Mechanism of TLR-4/NF-κB pathway in myocardial ischemia reperfusion injury of mouse
2016-07-25HaoChenRuoQingZhangXiaoGangWeiXiaoMinRenXiaoQianGaoDepartmentofCardiologyAffiliatedPeopleHospitalofHebeiMedicalUniversityHebei050000ChinaShijiazhuangNoHospitalShijiazhuangHebei050000ChinaDepartmentofInternalMedicineZheng
Hao Chen, Ruo-Qing Zhang, Xiao-Gang Wei, Xiao-Min Ren, Xiao-Qian GaoDepartment of Cardiology, Affiliated People’s Hospital of Hebei Medical University, Hebei 050000, ChinaShijiazhuang No.1 Hospital, Shijiazhuang, Hebei 050000, ChinaDepartment of Internal Medicine, Zhengding Hospital, Zhengding, Hebei 050000, China
ABSTRACT
Objective: To detect the expression of Toll-like receptor 4 (TLR-4) and NF-κB and to discuss the mechanism of TLR-4/NF-κB pathway in the myocardial ischemia reperfusion injury of mouse. Methods: TLR-4 mutant mice and wild homozygous mice were divided into the model group and sham group. Mice in the model group were given the ligation of left anterior descending coronary artery for the modeling, while mice in the sham group were not given the ligation after threading. The cardiac muscle tissues were collected for the morphological observation. The immuno histochemistry was employed to detect the expression of NF-κB, Western blot was used to detect the expression of TLR-4 and ELISA to detect the expression of serum infl ammatory factors. Results: The expression of NF-κB in TLR-4 null mice after the myocardial ischemia reperfusion was signifi cantly lower than that in wild homozygous mice. For the model group and sham group, the expression of TLR-4 in wild homozygous mice was all signifi cantly higher than that in TLR-4 null mice, while the expression of TLR-4 in TLR-4 null mice in the model group was signifi cantly higher than that in sham group, with the statistical difference (P<0.05). The expression of inflammatory factors in TLR-4 null mice and wild homozygous mice in the model group was signifi cantly higher than that in sham group. The expression of all factors in group A with TLR-4 null was signifi cantly lower than that in group B with wild homozygous type, with the statistical diff erence (P<0.05). Conclusions: TLR-4/NF-κB pathway is closely related to the myocardial ischemia reperfusion injury, which plays its role through the release of infl ammatory cytokines.
ARTICLE INFO
Article history:
Received 15 January 2016
Received in revised form 16 February 2016 Accepted 15 March 2016
Available online 20 May 2016
Mechanism of TLR-4/NF-κB pathway in myocardial ischemia reperfusion injury of mouse
Hao Chen1,2△*, Ruo-Qing Zhang1,2△, Xiao-Gang Wei1,2, Xiao-Min Ren1,2, Xiao-Qian Gao31Department of Cardiology, Affiliated People’s Hospital of Hebei Medical University, Hebei 050000, China
2Shijiazhuang No.1 Hospital, Shijiazhuang, Hebei 050000, China
3Department of Internal Medicine, Zhengding Hospital, Zhengding, Hebei 050000, China
ABSTRACT
Objective: To detect the expression of Toll-like receptor 4 (TLR-4) and NF-κB and to discuss the mechanism of TLR-4/NF-κB pathway in the myocardial ischemia reperfusion injury of mouse. Methods: TLR-4 mutant mice and wild homozygous mice were divided into the model group and sham group. Mice in the model group were given the ligation of left anterior descending coronary artery for the modeling, while mice in the sham group were not given the ligation after threading. The cardiac muscle tissues were collected for the morphological observation. The immuno histochemistry was employed to detect the expression of NF-κB, Western blot was used to detect the expression of TLR-4 and ELISA to detect the expression of serum infl ammatory factors. Results: The expression of NF-κB in TLR-4 null mice after the myocardial ischemia reperfusion was signifi cantly lower than that in wild homozygous mice. For the model group and sham group, the expression of TLR-4 in wild homozygous mice was all signifi cantly higher than that in TLR-4 null mice, while the expression of TLR-4 in TLR-4 null mice in the model group was signifi cantly higher than that in sham group, with the statistical difference (P<0.05). The expression of inflammatory factors in TLR-4 null mice and wild homozygous mice in the model group was signifi cantly higher than that in sham group. The expression of all factors in group A with TLR-4 null was signifi cantly lower than that in group B with wild homozygous type, with the statistical diff erence (P<0.05). Conclusions: TLR-4/NF-κB pathway is closely related to the myocardial ischemia reperfusion injury, which plays its role through the release of infl ammatory cytokines.
