树突状细胞多价核酸疫苗抗血吸虫感染作用及机制研究
2016-07-27沈定文罗金萍王若愚许培培余军林李雍龙
沈定文,罗金萍,王若愚,许培培,戴 波,余军林,李雍龙
树突状细胞多价核酸疫苗抗血吸虫感染作用及机制研究
沈定文1,罗金萍1,王若愚1,许培培1,戴波1,余军林1,李雍龙2
1.湖北科技学院基础医学院,咸宁437100;2.华中科技大学同济医学院,武汉430030
摘要:目的探讨基因转染对树突状细胞(DC)功能的影响及DC多价核酸疫苗抗血吸虫感染作用及机制。方法利用脂质体介导的基因转染技术将Sj26、Sj23和Sj14基因分别转染DC,流式细胞术(FCM)检测DC摄取抗原能力,混合淋巴细胞反应(MLR)检测DC对同种异体T淋巴细胞的刺激作用。Sj26、Sj23和Sj14基因转染DC分别和联合免疫BALB/c小鼠3次,末次免疫2周后,每只小鼠感染日本血吸虫尾蚴40条,小鼠感染血吸虫6周后,计数成虫和虫卵。ELISA法检测血清特异性IgG、血清干扰素-γ(IFN-γ)和白细胞介素-4(IL-4)及脾淋巴细胞培养上清IFN-γ、IL-4水平,噻唑蓝(MTT)法检测脾淋巴细胞的增殖。结果与真核表达载体pcDNA3转染DC和未处理DC相比较,基因转染DC摄取抗原的荧光强度明显降低(P<0.01),对同种异体T淋巴细胞的刺激指数明显升高(P<0.01)。各免疫组小鼠诱导的减虫率和减卵率均高于对照组(P<0.01),而基因转染DC联合免疫组小鼠抗血吸虫感染作用高于单一基因转染DC免疫组(P<0.001)。基因转染DC免疫组小鼠末次免疫2周后血清特异性IgG水平较免疫前显著升高(P<0.05),血清IFN-γ水平明显升高(P<0.01),而血清IL-4的水平无明显变化。与对照组比较,基因转染DC免疫组小鼠脾淋巴细胞经ConA和SEA刺激后培养上清IFN-γ水平显著增高,IL-4水平显著降低,刺激指数(SI)显著增高(P<0.001)。结论基因转染能促进DC的成熟,增强DC的活性,DC多价核酸疫苗可增强抗血吸虫感染作用,其作用机制以Th1型免疫应答为主。
关键词:日本血吸虫;树突状细胞;多价核酸疫苗;保护性免疫
Supported by the Natural Science Foundation of Hubei Province (No. 2012FFC01501), the Research Project of Hubei Provincial Department of Health (No. XF2012-13), and the Hubei Province Training Programs of Innovation and Entrepreneurship for Undergraduates (No. 201310927009)
我国湖沼地区及大山区血吸虫病疫情目前仍然十分严峻,成为亟待解决的公共卫生问题之一。随着免疫学和分子生物学技术的快速发展,核酸疫苗在血吸虫病防治方面具有广阔的前景,但是目前血吸虫疫苗候选分子均难以稳定达到WHO要求的50%的抗感染作用,如何增强核酸疫苗的免疫效果便成为当前血吸虫疫苗研究的焦点[1]。
树突状细胞(DC)在免疫应答的诱导中发挥关键作用,是具有最强提呈抗原功能的抗原提呈细胞(APC)。近年来DC在抗肿瘤、感染、移植免疫以及自身免疫病等方面得到了广泛的应用[2-5]。我们自2002年开始探讨以DC为载体,将Sj26、Sj23和Sj14三种血吸虫候选抗原编码基因分别转染DC,单独或联合免疫小鼠诱导抗血吸虫感染作用,并通过体内、体外实验研究其作用机制[6-7]。
1材料与方法
1.1细胞株与小鼠DC细胞株MTSC4(北京大学医学部陈慰峰教授惠赠);6~8周龄、雌性BALB/c小鼠(武汉市生物制品研究所)。
