Effects of Salmonella infection on hepatic damage following acute liver injury in rats
2016-08-26YongTaoLiChengBoYuDongYanJianRongHuangandLanJuanLiHangzhouChina
Yong-Tao Li, Cheng-Bo Yu, Dong Yan, Jian-Rong Huang and Lan-Juan LiHangzhou, China
Effects of Salmonella infection on hepatic damage following acute liver injury in rats
Yong-Tao Li, Cheng-Bo Yu, Dong Yan, Jian-Rong Huang and Lan-Juan Li
Hangzhou, China
BACKGROUND: Acute liver injury is a common clinical disorder associated with intestinal barrier injury and disturbance of intestinal microbiota. Probiotic supplementation has been reported to reduce liver injury; however, it is unclear whether enteropathogen infection exacerbates liver injury. The purpose of this study was to address this unanswered question using a rat model.
METHODS: Oral supplementation with Salmonella enterica serovar enteritidis (S. enteritidis) was given to rats for 7 days. Different degrees of acute liver injury were then induced by intraperitoneal injection of D-galactosamine. The presence and extent of liver injury was assayed by measuring the concentrations of serum alanine aminotransferase, aspartate aminotransferase, and total bilirubin. Histology was used to observe liver tissue damage. Additionally, we measured the changes in plasma endotoxin, serum cytokines and bacterial translocation to clarify the mechanisms underlying intestinal microbiota associated liver injury.
RESULTS: The levels of liver damage and endotoxin were significantly increased in the Salmonella infected rats with severe liver injury compared with the no infection ratswith severe liver injury (P<0.01); The peyer's patch CD3+T cell counts were increased significantly when the Salmonella infection with severe injury group was compared with the normal group (P<0.05). S. enteritidis pretreatment enhanced intestinal barrier impairment and bacterial translocation.
CONCLUSIONS: Oral S. enteritidis administration exacerbates acute liver injury, especially when injury was severe. Major factors of the exacerbation include inflammatory and oxidative stress injuries induced by the translocated bacteria and associated endotoxins, as well as over-activation of the immune system in the intestine and liver.
(Hepatobiliary Pancreat Dis Int 2016;15:399-405)
acute liver injury;
Salmonella enteritidis;
endotoxin;
cytokine;
bacterial translocation
Introduction
The gut and the liver are the key organs in nutrient absorption, metabolism and immune defense. The functions of the two organs affect each other through gut-liver axis.[1]Acute liver injury is commonly found in clinical practices. The liver injury often accompanies with intestinal barrier impairment and imbalance of intestinal microbiota. The imbalanced microbiota are often shown as excessive growth of aggressive bacteria and decrease of protective species, which contribute to the risk of spontaneous bacterial peritonitis and sepsis.[2]
The gut microbiota has been recognized as a significant factor influencing our health and well-being.[3]The main beneficial effects were achieved by stimulating the proliferation of epithelial cells, providing the host colonization resistance to invading pathogens and regulating intestinal immune system.[4, 5]Repeated alterations of the intestinal bacterial equilibrium can impair homeostasis,resulting in immune imbalance and the emergence of infectious inflammatory or allergic diseases.[6, 7]The ecological changes in intestinal system may also affect the liver function significantly. For example, liver and biliary abnormalities are common in patients with inflammatory bowel disease, celiac disease and in patients with jejunoileal bypass or short bowel syndrome.[1]A breakdown in intestinal barrier function and increased endotoxins result in the activation of macrophages in liver. The production of nitric oxide and cytokines also impairs liverfunction.[8]Several studies have demonstrated that probiotics, such as Lactobacillus and Bifidobacterium, can palliate liver injury through restoring the balance of intestinal microbiota and increase colonization resistance to enteropathogens.[9, 10]On the basis of these findings, we hypothesized that infection with enteropathogens would aggravate liver injury through interrupting the intestinal barrier, perturbing the balance of intestinal microbiota and enhancing bacteria translocation.
In this study, Salmonella, one of the most common food-borne bacteria,[11]was used to investigate whether oral infection exacerbates acute liver injury with different degrees of damages induced by D-galactosamine (GalN)and to what extent it affects. ketamine hydrochloride (50 mg/kg body weight) (Shanghai First Biochemical & Pharmaceutical Co., China) and ether inhalation.
