4Nanoparticles