ARTICLE INFO
Article history:
Received 15 January 2016
Received in revised form 16 February 2016 Accepted 15 March 2016
Available online 20 May 2016
Keywords:
1. Introduction
The concept of myocardial ischemia reperfusion injury was fi rstly proposed by Jennings et al in 1960 and then it had been emphasized by the medical profession since then. It’s proved that the reperfusion injury against the myocardial ultrastructure was some kind of irreversible myocardial necrosis [1] . As the common disease in the clinical practice, the pathology of myocardial ischemia reperfusion injury might be related to the complications after the operations such as the angioplasty and revascularization of cardiac coronary artery and the heart transplantation [2-4] .
Toll-like receptor (TLR) is the important protein that is involved in the nonspecifi c immunity. By recognizing the pathogen associated molecular patterns, it could induce the cytokine to play the role of anti-infection [5] . By now, over 10 subtypes of TLR have been found in the human body. The diff erent subtype can recognize the diff erent receptor and all TLR ligands can act as the immune adjuvant. According to the previous researches, the Toll-like receptor 4 (TLR-4) was involved in the infl ammatory response of myocardialischemia reperfusion injury, which could promote the formation of active oxygen free radicals and also activate many cytokine [6,7] . The nuclear factor κB (NF-κB) pathway was involved in the tissue injury and stress reaction, while the myocardial ischemia reperfusion could activate the NF-κB pathway in the further process of oxidative stress and calcium overload [8,9] . In this study, based on the building of mouse model of myocardial ischemia reperfusion injury, it was to discuss the mechanism of TLR-4 and NF-κB in the myocardial ischemia reperfusion injury of mice.
2. Materials and methods
2.1. Laboratory animals
Twenty male C3H/HeJ SPFTLR-4 null mice, with the age of 6-8 weeks and weight of (25 -30) g and 20 male wild homozygous mice, with the age of 6-8 weeks and weight of (25 -30) g were provided by the laboratory of Hebei Medical University. They were fed in the feeding room with the reasonable and controllable room temperature, light and humidity freely. The animal experiment and operation procedures of this study were reviewed and approved by the ethics committee of Laboratory Animal Center of Hebei Province.
2.2. Instruments and reagents
The automatic biochemical analyzer was purchased from Beckman Coulter (AU680), the biological and functional experimental system from Chengdu Taimeng (BL-420F), the high speed micro centrifuge from Shanghai Sangon Biotech (G508009), the precision singlechannel adjustable pipette from Shanghai Sangon Biotech (10-1 000) μL, the microwave oven from Sanyo (EM-F2108MS1), the biological microscope from Nikon (ECLIPSE80i), the UV spectrophotometer from Hanon Instruments (i8 dual beam), the gel imaging analysis system from UVP (GDS8000), the electronic balance from Sartorius, the ultra low temperature freezer from TCL -86 ℃; and the real-time quantitative PCR kit from Roche, the biochemical kit from Beckman Coulter, the IL-1, IL-6 and TNF-α ELISA kits from Beckman Coulter, TLR-4 primary antibody from Abcam, the rabbit anti-goat secondary antibody from Abcam, Tween20/TBS solution from Shanghai Rongbai, immunohistochemistry Max VisionTM kit from Maixin Biotech, Western blot kit from Maixin Biotech, citrate buff er antigen retrieval solution from Maixin Biotech andDAB color development kit from Maixin Biotech.
2.3. Experimental methods
2.3.1. Grouping
Fourty TLR-4 null mice were randomly divided into sham group (group AS) and myocardial ischemia reperfusion group (group A); while 40 wild homozygous mice were randomly divided into sham group (group BS) and myocardial ischemia reperfusion group (group B). Mice in the myocardial ischemia reperfusion group were given the ligation of left coronary artery for 45 min. After releasing the ligation thread, the reperfusion was performed for 180 min. For mice in sham group, only the stitching line was through the left coronary artery, but not for ligation, with the same remaining procedures as above.