Methods
Animals and Salmonella
Specific pathogen-free (SPF) male Sprague-Dawley rats weighing 180 to 220 g were purchased from Zhejiang Academy of Medical Sciences, Hangzhou, China. The experimental rats were individually caged at 21 ℃ and exposed to a 12 hours light/dark cycle. The rats were fed with sterilized water and standard rat chow. All animal experiments were conducted according to the Guide for the Care and Use of Laboratory Animals and approved by the Research, Animal Sources and Animal Care Committee of The First Affiliated Hospital, Zhejiang University School of Medicine. Salmonella enterica serovar enteritidis (S. enteritidis) was isolated from a patient and maintained in culture as described.[12]
Experimental design and animal treatment
Forty-eight rats were randomized into six groups (n=8/group): (i) Salmonella infection with no liver injury group (the S+no injury group); (ii) Salmonella infection with moderate liver injury group (the S+moderate injury group); (iii) Salmonella infection with severe liver injury group (the S+severe injury group); (iv) no infection and no liver injury group (the normal group); (v) no infection but with moderate liver injury group (the moderate injury group) and (vi) no infection but with severe liver injury group (the severe injury group). Salmonella infection was performed by gastric gavage with 2 mL/day (2.0 × 1010CFU/mL suspended in physiologic saline) of live S. enteritidis to the Salmonella infection groups. Animals in the no infection groups received 2 mL physiologic saline only. On day 7, the moderate liver injury was induced by intraperitoneal injection of 0.7 g/kg body weight of GalN (Sigma Chemical Co., St. Louis, MO, USA) and the severe liver injury with 1.1 g/kg GalN.[13]Twenty-four hours later,animals were sacrificed by intramuscular injection of
Assessment of liver injury and endotoxin measurement
Blood samples from the inferior vena cava were centrifuged at 3000 g/min for 15 minutes at room temperature. Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin (TBiL) were measured using a Hitachi 7600 automatic analyzer (Hitachi, Tokyo, Japan).
Blood from the inferior vena cava (0.5 mL) was placed in an endotoxin-free tube with heparin and centrifuged at 3000 g/min for 15 minutes at 4 ℃. Plasma endotoxin concentrations were determined using quantitative, chromogenic Limulus Amebocyte Lysate (LAL; Eihua Medical Co., Shanghai, China) according to the manufacturer's instructions. Endotoxin levels were expressed as ng/mL.
Bacterial translocation
Samples from the liver and mesenteric lymph node (MLN) tissues were weighed and placed in a sterile glass homogenizer containing a nine-fold amount of anaerobic buffer (PBS with 0.5 g cysteine HCl, 0.5 mL tween 80 and 0.5 g agar/L). They were homogenized and 50 μL homogenate was planted on a blood agar base (bioM-erieux, Inc., Durham, NC, USA) and an anaerobic blood agar base (bioMerieux) within 30 minutes of sample collection. The plates were incubated for 48 hours at 37 ℃in anaerobic or aerobic environments, respectively. Sterile swabs were passed over the parietal peritoneal surface and then cultured on the same medium as an index of aseptic technique. The number of CFU on each plate was counted and the number of bacteria in each sample was determined based on the original weight of the sample at the time of collection. The results were expressed as the mean log10CFU/g. Bacterial translocation (BT) was thought to occur if bacteria grew in the culture medium with the tissues homogenate.[14]
Liver histology
Liver tissue was fixed in 10% buffered formalin and embedded in paraffin. Five μm thick serial sections were stained with hematoxylin and eosin (HE) for histological analysis. The degree of liver injury and inflammation was semiquantitatively graded on a scale of 0 (absent), 1 (mild), 2 (moderate) and 3 (extensive).[15]At least three slides were studied in a blinded fashion from each specimen. Images were taken with a Philips light microscope (Philips Research, Eindhoven, The Netherlands) using a 20× objective.
Detection of apoptotic liver cells
Apoptotic liver cells were detected by the in situ terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) method according to the instructions of the In Situ Cell Death Detection Kit (Roche Diagnostics Corp, Basel, Switzerland). Positively stained nuclei were counted in 10 high-power fields (×200) for each animal. Results were reported as apoptotic index (AI)=apoptotic cell counts/total cells counts in the same field.
Measurement of serum cytokines
Serum levels of TNF-α, IFN-γ, IL-10 and IL-12 were determined using a quantitative enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems, MI, USA)according to the manufacturer's protocol. Results were expressed as pg/mL of serum.
Flow cytometry analysis of CD3+and CD4+T cells in PPs and peripheral blood
Single cell suspensions of peyer's patch (PPs) were obtained according to the methods described in a previous study.[14]The PP cell suspension was incubated with FITC-CD3 and PE-CD4 antibodies (Thermo Fisher Scientific, Waltham, MA, USA) on ice for 1 hour and then washed three times with PBS-1% BSA.
Fifty μL sodium citrate anti-coagulated blood were incubated with FITC-CD3 and PE-CD4 antibodies (Invitrogen) for 15 minutes at room temperature in the dark. The antibody-labeled samples were then analyzed using a flow cytometer (R&D Systems). Results were reported as ng/L of serum.