1.2Sj26、Sj23、Sj14基因转染DC制备PCR方法扩增Sj26、Sj23、Sj14基因片段,定向克隆至真核表达载体pcDNA3。参照文献[6],按LipofectamineTM2000试剂盒(Invitrogen公司)使用说明书,利用脂质体介导的基因转染技术将Sj26、Sj23、Sj14基因分别转染DC,常规方法培养传代。利用RT-PCR、SDS-PAGE和间接免疫荧光试验检测Sj26、Sj23和Sj14在DC中的表达。
1.3DC摄取抗原能力检测利用流式细胞术(FCM)分别检测Sj26、Sj23和Sj14基因转染DC摄取FITC标记羊抗鼠IgG的荧光强度,同时设pcDNA3转染DC和未处理DC对照。
1.4DC对同种异体T淋巴细胞刺激作用的检测
取正常BALB/c小鼠脾淋巴细胞作为反应细胞,混合淋巴细胞反应(MLR)分别检测Sj26、Sj23、Sj14、pcDNA3转染DC和未处理DC对淋巴细胞的刺激指数(SI),同时设RPMI-1640空白对照。SI=实验组A492值/对照组A492值。
1.5动物免疫与感染BALB/c小鼠80只,随机分为8组。免疫方案为,Sj26-Sj23-Sj14(A组)、Sj26-Sj23(B组)、Sj26(C组)、Sj23(D组)、Sj14基因转染DC(E组)分别经耳廓注射免疫BALB/c小鼠,免疫3次,间隔2周,同时设pcDNA3转染DC(F组)、未处理DC(G组)和RPMI-1640对照(H组)。感染方案为末次免疫2周后,每只小鼠感染日本血吸虫尾蚴40条。
1.6小鼠血清特异性IgG检测采用ELISA法进行,酶标仪测定吸光度(A491)值。
1.7抗血吸虫感染作用
1.7.1减虫率小鼠感染血吸虫6周后,门静脉灌注冲洗收集血吸虫成虫并计数。按公式计算减虫率:减虫率(%)=(对照组成虫均数-免疫组成虫均数)/对照组成虫均数×100%。
1.7.2减卵率取小鼠肝脏,称重,5%KOH 10 mL消化过夜,取0.1 mL计数血吸虫虫卵。按公式计算减卵率:减卵率(%)=(对照组每雌虫虫卵均数-免疫组每雌虫虫卵均数)/对照组每雌虫虫卵均数×100%。
1.8小鼠血清IFN-γ和IL-4检测按照IFN-γ和IL-4检测试剂盒(BD Biosciences pharmingen公司)使用说明书进行,酶标仪测定吸光度(A450)值。
1.9脾淋巴细胞培养上清IFN-γ和IL-4检测ELISA法分别检测小鼠脾淋巴细胞经SEA(10 μg/mL)和ConA(5 μg/mL)刺激后IFN-γ和IL-4水平,同时设RPMI-1640空白对照。
1.10脾淋巴细胞增殖噻唑蓝(MTT)法检测SEA(10 μg/mL)和ConA(5 μg/mL)刺激对脾淋巴细胞的增殖情况,同时设RPMI-1640空白对照,酶标仪测定吸光度(A560)值。按公式计算SI:SI=刺激孔A560均值/对照孔A560均值。
1.11统计分析数据分析采用SPSS 16.0版本F检验和t检验。
2结果
2.1Sj26、Sj23和Sj14在DC中的表达RT-PCR、SDS-PAGE和间接免疫荧光法检测结果显示,Sj26、Sj23和Sj14基因转染成功并在DC中获得表达(图1-3)。
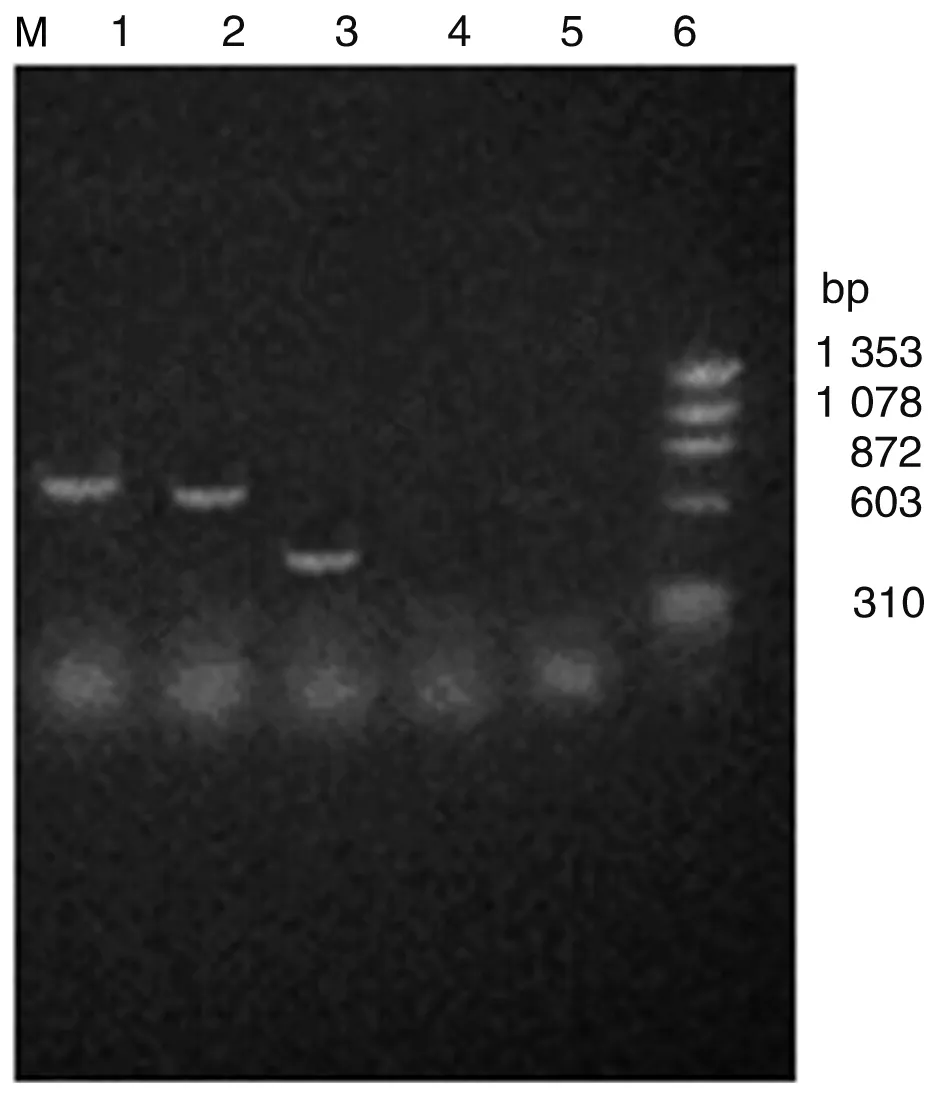
1:Product of pcDNA3-Sj26 transferred DC;2: Product of pcDNA3-Sj23 transferred DC;3: Product of pcDNA3-Sj14 transferred DC;4: Product of pcDNA3 transferred DC; 5: Product of untreated DC; 6: DNA marker.