2.3.2. Modeling
The fasting was applied to mice in all groups for 12 h and they were weighted before the anesthesia. The intraperitoneal injection of 1 g/ kg urethane was performed for the anesthesia. Mice were fi xed on the operation table at the supine position. The limbs were connected with the electrodes. The electrocardiogram change was monitored and the ventilator support was provided. After removing the hair on the chest and the disinfection, the incision was performed on the left chest, upper to the posterior margin line of forelimbs and lower to the fi fth intercostal space. Afterwards, the skin was cut off and separated layer by layer to expose the 3rd and 4th ribs. The ribs were distracted to expose the heart. By opening the left atrial appendage gently, it was to fi nd the left coronary artery in the cardiac muscle. Afterwards, the needle was inserted through the pulmonary conus and then came out from the right side of left atrial appendage. The suture lines were coated with the polyethylene tube. After the rapid ligation, the anterior wall of heart became bluish gradually. The ECG Ⅱ lead was observed. The ST segment elevation indicated the successful ischemia. After 45 min of ligation, the ligation lines were released. The heart surface became red again and ST segment was gradually depressed, which indicated that the heart had recovered the reperfusion. Then the skin was stitched layer by layer and the chest was closed, with the reperfusion for 180 min. The operation procedures for sham group were the same as above. But after the suture line running through, the ligation was not applied to the left coronary artery [10,11] .
2.3.3. Morphological observation of cardiac muscle
After the modeling, the cardiac muscle was taken out. One part was frozen quickly and cut into pieces to be stored at -20 ℃, with the thickness of 1 mm. It was then incubated in 1% triphenyltetrazolium chloride solution and at 37 ℃ for 20 min and fixed with 4% paraformaldehyde. The cardiac muscle tissue with the ischemic infarction appeared to be pale; while the cardiac muscle tissue without the ischemic infarction appeared to be red. The other part was embedded with paraffi n and then sliced into pieces with the thickness of 4 μm. After HE staining, dehydration and clarifying, it was observed under the optical microscope.
2.3.4. Expression of NF-κB detected by immunohistochemical assay
The above paraffi n sections were dried in the oven at 60 ℃. Then it was deparaffi naged with dimethylbenzene for 10 min and dehydratedwith gradient alcohol for 5 min. After being immersed in PBS buff er, it was then heated in the microwave oven and the citrate antigen retrieval buff er was added for the retrieval, with pH 6.0, at 750 W for 5 min and at 150 W for 15 min. It was washed with PBS and then dried. After adding 50 μL endogenous biotin antagonist, it was then incubated at the room temperature for 10 min and washed with PBS for 3 min. Afterwards, the primary antibody was added and it was then incubated in the refrigerator at 4 ℃ over night and washed with PBS; after adding the secondary antibody, it was incubated at the room temperature for 40 min and then washed with PBS. Finally, DAB color development was performed and the reaction of color development was observed under ECLIPSE80i microscope. The scoring criteria as follows: no expression as 0 point; 0%-25% as 1 point; 26%-50% as 2 points; and ≥51% as 3 points. 0-1 point referred to the low expression and ≥2 points to the high expression.
2.3.5. Expression of TLR-4 detected by Western blot
The ischemic cardiac muscle tissue of mice was collected to be washed with PBS and then cut into pieces. After being fully lysed, it was centrifuged for 5 min to separate the supernatant. The determination of content was performed for the collected histones. After 2 h of electrophoresis, the transfer was performed. Afterwards, the membrane was immersed in Tween20/TBS buffer to be oscillated in the blocking buf fer. After adding the primary antibody and secondary antibody respectively, it was washed and then colored with DAB. The gel imaging analysis system was employed for the scanning to calculate the net optical density of research object.
2.3.6. Expressuon of inflammatory factors detected by ELISA method
After the modeling, the venous blood was collected by 3 mL from cardiac muscle. It was anticoagulated with heparin, centrifuged at 3 000 r/min for 10 min, and stored at -80 ℃. The IL-1, IL-6 and TNF-α ELISA kits (double-antibody sandwich enzyme-linked immunosorbent assay) were employed to detect the expression of infl ammatory factors in the serum.
2.4. Statistical analysis
The research data was treated with SPSS 21.0. The measurement data was expressed by mean±SD. The one-way ANOVA analysis or student’s t test was employed for the comparison between groups. P<0.05 indicated the statistical diff erence.