Statistical analysis
Data were analyzed using Student's t tests or one-way ANOVA for parametric samples, and non-parametric Mann-Whitney U tests for nonparametric samples. Spearman rank correlations for correlation analyses and the incidence of bacteria translocation were statistically evaluated using Fisher's exact Chi-square tests with SPSS software (SPSS 16.5, SPSS Inc. Chicago, IL, USA). A P<0.05 was considered statistically significant.
Results
Salmonella infection exacerbates damage to liver function
The Salmonella infection in the S+severe injury group caused significant increases in serum ALT, AST and TBiL compared with the severe injury rats (P<0.01, Table 1). AST and TBiL increased in the S+moderate injury group compared with the moderate injury animals (P<0.05,Table 1). This indicated that S. enteritidis infection has no apparent effect on normal rats, but exaggerates liver injury, especially in rats with severely damaged liver.
Endotoxin levels in the S+moderate injury group and the S+severe injury group were significantly elevated (P<0.05 and P<0.01, respectively) compared with the normal group or the severe injury group. The endotoxin levels were increased slightly in the moderate injury group and the severe injury group compared with the normal animals.
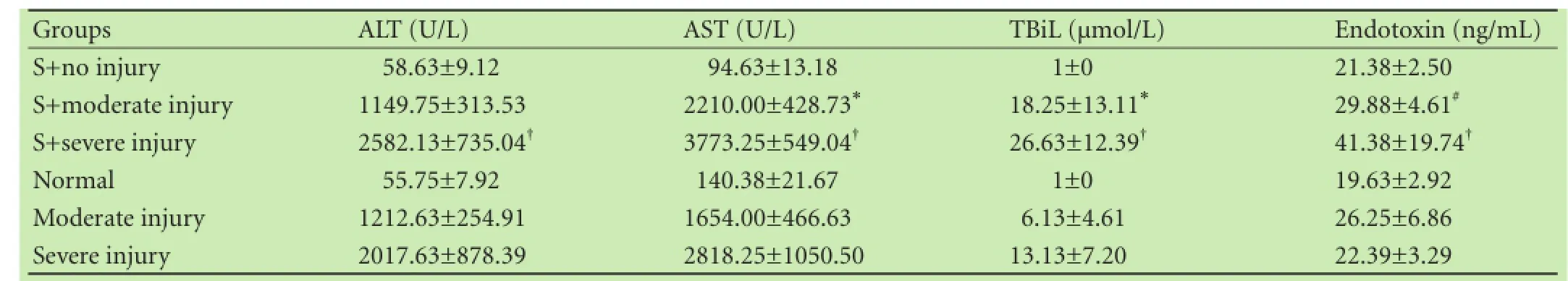
Table 1. Measurement of liver enzymes, TBiL and endotoxin (mean±SD)
Salmonella infection enhances liver structure damage
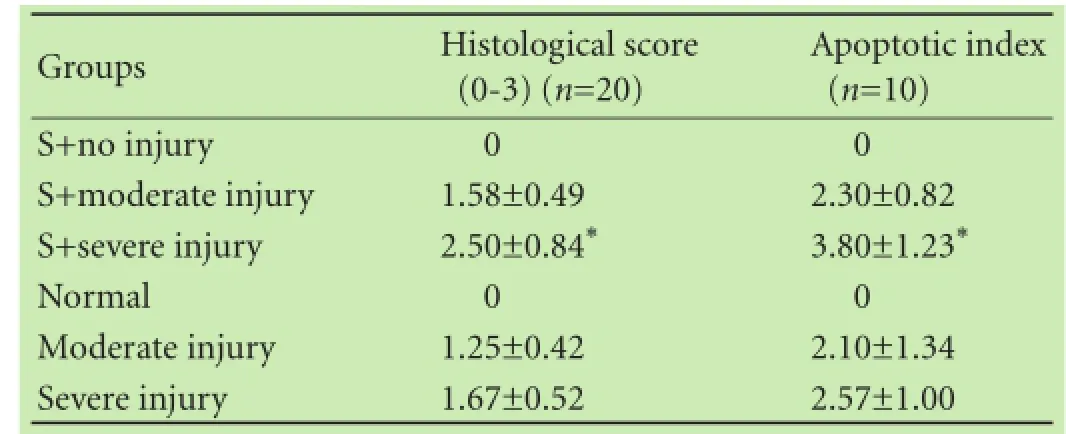
Liver tissues from the S+moderate injury group,the moderate injury group, the S+severe injury group and the severe injury group exhibited cellular swelling,vacuolization, pan lobular focal hepatocellular necrosis,pronounced polymorphonuclear (PMN) cell infiltration and microabscess formation in the parenchyma. Lesions were more extensive in the S+severe injury group and the severe injury group (Fig. 1) with extensive and massive hepatocellular necrosis and prominent hemorrhage. Hepatic lobules almost disappeared in the S+severe injury group tissues with higher histological scores (Table 2;P<0.05) compared with tissues from the severe injury group. The AI in the S+severe injury group was higher than that in the severe injury group (Table 2, Fig. 2;P<0.05). These results strongly suggested that Salmonellainfection enhances structural damage to the liver and induces apoptotic cell death.