图1RT-PCR检测mRNA的表达
Fig. 1Expression of mRNA detected by RT-PCR
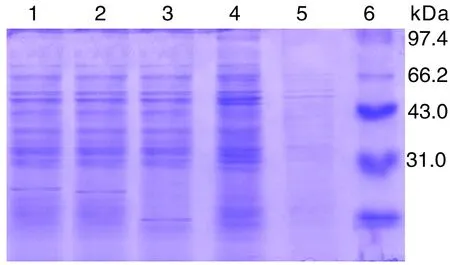
1: Product of pcDNA3-Sj26 transferred DC; 2: Product of pcDNA3-Sj23 transferred DC;3: Product of pcDNA3-Sj14 transferred DC;4: Product of pcDNA3 transferred DC;5: Product of untreated DC;6: Protein marker.
图2基因转染DC表达产物的SDS-PAGE分析
Fig. 2SDS-PAGE analysis of expression products of gene transferred DC
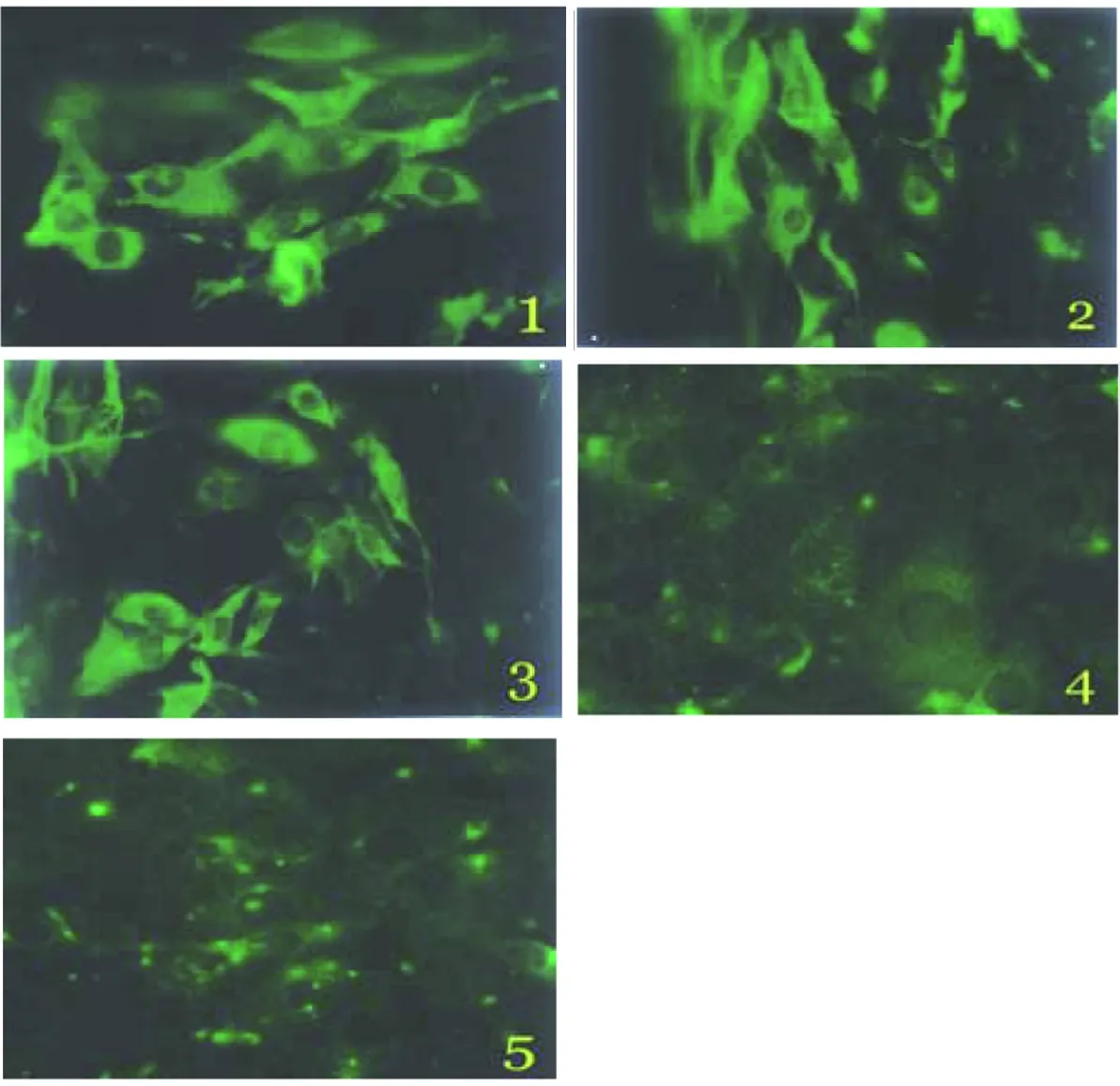
1: pcDNA3-Sj26 transferred DC; 2: pcDNA3-Sj23 transferred DC; 3: pcDNA3-Sj14 transferred DC;4: pcDNA3 transferred DC;5: untreated DC.
