不同浓度去氢表雄酮对小鼠蜕膜化子宫内膜基质细胞增殖及Dtprp mRNA表达的影响
2016-08-31蒋凌云覃爱平欧奇志靳玉甫杭馥覃金春谢伟莫馥华
蒋凌云,覃爱平,欧奇志,靳玉甫,杭馥,覃金春,谢伟,莫馥华
(广西医科大学第一附属医院生殖医学研究中心,南宁530021)
不同浓度去氢表雄酮对小鼠蜕膜化子宫内膜基质细胞增殖及Dtprp mRNA表达的影响
蒋凌云,覃爱平,欧奇志,靳玉甫,杭馥,覃金春,谢伟,莫馥华
(广西医科大学第一附属医院生殖医学研究中心,南宁530021)
目的观察不同浓度去氢表雄酮(DHEA)对小鼠蜕膜化子宫内膜基质细胞增殖及蜕膜催乳素相关蛋白(Dtprp)mRNA表达的影响。方法体外分离培养妊娠第4天小鼠的子宫内膜基质细胞。将细胞分为实验1组、实验2组、实验3组、实验4组、模型组、对照组;各实验组与模型组以E2、P4诱导蜕膜化,实验1、2、3、4组分别加入0.1、1、10、50 μmol/L的DHEA,模型组不加DHEA,对照组培养液不给予E2、P4及DHEA;培养72 h后采用real-time PCR法检测细胞中的Dtprp mRNA,分别于培养24、48 h后倒置显微镜下观察细胞形态变化。取各实验组、模型组的细胞悬液接种于96孔板,调零组不接种细胞;培养72 h后检测细胞增殖能力。结果实验1、2、3、4组细胞中Dtprp mRNA相对表达量分别为0.978 0±0.258 0、0.764 0±0.135 0、0.250 0±0.022 0、0.013 0±0.000 0,模型组与对照组分别为1.000 0±0.000 0、0.000 2±0.000 0;实验3、4组Dtprp mRNA相对表达量与模型组相比,P均<0.05;实验2组与实验4组相比,P<0.05。对照组细胞呈成纤维细胞样;模型组细胞形态更饱满,胞质增多;实验1、2组细胞形态与模型组近似,实验3、4组细胞呈多角形,胞质减少。实验1、2、3、4组细胞增殖能力分别为1.222 8±0.038 2、0.790 5±0.207 1、0.592 5±0.229 8、0.265 8±0.037 4,模型组为1.149 7±0.106 2,调零组校正A值为0.087 1±0.006 3;实验3、4组与模型组相比,P均<0.05;实验1组与实验2、3、4组相比,P均<0.05;实验2组与实验4组相比,P<0.05。结论0.1、1 μmol/L DHEA对蜕膜化子宫内膜基质细胞增殖及Dtprp mRNA表达无明显影响,而10、50 μmol/L DHEA可抑制细胞增殖,并下调Dtprp mRNA表达。
去氢表雄酮;子宫内膜基质细胞;蜕膜细胞反应;蜕膜催乳素相关蛋白
蜕膜化是子宫内膜基质细胞分化为蜕膜细胞的过程,对胚胎着床和妊娠有重要作用[1,2]。去氢表雄酮(DHEA)是人体最丰富的甾体激素,具有调节血管内皮细胞功能、改善胰岛素敏感性、改善中枢神经系统功能、调节免疫及血脂代谢等多种生物学效应[3]。国外研究[4]证实高浓度(50、100 μmol/L)DHEA可抑制子宫内膜蜕膜化,不利于胚胎着床和妊娠。2013年9月~2015年7月,我们观察了不同浓度DHEA对体外蜕膜化小鼠子宫内膜基质细胞增殖及蜕膜化标志物[5]蜕膜催乳素相关蛋白(Dtprp)mRNA表达的影响,现报告如下。
1 材料与方法
1.1实验动物清洁级中国昆明小白鼠400只(广西医科大学实验动物中心),6~8周龄,体质量30~35 g,于发情当日雌雄2∶1合笼,次日晨8:00~9:00检查阴栓,发现阴栓计为妊娠第1天。
1.2小鼠子宫内膜基质细胞的分离与鉴定参考文献[6]方法并略作改动。取妊娠第4天小鼠,断头处死后取子宫,剔除多余脂肪及结缔组织;用含100 IU/mL双抗的PBS冲洗2遍,剪开双侧子宫角暴露宫腔,剪碎移入15 mL离心管,离心去除PBS;加入与组织碎块等体积的消化液,在37 ℃的CO2温箱消化2 h,每15 min振荡30 s;用含10%普通胎牛血清的无酚红DMEM/F12培养液终止消化,吸管吹打数次;静置离心管3 min,吸取上层细胞悬液,过200目滤网,PBS冲洗离心管内组织块2次,冲洗液过200目滤网;将上述细胞滤液1 000 r/min离心7 min,弃上清,用加双抗的含10%普通胎牛血清DMEM/F12培养液重悬,台盼蓝染色,细胞以2×105/孔接种入24孔板、12×105/瓶接种入T25培养瓶,置于37 ℃、5%CO2培养箱培养。1~2 h内基质细胞贴壁后吸出原培养液,依次用PBS和新鲜待加入培养液润洗2次,加入新鲜培养液继续培养。待24孔板中细胞爬满80%板底面积时,进行免疫细胞荧光染色[7],鉴定子宫内膜基质细胞。