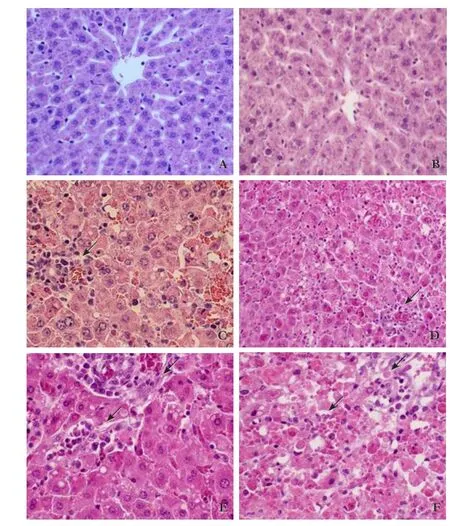
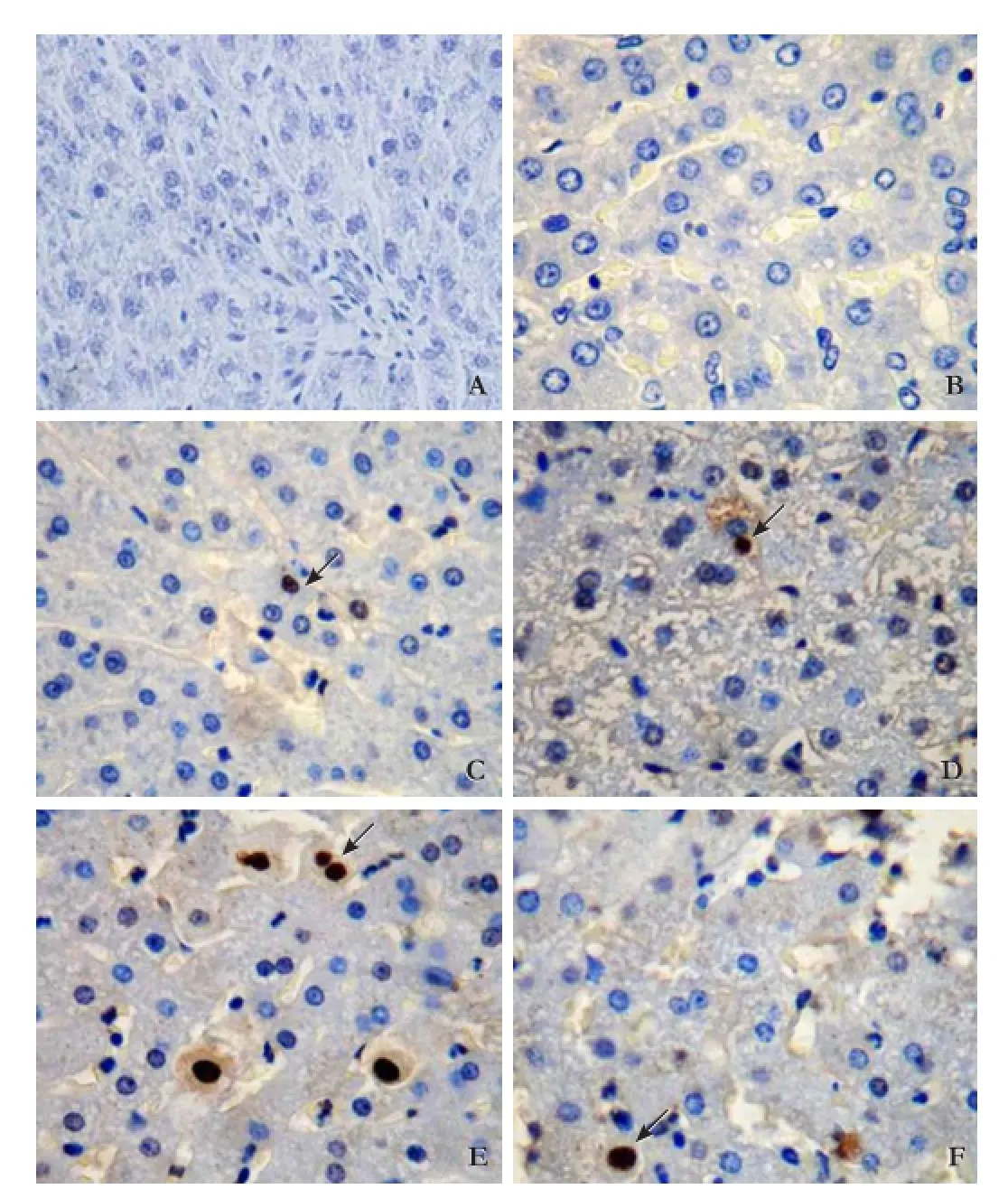
Fig. 1. Histological analysis of liver sections, showing HE stained liver section of (A) the normal group; (B) S+no injury group; (C)the moderate injury group; (D) S+moderate injury group; (E) severe injury group; (F) S+severe injury group. Lesions were more extensive in the S+moderate injury, moderate injury, S+severe injury,and severe injury groups (arrows). Original magnification × 200.