图3间接免疫荧光试验检测Sj26、Sj23和Sj14在DC中的表达(200×)
Fig. 3Expression of Sj26、Sj23 and Sj14 in DC detected by indirect immunofluorescence assay(200×)
2.2DC摄取抗原能力与pcDNA3转染DC和未处理DC相比较,基因转染DC摄取抗原的荧光强度明显降低(P<0.01),表明基因转染后,促进了DC的成熟,其摄取抗原能力明显降低(图4)。
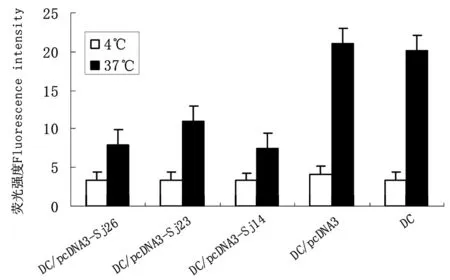
图4 基因转染DC摄取抗原的能力
Fig. 4The ability of antigen absorbing of S. japonicum encoding gene transferred DC
2.3DC对同种异体T淋巴细胞的刺激作用基因转染DC对同种异体T淋巴细胞的SI明显高于pcDNA3转染DC和未处理DC(P<0.01),表明基因转染后,增强了DC的生物学活性(图5)。
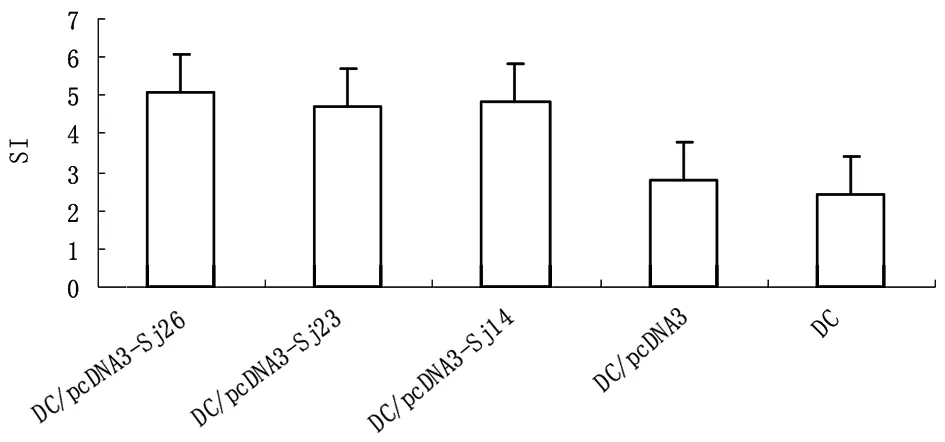
图5 基因转染DC对同种异体T淋巴细胞的刺激作用
Fig. 5The effect of gene transferred DC on stimulating allogenetic T lymphocyte
2.4抗血吸虫感染作用各免疫组小鼠抗血吸虫感染作用与RPMI-1640对照组相比较,差异有统计学意义(P<0.01),而基因转染DC联合免疫组小鼠抗血吸虫感染作用明显高于单一基因转染DC免疫组(P<0.001)(表1)。
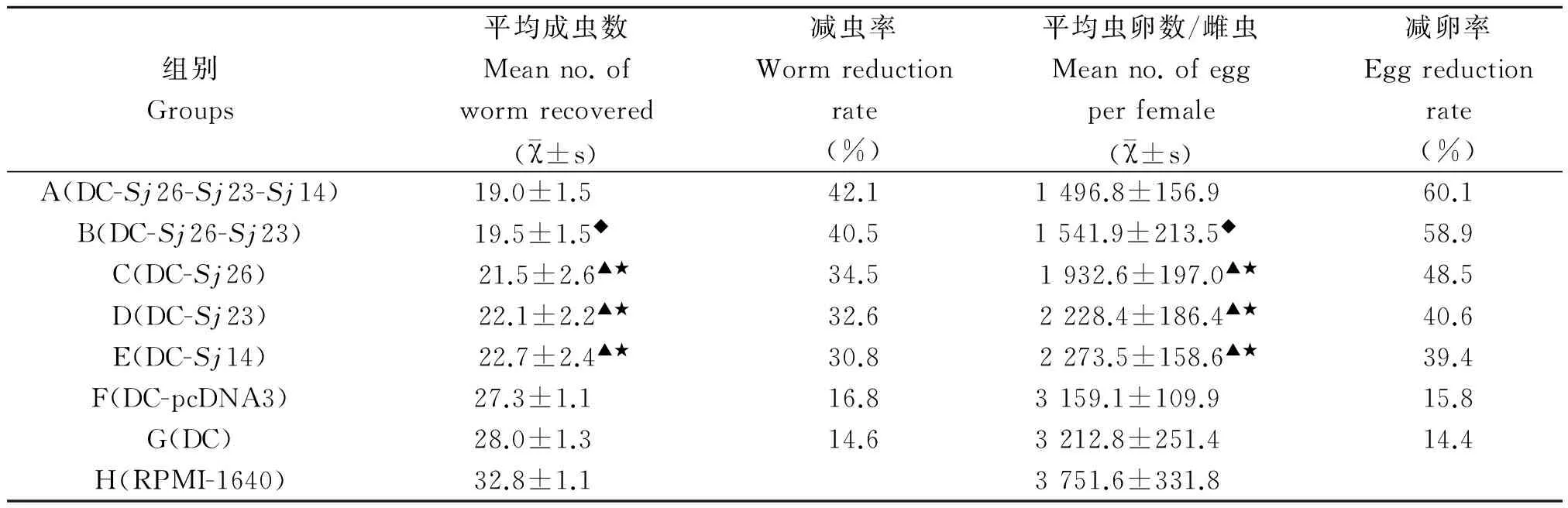
表1 基因转染DC免疫BALB/c小鼠抗血吸虫感染作用
Note:★Compared with group A,P<0.001;▲Compared with group B,P<0.001;◆Compared with group A,P>0.05.