1.3DHEA对子宫内膜基质细胞中Dtprp mRNA表达及细胞形态的影响观察
1.3.1细胞分组与DHEA用法将细胞分为实验1组、实验2组、实验3组、实验4组、模型组与对照组。实验1、2、3、4组与模型组以10 nmol/L E2、1 μmol/L P4诱导蜕膜化,其中实验1、2、3、4组分别加入0.1、1、10、50 μmol/L的DHEA,模型组不加DHEA;对照组培养液不给予E2、P4及DHEA处理,加入0.1%乙醇。各组每隔24 h换培养液。培养72 h后检测Dtprp mRNA。
1.3.2各组细胞中Dtprp mRNA检测提取细胞总RNA,逆转录为cDNA,进行real-time PCR反应。Dtprp mRNA上游引物序列为5′-AGAGCCAGAAATCACTGCCACTCTA-3′,下游引物序列为5′-AGGAGTGATCCATGCACCCATAA-3′;内参GAPDH上游引物序列为5′- GGTGAAGGTCGGTGTGAACG-3′,下游引物序列为5′-CTCGCTCCTGGAAGATGGTG-3′。以2-ΔΔCt表示目的基因相对表达量。
1.3.3细胞形态观察分别于培养24、48 h后,在倒置显微镜下观察细胞形态变化。
1.4DHEA对子宫内膜基质细胞增殖的影响观察取“1.3.1”中各实验组、模型组的细胞悬液,接种于96孔板,细胞约8×104个/孔。调零组不接种细胞,只加入含E2、P4的1%碳吸附胎牛血清DMEM/F12培养液。置于37 ℃、5% CO2培养箱中培养。培养72 h后吸出各孔培养液,每孔加入无酚红DMEM/F12培养液100 μL及CCK-8试剂10 μL,继续培养2 h,ELISA法检测吸光度值(A460值),将模型组及实验组各孔测得的A460值减去调零孔A460值,得到校正的A值,表示细胞增殖能力。
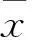
2 结果
2.1各组细胞中Dtprp mRNA表达比较实验1、2、3、4组细胞中Dtprp mRNA相对表达量分别为0.978 0±0.258 0、0.764 0±0.135 0、0.250 0±0.022 0、0.013 0±0.000 0,模型组与对照组分别为1.000 0±0.000 0、0.000 2±0.000 0;实验3、4组Dtprp mRNA相对表达量与模型组相比,P均<0.05;实验2组与实验4组相比,P<0.05。
2.2各组细胞形态变化对照组子宫内膜基质细胞生长旺盛,呈长梭形;模型组细胞出现蜕膜化,形态更饱满,胞质增多;实验1、2组细胞形态与模型组近似,实验3、4组细胞呈多角形,胞质明显减少。
2.3各组细胞增殖能力比较实验1、2、3、4组增殖能力分别为1.222 8±0.038 2、0.790 5±0.207 1、0.592 5±0.229 8、0.265 8±0.037 4,模型组增殖能力为1.149 7±0.106 2,调零组校正A值为0.087 1±0.006 3;实验3、4组与模型组相比,P均<0.05;实验1组与实验2、3、4组相比,P均<0.05;实验2组与实验4组相比,P<0.05。
3 讨论
DHEA主要在人肾上腺皮质网状带合成,大部分以硫酸去氢表雄酮的形式进入血循环。DHEA有广泛的生物学效应。蜕膜化是子宫内膜基质细胞分化为蜕膜细胞的过程。研究[2]证实,蜕膜组织能提供胚胎发育所需的生长因子和细胞因子,调节滋养细胞侵袭,并发挥免疫调节作用。有研究[8]指出,DHEA可用于绝经后女性预防骨质疏松及改善阴道萎缩的替代治疗。也有学者[9]将DHEA用于卵巢低反应性患者从而改善卵巢反应性,但效果和安全性有待进一步验证。
正常人血清DHEA水平一般在30 nmol/L以下[10],多囊卵巢综合征患者血清DHEA水平可达正常人的2倍。国外研究显示,100 μmol/L DHEA可抑制肾上腺髓质祖细胞增殖,而0.1、1、10 μmol/L DHEA对细胞活性无显著影响[11]。药理浓度(1~200 μmol/L)的DHEA可抑制HepG2细胞系增殖,而生理浓度(1 μmol/L以下)的DHEA可促进细胞增殖[12]。有学者[4]发现,10 μmol/L及以上浓度的DHEA可抑制细胞葡萄糖-6-磷酸脱氢酶的活性。我们推测不同浓度DHEA对子宫内膜基质细胞葡萄糖-6-磷酸脱氢酶活性的抑制作用存在差别。