Table 2. Histological scores and apoptotic index of liver
*: P<0.05, vs the severe injury group.

Table 3. Bacterial translocation to the liver and MLNs in different groups (log10CFU/g tissue, aerobic/anaerobic in parentheses)
Salmonella infection facilitates bacterial translocation
Bacterial translocation to the liver and MLNs in the S+severe injury group and the S+moderate injury group was increased significantly when compared with the normal group (Table 3; P<0.05). Only a small number of bacteria was detected in the MLNs of the S+no injury group and the normal group. These results suggested that Salmonella infection significantly facilitates bacterial translocation, especially to the liver.
Salmonella infection induces the host's anti-salmonella response
IL-12, IFN-γ, TNF-α or IL-10 levels in the S+no injury group tended to be increased compared with the normal group, but the increase did not reach significant level. This suggested that salmonella infection induces an antisalmonella response in the host organism. IFN-γ, IL-10and IL-12 were increased significantly in the S+ severe injury group compared with those in the normal group. TNF-α increased significantly in the S+moderate injury group, the S+severe injury rats and severe injury animals compared with that in the normal group (Table 4).
Salmonella infection suppresses the systemic immune response, especially in the severely injured liver
CD3+and CD4+T cells were decreased in the peripheral blood while PP CD3+T cells were increased significantly in the S+severe injury group (P<0.05) compared with those in the normal group, suggesting that the systemic immune response was suppressed but that the intestinal immune response was activated in the S+severe injury group (Table 5). CD3+and CD4+T cell counts in PPs were positively correlated with the number of PPs in individual animals in the S+moderate injury group and the S+severe injury group. The number of PPs in the whole small intestine was greater (Table 5; P<0.05) in the S+severe injury group compared with those in the normal group.
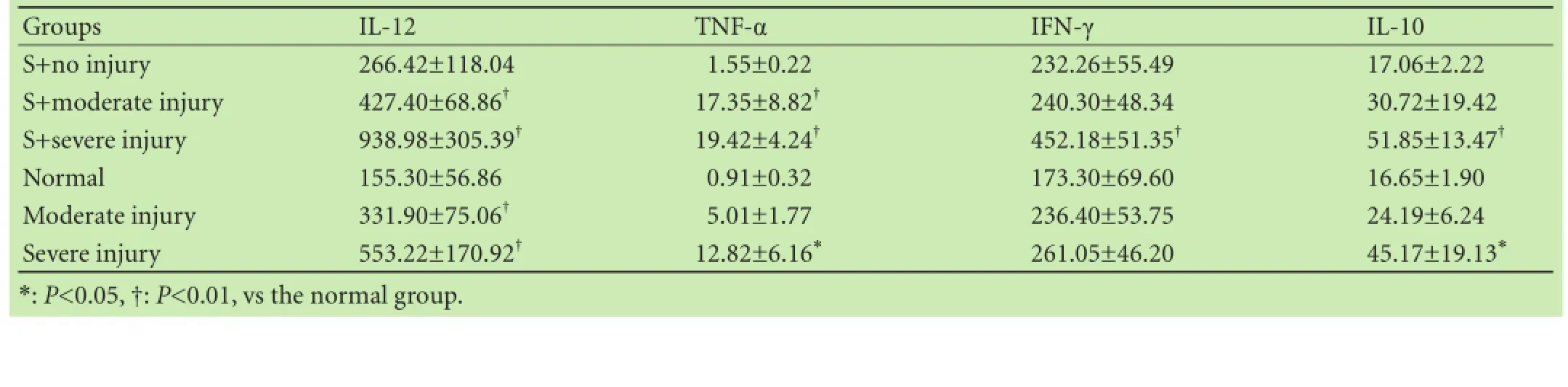
Table 4. Serum concentrations of cytokines (mean±SD, pg/mL)
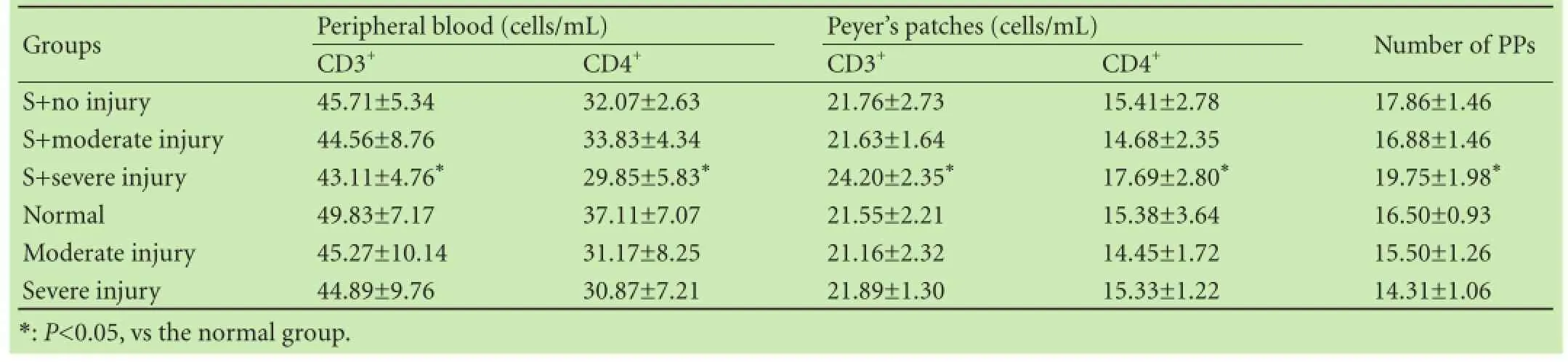
Table 5. CD3+T cells and CD4+T cells in peripheral blood and peyer's patches (PPs) and the number of PPs in the entire small intestinal (mean±SD)
Discussion
Previous studies have demonstrated that gut microbiota modifications had different effects on the prevention or exacerbation of acute liver injury.[14]In this study, we demonstrated that oral Salmonella enteritidis administration exacerbated liver injury, as evidenced by increasing liver enzyme activities, hepatic histological scores and apoptotic cell counts. This was especially clear when the liver was severely insulted.