2.5血清IgG水平基因转染DC免疫小鼠后,血清IgG水平较免疫前明显升高(P<0.05)。基因转染DC联合免疫组小鼠IgG水平明显高于单一基因转染DC免疫组(P<0.01)。感染6周后,各组小鼠血清IgG水平差异无统计学意义(图6)。
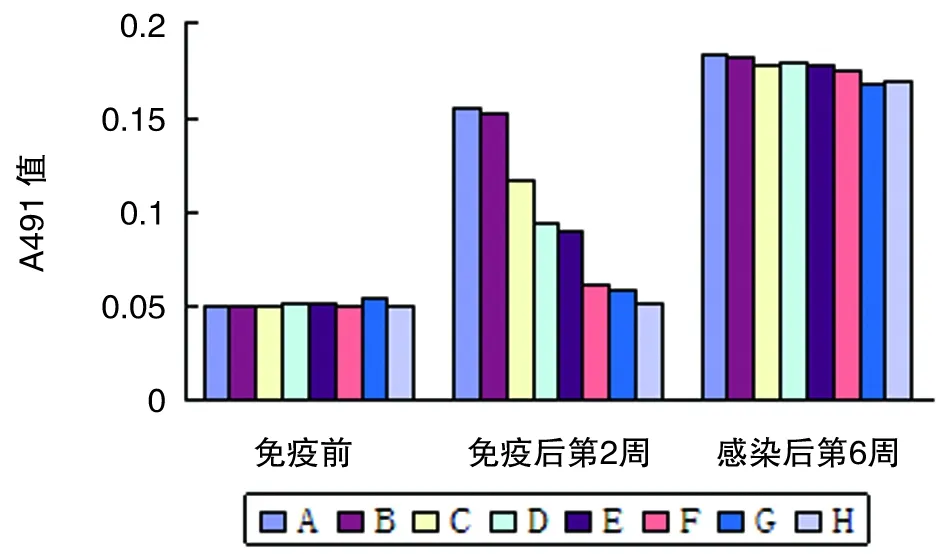
图6 各组BALB/c小鼠血清IgG水平
Fig.6The level of IgG in BALB/c mice sera of each group
2.6血清IFN-γ水平BALB/c小鼠经基因转染DC免疫后,血清IFN-γ含量明显升高(与免疫前相比较,P<0.01),且明显高于pcDNA3转染DC和未处理DC对照组(P<0.01),基因转染DC联合免疫组小鼠血清IFN-γ水平明显高于单一基因转染DC免疫组(与Sj26相比,P<0.05,与Sj23和Sj14相比较,P<0.01)(图7)。
2.7血清IL-4水平各组BALB/c小鼠免疫前、后血清IL-4水平差异无统计学意义(图8)。
2.8脾淋巴细胞培养上清IFN-γ水平与RPMI-1640对照组相比较,基因转染DC免疫组小鼠脾淋巴细胞经ConA和SEA刺激后培养上清IFN-γ水平显著增高(P<0.001)。基因转染DC联合免疫组小鼠脾淋巴细胞经ConA和SEA刺激后培养上清IFN-γ水平明显高于单一基因转染DC免疫组(P<0.01)(图9)。
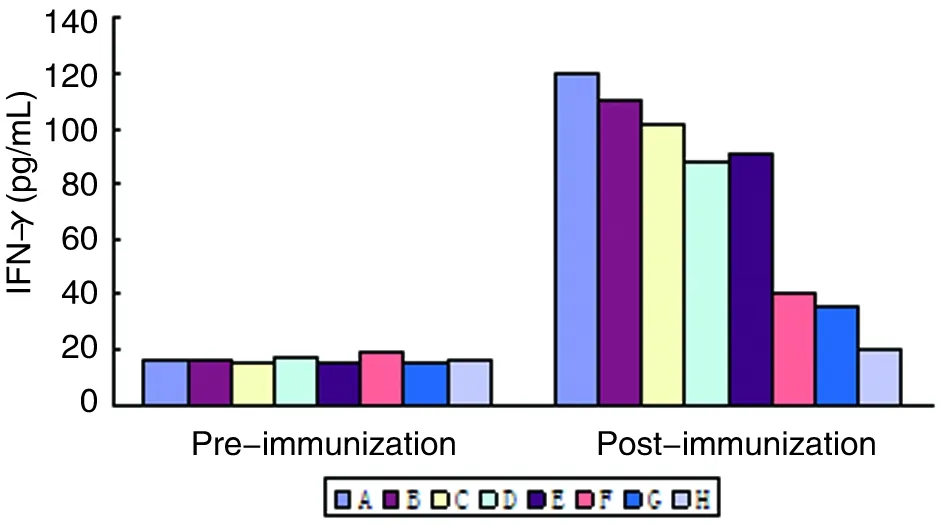
图7 各组BALB/c小鼠血清IFN-γ水平
Fig.7The level of IFN-γ in BALB/c mice sera of each group
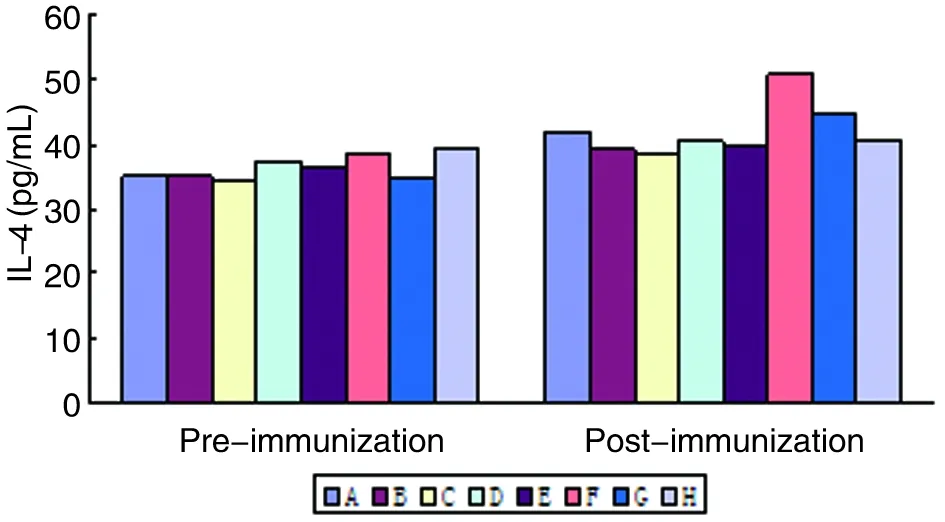
图8 各组BALB/c小鼠血清IL-4水平
Fig. 8The level of IL-4 in BALB/c mice sera of each group
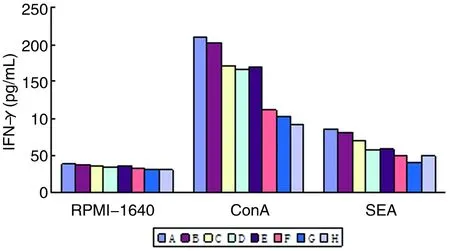
图9 各组BALB/c小鼠脾淋巴细胞培养上清IFN-γ水平
Fig. 9The level of IFN-γ in BALB/c mice spleen lymphocytes culture supernatant of each group
2.9脾淋巴细胞培养上清IL-4水平与RPMI-1640对照组比较,基因转染DC免疫组小鼠脾淋巴细胞经ConA和SEA刺激后培养上清IL-4水平显著降低(P<0.001)。基因转染DC联合免疫组小鼠脾淋巴细胞经ConA和SEA刺激后培养上清IL-4水平低于单一基因转染DC免疫组(与Sj26相比,P<0.05,与Sj23和Sj14相比较,P<0.01)(图10)。
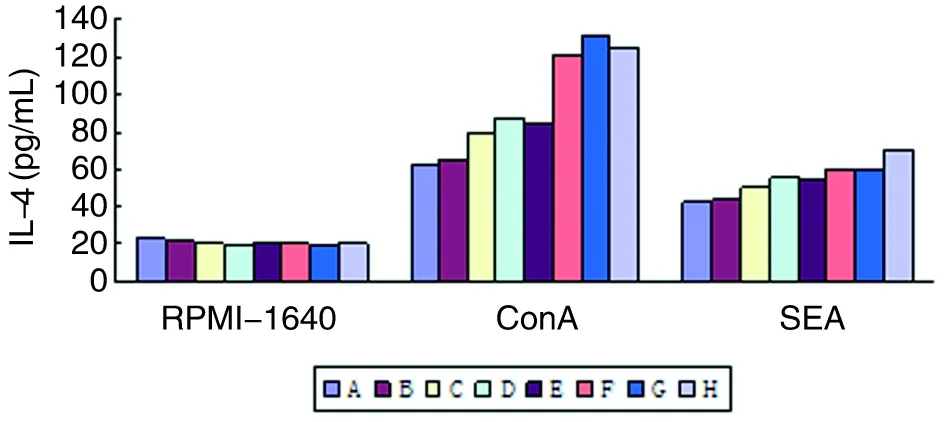
图10 各组BALB/c小鼠脾淋巴细胞培养上清IL-4水平
Fig.10The level of IL-4 in BALB/c mice spleen lymphocytes culture supernatant of each group
2.10脾淋巴细胞增殖与RPMI-1640对照组相比较,基因转染DC免疫组小鼠脾淋巴细胞经ConA和SEA刺激后的SI显著升高(P<0.001),而pcDNA3转染DC和未处理DC组差异无统计学意义。基因转染DC联合免疫组小鼠脾淋巴细胞经ConA和SEA刺激后的SI明显高于单一基因转染DC免疫组(P<0.01)(图11)。
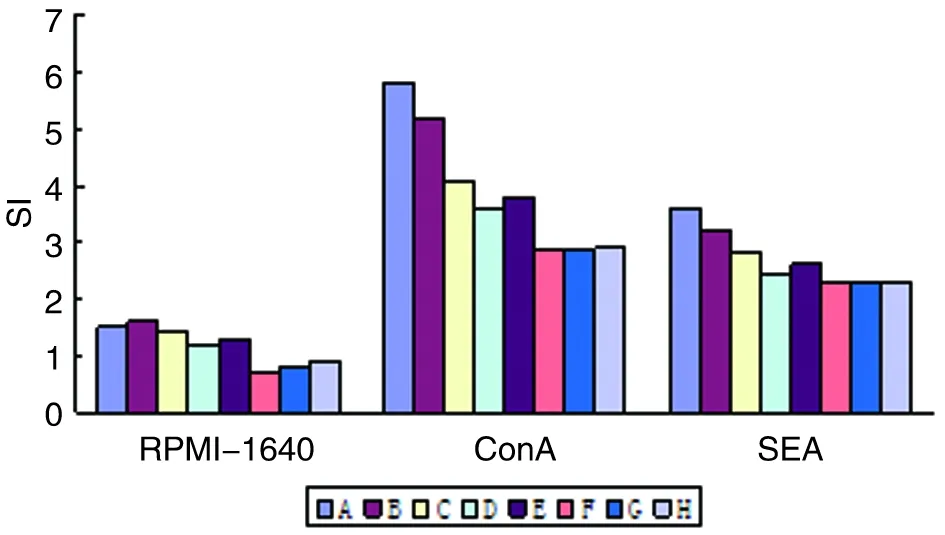
图11 各组BALB/c小鼠脾淋巴细胞SI
Fig.11The SI of spleen lymphocytes in BALB/c mice of each group
3讨论
随着免疫学和分子生物学技术的不断发展,用疫苗预防血吸虫病已成为可能。目前国际上公认有发展前途的血吸虫疫苗候选抗原有6种,即谷胱苷肽S转移酶(GST),血吸虫97 kDa副肌球蛋白(Sm97),磷酸丙糖异构酶(TPI),照射疫苗5(IRV5),Sm37甘油磷酸脱氢酶(GAPDH)和Sm14脂肪酸结合蛋白(FABP)。虽然血吸虫疫苗的研究工作取得了初步的进展,但真正应用于临床还需进行更多的研究,目前仅有GST进入临床I期试验[1]。
现有的血吸虫疫苗候选分子抗感染作用有限,探索新的疫苗候选抗原,改进免疫方法和途径等以提高免疫作用,其意义显得尤为重要而迫切。DC是体内功能最强的抗原提呈细胞,在免疫应答的诱导中发挥关键作用,能有效活化未致敏T淋巴细胞。抗原提呈效率高,少量抗原和少量DC便足以激活T淋巴细胞,核酸疫苗若能以DC为载体可望获得良好的免疫效果。