本研究利用原代分离培养的小鼠子宫内膜基质细胞,在培养液中加入雌、孕激素诱导蜕膜化,发现不同浓度DHEA对小鼠子宫内膜基质细胞增殖和体外蜕膜化的影响程度不同。实验3、4组Dtprp mRNA相对表达量及细胞增殖能力低于模型组,细胞呈多角形、胞质减少,提示0.1、1 μmol/L DHEA不抑制子宫内膜基质细胞增殖和体外蜕膜化,而10、50 μmol/L DHEA则可明显抑制细胞增殖及体外蜕膜化。我们推测,人在疾病状态下补充DHEA可能不会对子宫内膜蜕膜化产生影响。然而,DHEA的生理功能及作用机制仍未完全阐明,上述结论仍需要进一步去证实。
[1] Zhu H,Hou CC,Luo LF,et al.Endometrial stromal cells and decidualized stromal cells: origins,transformation and functions[J].Gene,2014,551(1):1-14.
[2] Ramathal CY,Bagchi IC,Taylor RN,et al.Endometrial decidualization: of mice and men[J].Semin Reprod Med,2010,28(1):17-26.
[3] Traish AM,Kang HP,Saad F,et al.Dehydroepiandrosterone (DHEA)--a precursor steroid or an active hormone in human physiology[J].J Sex Med,2011,8(11):2960-2982.
[4] Frolova AI,O′Neill K,Moley KH.Dehydroepiandrosterone inhibits glucose flux through the pentose phosphate pathway in human and mouse endometrial stromal cells,preventing decidualization and implantation[J].Mol Endocrinol,2011,25(8):1444-1455.
[5] Kimura F,Takakura K,Takebayashi K,et al.Messenger ribonucleic acid for the mouse decidual prolactin is present and induced during in vitro decidualization of endometrial stromal cells[J].Gynecol Endocrinol,2001,15(6):426-432.
[6] Zhao KQ,Lin HY,Zhu C,et al.Maternal Smad3 deficiency compromises decidualization in mice[J].J Cell Biochem,2012,113(10):3266-3275.
[7] Zhang L,Guo W,Chen Q,et al.Adam12 plays a role during uterine decidualization in mice[J].Cell Tissue Res,2009,338(3):413-421.
[8] Lin LT,Tsui KH,Wang PH.Clinical application of dehydroepiandrosterone in reproduction: A review of the evidence[J].J Chin Med Assoc,2015,78(8):446-453.
[9] Rutkowski K,Sowa P,Rutkowska-Talipska J,et al.Dehydroepiandrosterone (DHEA): hypes and hopes[J].Drugs,2014,74(11):1195-1207.