Salmonella infection disrupts epithelial barrier integrity, enhances bacterial translocation and promotes the migration of PMNs which evoke a rapid local inflammatory response to block bacterial replication.[16, 17]However, we observed no significant increase of PMN infiltration in Salmonella+ GalN (the S+moderate injury group and the S+severe injury group) when compared with GalN only groups (the moderate injury group and the severe injury group). This may explain the higher bacterial translocation and lack of diarrhea observed in the Salmonella+GalN groups.[18]It is thought that PPs,the main inductive site of gut immunity against infection, especially in the ileum, are preferentially colonized by Salmonella.[14]Salmonella infection and severe liver injury (the S+severe injury group) caused PP enlargement and lymphoid follicle proliferation, as well as a significant increase in the number of PPs and CD3+T cells. This suggested a strong inflammatory response occurred within the PPs, providing a mechanism through which PPs may participate in the immune defense against Salmonella infection. Although local inflammation and histological lesions of the ileum were observed in the S+no injury group, bacterial translocation and liver structure and function were not affected. However,excessive pathogen induced host inflammatory responsesin intestinal barrier lesions can lead to further impairment and result in devastating consequences by promoting bacterial translocation to extraintestinal sites. Furthermore, increased intestinal immune responses could shift the balance between the protective microbiota and pathogenic bacteria.[19]This hypothesis is consistent with our observations that bacterial translocation and plasma endotoxin levels were elevated in the Salmonella+GalN groups. Moreover, systemic immune response suppression could also contribute to bacteria translocation.
A previous study[20]demonstrated that it was primarily S. enteritidis flagellin, not lipopolysaccharide, that caused oxidative liver injury when systemically administered. This effect was significantly more profound after 8 h. Both flagellin and lipopolysaccharide induced a significant increase in plasma ALT, but a more pronounced increase in AST was observed in mice receiving flagellin. These results support the observations of the current study that, although liver function was significantly impaired, serum cytokines including TNF-α were comparable in the S+severe injury group and the severe injury group. Furthermore, in both of these groups, TNF-α increased significantly when compared with the normal group. This increase may have been due to the existence of an “endotoxin tolerance” in the livers of the S+severe injury group.[21, 22]
In our study, endotoxins were increased slightly in the moderate injury group, the severe injury group and the S+no injury group. The reason for these increases may be a transient increase in circulating endotoxin induced by D-galactosamine.[23, 24]During acute liver injury without exogenous infection, translocated bacteria and endotoxin are insufficient to cause a striking elevation of endotoxin; however, in normal tissues, translocated endotoxin can be rapidly cleared by Kupffer cells.