Schraml等(2015)和Mildner等(2014)研究证实,未成熟DC具有较强的抗原内吞及加工处理能力,当DC受到某些因子(如TNF-α、LPS、IL-1β等)刺激或摄取抗原后,可分化成熟。成熟DC低表达与吞噬有关的受体,高表达MHCⅡ、CD86、CD80、CD40、CD54等分子,特征性标志主要是CD83和CD25,其摄取、加工抗原的能力反而降低,而抗原提呈能力和刺激T淋巴细胞的作用增强[8-9]。本实验结果与pcDNA3转染DC和未处理DC相比较,基因转染DC摄取抗原的荧光强度明显降低,对T淋巴细胞的SI明显高于pcDNA3转染DC和未处理DC,表明基因转染DC后促进了DC的分化成熟,增强了DC的生物学活性。
血吸虫抗原成分复杂多样,感染后的保护性免疫作用机制目前尚不完全清楚,迄今为止,单价抗感染疫苗候选分子诱导的抗感染作用很少超过50%。将含有不同抗原基因的质粒混合起来进行联合免疫的多价核酸疫苗,可综合各种核酸疫苗的协同作用,刺激机体有效的免疫应答,诱导宿主产生更完全的抗感染作用。本实验结果基因转染DC联合免疫组小鼠诱导的抗感染作用明显高于单一基因转染DC免疫组,DC多价核酸疫苗联合免疫可协同增强抗日本血吸虫感染作用。
虫卵是血吸虫病的主要致病因子,所致的虫卵肉芽肿和纤维化是血吸虫病的主要病变,虫卵肉芽肿的形成是宿主对致病因子的一种免疫应答。Pearce等研究证实,小鼠血吸虫病的免疫病理与Tp型免疫应答有关,而特异性抗原诱导宿主的抗感染作用则与Th1型免疫应答有关[10],虫卵大量沉积前应用Th1型免疫应答为主的疫苗加以干预,在诱导抗血吸虫感染方面有极其重要的作用。本实验结果小鼠经基因转染DC免疫后血清特异性IgG、IFN-γ明显升高,而血清IL-4无明显变化,与RPMI-1640对照组比较,基因转染DC免疫组小鼠脾淋巴细胞经ConA和SEA刺激后IFN-γ水平显著增高,IL-4水平显著降低,SI显著增高,表明Th1型免疫应答在DC多价核酸疫苗诱导的抗日本血吸虫感染中起主要作用。
参考文献:
[1]Kouriba B, Traore B, Diemert D, et al. Immunity in human schistosomiasis: hope for a vaccine[J]. Med Trop, 2010, 70(2): 189-197. DOI: 10.4161/hv.25787
[2]Akiyama H, Miller C, Patel HV, et al. Virus particle release from glycosphingolipid-enriched microdomains is essential for dendritic cell-mediated capture and transfer of HIV-1 and henipavirus[J]. J Virol, 2014, 88(16): 8813-8825. DOI: 10.1128/JVI.00992-14
[3]Tagliamonte M, Petrizzo A, Tornesello ML, et al. Antigen-specific vaccines for cancer treatment[J]. Hum Vaccin Immunother, 2014, 10(11): 3332-3346. DOI: 10.4161/21645515.2014.973317
[4]Zhuang Q, Lakkis FG. Dendritic cells and innate immunity in kidney transplantation[J]. Kidney Int, 2015, 87(4): 712-718. DOI: 10.1038/ki.2014.430
[5]Mackern-Oberti JP, Llanos C, Vega F, et al. Role of dendritic cells in the initiation, progress and modulation of systemic autoimmune diseases[J]. Autoimmun Rev, 2015, 14(2): 127-139. DOI: 10.1016/j.autrev.2014.10.010
[6]Shen DW, Luo JP, Li YL. Studies on protective immunity ofSj26 gene transfer dendritic cells againstSchistosomajaponicum[J]. Chin J Public Health, 2005, 21(11): 1393-1394. DOI: 10.3321/j.issn:1001-0580.2005.11.036(in Chinese)
沈定文,罗金萍,李雍龙.Sj26基因转染树突状细胞抗血吸虫感染作用[J]. 中国公共卫生,2005,21(11):1393-1394. DOI: 10.3321/j.issn:1001-0580.2005.11.036