[10] Warner M,Gustafsson JA.DHEA - a precursor of ERbeta ligands[J].J Steroid Biochem Mol Biol,2015,145:245-247.
[11] Chung KF,Qin N,Androutsellis-Theotokis A,et al.Effects of dehydroepiandrosterone on proliferation and differentiation of chromaffin progenitor cells[J].Mol Cell Endocrinol,2011,336(1-2):141-148.
[12] Teng Y,Litchfield LM,Ivanova MM,et al.Dehydroepiandrosterone-induces miR-21 transcription in HepG2 cells through estrogen receptor beta and androgen receptor[J].Mol Cell Endocrinol,2014,392(1-2):23-36.
Effects of different concentrations of dehydroepiandrosterone on proliferation and Dtprp mRNA expression of decidualized endometrial stromal cells in mice
JIANG Lingyun,QIN Aiping,OU Qizhi,JIN Yufu,HANG Fu,QIN Jinchun,XIE Wei,MO Fuhua
(The First Affiliated Hospital of Guangxi Medical University,Nanning 530021,China)
ObjectiveTo observe the effects of different concentrations of dehydroepiandrosterone (DHEA) on proliferation and decidual prolactin-related protein (Dtprp) mRNA expression of decidualized endometrial stromal cells in mice.MethodsPrimary endometrial stromal cells were isolated and cultured in vitro from mice on day 4 of pregnancy.The cells were divided into the experimental group 1,group 2,group 3,group 4,model group and control group.The experimental groups and the model group were treated with estradiol (E2) and progesterone (P4) to induce in vitro decidualization.The experimental groups 1,2,3 and 4 were exposed to 0.1,1,10 and 50 μmol/L DHEA,respectively.The control group was not treated with E2,P4and DHEA.The expression of Dtprp mRNA was detected by real-time PCR after 72-hour culture.Morphological changes were observed under inverted microscope after 24 and 48 h.The cells of the experimental groups and model group were cultured in 96-well plates except the blank wells.The cell proliferation ability was measured using a cell counting kit-8 after 72 h.ResultsThe relative expression of Dtprp mRNA in the experimental groups 1,2,3 and 4 was 0.978 0±0.258 0,0.764 0±0.135 0,0.250 0± 0.022 0 and 0.013 0±0.000 0,respectively; while that in the model group and control group was 1.000 0±0.000 0 and 0.000 2±0.000 0.The expression of Dtprp mRNA in both group 3 and group 4 was lower than that of the model group (all P<0.05).Significant difference was found between the experimental group 2 and group 4 (P<0.05).The cells in the control group showed a fibroblast-like appearance.Cells in the model group enlarged and had an increased amount of cytoplasm.The appearance of cells in the experimental group 1 and group 2 was similar to that of the model group.Cells in the experimental group 3 and 4 became polygonal with less cytoplasm.The corrected absorbance of the experimental groups 1,2,3,4,model group and backgroud absorbance were 1.222 8± 0.038 2,0.790 5±0.207 1,0.592 5±0.229 8,0.265 8±0.037 4,1.149 7±0.106 2 and 0.087 1±0.006 3,respectively.Significant difference was found between experimental groups 3,4 and the model group,between experimental group 1 and groups 2,3 and 4,between experimental group 2 and group 4 (all P<0.05).ConclusionThe proliferation and Dtprp mRNA expression of mouse endometrial stromal cells are not significantly affected by 0.1 and 1 μmol/L DHEA,while 10 and 50 μmol/L DHEA inhibits cell proliferation and down-regulates Dtprp mRNA expression.
dehydroepiandrosterone; endometrial stromal cells; decidual reaction; decidual prolactin-related protein
国家自然科学基金资助项目(81260096)。
蒋凌云(1989-),女,在读硕士研究生,主要研究方向为生殖内分泌。E-mail:jianglingyun2013@sina.com
简介:覃爱平(1963-),女,教授,主要研究方向为生殖内分泌、辅助生殖技术。E-mail: aini02456@126.com
10.3969/j.issn.1002-266X.2016.19.002
R339.2
A
1002-266X(2016)19-0005-03
2015-11-24)