Cytokines are the key communication molecules for defense against Salmonella. Previous investigations have reported that IL-1α, IFN-γ, TNF-α, IL-12 and IL-18 have protective functions, whereas the anti-inflammatory cytokines IL-4 and IL-10 inhibit the host defense against Salmonella.[25, 26]IFN-γ plays a key role in Salmonella defense by increasing epithelial resistance to bacterial infection and limiting the intracellular growth of Salmonella.[27]Our results indicated that IL-12 and IFN-γ levels in the S+no injury group were increased significantly compared with those in the normal group. In contrast, IL-10 was not increased. These results indicated that Salmonella infection induced an intense anti-Salmonella response in the host. IFN-γ was increased in the S+moderate injury group but not in the S+severe injury group compared with that in the normal group, whereas IL-10 was increased profoundly in the S+severe injury group compared with that in the S+no injury group. These results suggested that the anti-Salmonella response was suppressed in the severely injured group. TNF-α was increased significantly in the S+moderate injury group and the S+severe injury group compared with that in the normal group, and its levels were significantly increased in moderate injury group and in the severe injury group compared with normal animals, suggesting that TNF-α plays a key role in the deleterious effects of Salmonella infection and liver injury (in the Salmonella+GalN groups).
We propose the paradigm described below to explain the aggravation of liver injury by Salmonella infection. Proinflammatory mediators induced by acute liver injury promote tissue damage in the intestine. These mediators also disturb the intestinal microbiota, and promote an overgrowth of aggressive pathogenic bacteria. Salmonella infection exacerbates the intestinal injury and microbiota imbalance by activating the intestinal immune system and releasing proinflammatory cytokines. In turn, the proinflammatory cytokines enhance intestinal injury and promote bacterial and endotoxin translocation. When these harmful substances arrive in the liver, they activate hepatic immune cells and induce the production of large quantities of proinflammatory cytokines (such as IL-12 and TNF-α) exacerbate liver injury by causing hepatocyte cell death and inducing oxidative stress. PMN infiltration and inflammatory injury could be the key early stage factors in liver injury, while oxidative stress injury may play a key role in later stages.
This study provides new insights concerning our understanding of intestinal microbiota homeostasis and its importance during liver injury. Furthermore, our findings emphasize the importance of preventing enteropathogen infection in patients with liver injury.
Acknowledgment: We thank Dr. Ping Wang for excellent technical assistance.
Contributors: LLJ conceived and designed the experiments. LYT and YCB performed the experiments. LYT drafted the paper. YD and HJR analyzed the data. All authors contributed to the design and interpretation of the study and to further drafts. LLJ is the guarantor.
Funding: The study was supported by a grant from the National Basic Research Program (973) of China (2013CB531401).
Ethical approval: This study was approved by the Research, Animal Sources and Animal Care Committee of The First Affiliated Hospital, Zhejiang University School of Medicine (2015193).
Competing interest: No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
1 Visschers RG, Luyer MD, Schaap FG, Olde Damink SW, Soeters PB. The gut-liver axis. Curr Opin Clin Nutr Metab Care 2013;16:576-581.
2 Othman M, Agüero R, Lin HC. Alterations in intestinal microbial flora and human disease. Curr Opin Gastroenterol 2008;24:11-16.
3 Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science 2005;307:1915-1920.
4 Chiba Y, Shida K, Nagata S, Wada M, Bian L, Wang C, et al. Well-controlled proinflammatory cytokine responses of Peyer' s patch cells to probiotic Lactobacillus casei. Immunology 2010;130:352-362.
5 Pasparakis M. Role of NF-κB in epithelial biology. Immunol Rev 2012;246:346-358.
6 Ai TL, Solomon BD, Hsieh CS. T-cell selection and intestinal homeostasis. Immunol Rev 2014;259:60-74.
7 Wu ZW, Ling ZX, Lu HF, Zuo J, Sheng JF, Zheng SS, et al. Changes of gut bacteria and immune parameters in liver transplant recipients. Hepatobiliary Pancreat Dis Int 2012;11:40-50.
8 Kirpich IA, Marsano LS, McClain CJ. Gut-liver axis, nutrition, and non-alcoholic fatty liver disease. Clin Biochem 2015;48:923-930.
9 Xing HC, Li LJ, Xu KJ, Shen T, Chen YB, Sheng JF, et al. Protective role of supplement with foreign Bifidobacterium and Lactobacillus in experimental hepatic ischemia-reperfusion injury. J Gastroenterol Hepatol 2006;21:647-656.
10 Bezirtzoglou E, Stavropoulou E. Immunology and probiotic impact of the newborn and young children intestinal microflora. Anaerobe 2011;17:369-374.
11 Ghosal A, Jellbauer S, Kapadia R, Raffatellu M, Said HM. Salmonella infection inhibits intestinal biotin transport: cellular and molecular mechanisms. Am J Physiol Gastrointest Liver Physiol 2015;309:G123-131.