[7]Shen DW, Luo JP. Mechanism of protective immunity against infection ofSchistosomajaponicuminduced by dendritic cell DNA multivalent vaccine[J]. Chin J Zoonoses, 2012, 28(10): 996-999. DOI: 10.3969/j.issn.1002-2694.2012.10.007(in Chinese)
沈定文,罗金萍. 树突状细胞DNA疫苗抗日本血吸虫感染作用机制的研究[J]. 中国人兽共患病学报,2012,28(10):996-999. DOI: 10.3969/j.issn.1002-2694.2012.10.007
[8]Schraml BU, Reise SC. Defining dendritic cells[J]. Curr Opin Immunol, 2015, 32: 13-20. DOI: 10.1016/j.coi.2014.11.001
[9]Mildner A, Jung S. Development and function of dendritic cell subsets[J]. Immunity, 2014, 40(5): 642-656. DOI: 10.1016/j.immuni.2014.04.016
[10]Pearce EJ, Kane CM, Sun J, et al. Tp response polarization during infection with the helminth parasiteSchistosomamansoni[J]. Immunol Rev, 2004, 201: 117-126.
DOI:10.3969/j.issn.1002-2694.2016.04.017
中图分类号:R532.21
文献标识码:B
文章编号:1002-2694(2016)04-0406-06
收稿日期:2015-07-17修回日期:2015-12-15
Protective immunity and mechanism of dendritic cell multivalent nucleic acid vaccine against infection of Schistosoma japonicum
SHEN Ding-wen1,LUO Jin-ping1,WANG Ruo-yu1,XU Pei-pei1,DAI Bo1,YU Jun-lin1,LI Yong-long2
(1.BasicMedicalCollege,HubeiUniversityofScienceandTechnology,Xianning437100,China;2.TongjiMedicalCollege,HuazhongUniversityofScienceandTechnology,Wuhan430030,China)
Abstract:To study the effect of gene transfer on the functions of dendritic cell (DC) and protective immunity and mechanism of DC multivalent nucleic acid vaccine against infection of Schistosoma japonicum, DCs were transfected with recombinant eukaryotic expression vector pcDNA3-Sj26, pcDNA3-Sj23 and pcDNA3-Sj14. The ability of DC to absorb antigen was determined by flow cytometry (FCM). The mixed lymphocyte reaction (MLR) was used to detect the effect of gene transferred DC on stimulating allogenetic T lymphocyte. BALB/c mice were immunized three times with Sj26, Sj23 and Sj14 gene transferred DC, alone or combination, and infected with 40 cercariae of S. japonicum per mouse 2 weeks after the last immunization. The number of adult worm and the