3. Results
3.1. Behavior and survival of mice
Mice had the smooth breathing during the anesthesia. Mice in the operation group had the deep and slow breathing after the ligation of left coronary artery and the shallow and fast breathing after restoring the reperfusion with the hair standing. There had been no anesthetic accident during the modeling. The survival rate of mice in group A after the reperfusion was 95.00%, while the survival rate of mice in group B after the reperfusion was 90.00%. Because of no ligation of coronary artery for mice in group AS and group BS, their survival rate was all 100.00%. Mice in sham group had the ischemia and infarction area. The ischemia and infarction area in TLR-4 null mice after the myocardial ischemia reperfusion was significantly less than that in wild homozygous mice, with the statistical diff erence (P<0.05) (Table 1).

Table 1Comparison of heart condition after myocardial ischemia reperfusion of mice.
3.2. Morphological observation of cardiac muscle
After MTT staining, the normal cardiac muscle tissue appeared to be red and part of cardiac muscle appeared to be pale, which was caused by the myocardial infarction after myocardial ischemia reperfusion. After HE staining, the structure of cardiac muscle for mice in group AS and group BS was normal under the microscope, with the orderly arrangement of fi ber and without the infi ltration of inflammatory cells. The structure of cardiac muscle for mice in group A was regular, with a bit fiber failure, relatively wide intercellular space and some infl ammatory cells; while the structure of cardiac muscle for mice in group B was in mess, with a lot of fi ber failure and fi brinolysis, signifi cantly wide intercellular space and a great number of infl ammatory cells.
3.3. Expression of NF-κB
The immunohistochemistry was employed to detect the expression of NF-κB. There was no expression of NF-κB in the cardiac muscle tissue for mice in group AS and group BS; while there were cytoplasms of cardiac muscle cells and the brown particles in the cell nucleus for mice in group A and group B, as the positive expression of NF-κB. After the myocardial ischemia reperfusion, the expression of NF-κB in TLR-4 null mice (14 mice with low expression and 5 mice with high expression) was signifi cantly lower than that in wild homozygous mice (6 mice with low expression and 12 mice with high expression), with the signifi cant diff erence (P<0.05) (Table 2).
3.4. Expression of TLR-4
The gray level ratio of TLR-4 in wild homozygous mice in the model group and sham group was (77.3±11.54) and (32.48±7.62) respectively, which was signifi cantly higher than that in TLR-4 null mice, while the gray level ratio of TLR-4 in TLR-4 null mice in the model group was (30.71±7.57), which was signifi cantly higher than that of (9.89±3.36) in sham group, with the statistical difference (P<0.05) (Table 2).
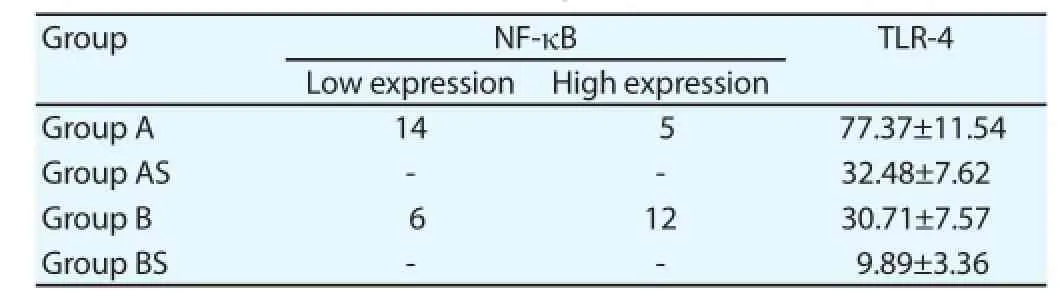
Table 2Expression of NF-κB and TLR-4 in each group.
3.5. ELISA to detect the expression of inflammatory factors
These two types of mice all had the different degree of infl ammatory response after the myocardial ischemia reperfusion, with the certain increase of IL-1, IL-6 and TNF-α. The expression of infl ammatory factors for TLR-4 null mice and wild homozygous mice in the model group was signifi cantly higher than that in sham group, while the expression of these factors for TLR-4 null mice in group A was signifi cantly lower than that for wild homozygous mice in group B, with the statistical diff erence (P<0.05) (Table 3).