12 Fayol-Messaoudi D, Berger CN, Coconnier-Polter MH, Liévin-Le Moal V, Servin AL. pH-, Lactic acid-, and non-lactic aciddependent activities of probiotic Lactobacilli against Salmonella enterica Serovar Typhimurium. Appl Environ Microbiol 2005;71:6008-6013.
13 Nie XH, Han T, Ha FU, Liang N, Wang SH, Zhu ZY, et al. Comparison of the effects of the pretreatment and treatment with RhIL-11 on acute liver failure induced by D-galactosamine. Eur Rev Med Pharmacol Sci 2014;18:1142-1150.
14 Li YT, Wang L, Chen Y, Chen YB, Wang HY, Wu ZW, et al. Effects of gut microflora on hepatic damage after acute liver injury in rats. J Trauma 2010;68:76-83.
15 Deutschman CS, Cereda M, Ochroch EA, Raj NR. Sepsis-induced cholestasis, steatosis, hepatocellular injury, and impaired hepatocellular regeneration are enhanced in interleukin-6 -/-mice. Crit Care Med 2006;34:2613-2620.
16 Wick MJ. Innate immune control of Salmonella enterica serovar Typhimurium: mechanisms contributing to combating systemic Salmonella infection. J Innate Immun 2011;3:543-549.
17 Agbor TA, Demma ZC, Mumy KL, Bien JD, McCormick BA. The ERM protein, ezrin, regulates neutrophil transmigration by modulating the apical localization of MRP2 in response to the SipA effector protein during Salmonella Typhimurium infection. Cell Microbiol 2011;13:2007-2021.
18 Marchelletta RR, Gareau MG, McCole DF, Okamoto S, Roel E,Klinkenberg R, et al. Altered expression and localization of ion transporters contribute to diarrhea in mice with Salmonellainduced enteritis. Gastroenterology 2013;145:1358-1368.
19 Ferreira RB, Gill N, Willing BP, Antunes LC, Russell SL, Croxen MA, et al. The intestinal microbiota plays a role in Salmonellainduced colitis independent of pathogen colonization. PLoS One 2011;6:e20338.
20 Eguchi M, Sekiya Y, Suzuki M, Yamamoto T, Matsui H. An oral Salmonella vaccine promotes the down-regulation of cell surface Toll-like receptor 4 (TLR4) and TLR2 expression in mice. FEMS Immunol Med Microbiol 2007;50:300-308.
21 Futoma-Kołoch B, Godlewska U, Guz-Regner K, Dorotkiewicz-Jach A, Klausa E, Rybka J, et al. Presumable role of outer membrane proteins of Salmonella containing sialylated lipopolysaccharides serovar Ngozi, sv. Isaszeg and subspecies arizonae in determining susceptibility to human serum. Gut Pathog 2015;7:18.
22 De Maio A, Vazquez D. Extracellular heat shock proteins: a new location, a new function. Shock 2013;40:239-246.
23 De Maio A. Extracellular Hsp70: export and function. Curr Protein Pept Sci 2014;15:225-231.
24 Kim SJ, Choi HS, Cho HI, Jin YW, Lee EK, Ahn JY, et al. Protective effect of wild ginseng cambial meristematic cells on d-galactosamine-induced hepatotoxicity in rats. J Ginseng Res 2015;39:376-383.
25 Knodler LA, Crowley SM, Sham HP, Yang H, Wrande M, Ma C,et al. Noncanonical inflammasome activation of caspase-4/caspase-11 mediates epithelial defenses against enteric bacterial pathogens. Cell Host Microbe 2014;16:249-256.
26 Owen KA, Anderson CJ, Casanova JE. Salmonella Suppresses the TRIF-Dependent Type I Interferon Response in Macrophages. MBio 2016;7:e02051-15.
27 Gal-Mor O, Suez J, Elhadad D, Porwollik S, Leshem E, Valinsky L, et al. Molecular and cellular characterization of a Salmonella enterica serovar Paratyphi a outbreak strain and the human immune response to infection. Clin Vaccine Immunol 2012;19:146-156.
Accepted after revision May 17, 2016
Author Affiliations: State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, and Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003, China (Li YT, Yu CB,Yan D, Huang JR and Li LJ)
Lan-Juan Li, MD, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, and Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou 310003,China (Email: ljli@zju.edu.cn)
© 2016, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(16)60113-3
Published online July 4, 2016.
February 19, 2016
杂志排行
Hepatobiliary & Pancreatic Diseases International的其它文章
- Hepatobiliary & Pancreatic Diseases International
- Dietary supplementation in patients with alcoholic liver disease: a review on current evidence
- Combined hepatectomy and radiofrequency ablation versus TACE in improving survival of patients with unresectable BCLC stage B HCC
- Long-term follow-up of children and adolescents with primary sclerosing cholangitis and autoimmune sclerosing cholangitis
- Letters to the Editor
- Kidney transplantation after liver transplantation