egg number in liver were calculated six weeks after infecting. ELISA was used to detect the levels of specific IgG, IFN-γ and IL-4 in sera from each mice group. The level of IFN-γ and IL-4 in the culture supernatant of spleen lymphocytes stimulated with soluble egg antigen (SEA) and ConA were quantified by ELISA. The proliferation of spleen lymphocytes were measured by the method of MTT. Compared with pcDNA3 transferred DC and untreated DC, the fluorescence intensity of antigen absorbing decreased significantly in S. japonicum encoding gene-transferred DC (P<0.01), and the stimulating index (SI) increased significantly (P<0.01). The rate of worm reduction and egg reduction in each group of immunization were higher than that of control group (P<0.01), while protective immunity induced by gene transfer DC were significantly higher than that of gene transfer DC alone (P<0.001).Compared with previous immunization, the levels of specific IgG increased significantly in sera from group of S. japonicum encoding gene-transferred DC 2 weeks after the last immunization (P<0.05), the levels of IFN-γ increased significantly (P<0.01), while the levels of IL-4 were not significantly different. In response to ConA and SEA, the level of IFN-γ in the culture supernatant of spleen lymphocytes from group of S. japonicum encoding gene-transferred DC increased significantly, the level of IL-4 decreased significantly, while SI increased significantly (compared with control group, P<0.001). Results indicated that S. japonicum encoding gene transfer can promote DC maturation and enhance the biologic activity of DC. DC multivalent nucleic acid vaccine could induce and enhance protective immunity against infection of S. japonicum. Predominant Th1 type immune response might play an important role in the protective immunity induced by gene-transferred DC against infection of S. japonicum.
Keywords:Schistosoma japonicum; dendritic cell; multivalent nucleic acid vaccine; protective immunity
湖北省自然科学基金项目(No.2012FFC01501),湖北省卫生厅科研项目(No.XF2012-13)和湖北省大学生创新创业训练计划项目(No.201310927009)联合资助