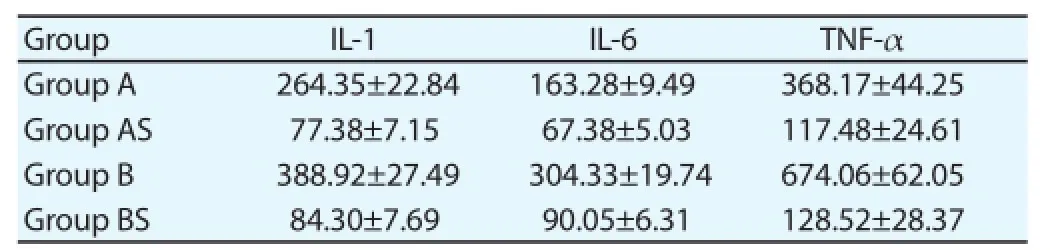
Table 3Expression of infl ammatory factors (pg/mL).
4. Discussion
The ischemia is some kind of pathological manifestation of inadequate blood fl ow caused by the defi ciency of blood supply. The ischemic diseases refer to ones because of the tissue ischemia, which can be found in many tissues and organs. The reperfusion is the process that the blood supply is restored after the ischemia of tissues [12,13] . But after the interruption of blood supply in the tissues and organs and restoring the blood fl ow, the function of tissues and organs was not recovered, but the diseases were worsened and the function disorder and structure injury were aggravated [14] . The myocardial ischemia reperfusion injury was related to many factors. The advances in the science and technology and techniques of electrophysiology, genomes and proteomes all provide the support for the research on the mechanism of myocardial ischemia reperfusion injury.
The infl ammatory cytokines play the key role in the myocardial ischemia reperfusion injury. Most members of interleukin-1 family are the pro-inflammatory cytokines, which could stimulate the infl ammatory and autoimmune response [15] . In the normal organic environment, the expression of IL-1 was extremely low; but after the ischemic reperfusion, its expression was signifi cantly increased. The research presumed that it was related to the activation of oxygen free radicals [16] . IL-6 is also the big family of infl ammatory factors. According to the previous research, in the process of myocardial ischemia reperfusion, NO was produced by reducing the cardiac muscle cells. NO could be reacted with the free radicals of superoxide anions to produce the peroxynitrite anions. As the substance with the strong oxidizability and toxicity, it could cause the peroxidation of membrane lipid and the oxidative damage of other cellular components and thus damage the cardiac muscle [17] . TNF-α has the function of signal transduction, which can activate NF-κB. Its injury mechanism is not only related to the oxygen free radicals and NO, but also to the overload of calcium channel. The transmembrane influx of calcium ions can accumulate a great number of oxygen free radicals in cells, while the excessive oxygen free radicals can induce the increase of free calcium in the cytoplasm. Such vicious circle would worsen the injury of cardiac muscle till the cell death [18] . The results of this study also indicated that the expression of infl ammatory factors for mice in the model group was signifi cantly up-regulated, which was closely related to the injury mechanism of involved cardiac muscle.
The expression of TLR-4 can be found in the cardiac muscle cells and vascular endothelial cells, which is closely related to the myocardial ischemia reperfusion injury. It mainly plays its role by mediating the MyD88 dependent and independent pathways. Through the MyD88 dependent pathway, it could activate the NF-κB, cause its transfer in the nucleus and activate the transcription of cytokine to release the related infl ammatory cytokines [19] . The production of cytokine could also play the role of positive feedback to further activate the NF-κB. Meanwhile, there was the certain regulation of negative feedback. When the activation of NF-κB was inhibited, the mediation of TLR-4 was reduced and the expression was blocked as well [20] .
In this study, the myocardial ischemia reperfusion injury in TLR-4 null mice was lower than that in wild mice. The reason might be that the decreased expression of TLR-4 could inhibit the mediation of MyD88 pathway to reduce the activation of NF-κB, affect the release of related inflammatory cytokines and thus relieve the injury of cardiac muscle mediated by cytokine [21] . Meanwhile, the expression of TLR-4, NF-κB and cytokine for TLR-4 null mice in the model group was all higher than that in sham group, which also proved that the up-regualted expression of TLR-4 was closely related to the myocardial ischemia reperfusion injury[22]. In the process of myocardial ischemia reperfusion injury of mice, the TLR-4/NF-κBpathway to the release of cytokine is the important cascade reaction. The change of any link may aff ect the degree of injury. It thus also points out the direction of prevention and treatment of myocardial ischemia reperfusion injury. The further researches will discuss whether there is a certain regulatory mechanism between TLR-4 and cytokine.
Conflict of interest statement
We declare that we have no confl ict of interest.
References
[1] Jennings RB, Sommers HM, Smyth GA, Flack HA, Linn H. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch Pathol 1960; 7(70): 68-78.
[2] Shao Y, Wu QN, Zhou J, Yue W, Chao JG. Protective effects of total fl avones from Lophatherum gracile on myocardial ischemia-reperfusion injury in rats. Chin Pharmacol Bulletin 2013; 2: 241-247.
[3] Liu J, Li C, Yin Y, Yu L, Zhang LH, Ma H. Acetaldehyde dehydrogenase-2 activation inhibits myocardial hydroxy stress and ischemia-reperfusion injury of mice with diabetes. Chin Heart J 2014; 5: 497-501.
[4] Gao JB, Zhang Y, Lou JS. Long-term cardioprotective eff ects of multiple courses noninvasive delayed limb ischemic preconditioning against myocardial ischemia-reperfusion injury in rats. J Clin Cardiol 2014; 5: 439-442.
[5] Sun XF, Wang HX, Liang LJ, Lu ML, Gu JY, He HY. Inhibition of Astragalus polysaccharide in lipopolysccharide-induced cardiomyocyte hypertrophy in rats through the TLR4/NF-κB signal transduction. Chin Pharmacol Bulletin 2013; 2: 208-212.
[6] Foley NM, Wang J, Redmond HP, Wang JH. Current knowledge and future directions of TLR and NOD signaling in sepsis. Military Med Res 2014; 4: 217-228.
[7] Li YW, Xie GR, Li L, Jiang ZS, Yue ZS, Pan ZY. The effect of TLR4/ MyD88/NF-κB signaling pathway on proliferation and apoptosis in human nasopharyngeal carcinoma 5-8F cells induced by LPS. J Clin Otorhinolaryngol Head Neck Surg 2015; 11: 1012-1015.
[8] Zhu HP. Eff ects of sulforaphane on proliferation, cell cycle and apoptosis of LNCaP and expressions of IGFBP3 and NF-κB. J Zhengzhou Univ (Med Sci)2014; 6: 801-804.
[9] Shi XJ, Wang GP, Tao GZ. Mechanism of atorvastatin that inhibts the infl ammatory response and apoptosis of ischemiare perfusion cardiac muscle in rats by inhibiting NF-κB. J Clin Cardiol 2015; 7: 788-790.
[10] Cao J, Xie H, Sun Y, Zhu J, Ying M, Qiao S, et al. Sevoflurane postconditioning reduces rat myocardial ischemia reperfusion injury through an increase in NOS and a decrease in phopshorylated NHE1 levels. Int J Mol Med 2015; 36: 1529-1537. doi: 10.3892/ijmm.2015.2366.
[11] Wu N, Zhang X, Jia P, Jia D. Hypercholesterolemia aggravates myocardial ischemia reperfusion injury via activating endoplasmic reticulum stress-mediated apoptosis. Exp Mol Pathol 2015; 99(3): 449-454.
[12] Guo WN, Yin Y, Ma H. A discussion on selection of death types of cardiac muscle cells and its mechanism after ischemia/reperfusion. Chin Heart J 2015; 4: 470-473.
[13] Yang CJ, Yang J, Fan ZX, Yang J. Activating transcription factor 3-an endogenous inhibitor of myocardial ischemia-reperfusion injury (Review). Mol Med Rep 2015; 13(1): 9-12.
[14] Yang J, Guo X, Yang J, Ding JW, Li S, Yang R, et al. RP105 protects against apoptosis in ischemia/reperfusion-induced myocardial damage in rats by suppressing TLR4-mediated signaling pathways. Cell Physiol Biochem 2015; 36(6): 2137-2148.
[15] Wang M, Cao BZ. Expression of ICAM-1, NF-κB p65, TNF-α and IL-1β in brain tissue of chronic hypoperfusion rats. J Apoplexy Nervous Dis 2012; 11: 974-978.
[16] Gan N, Yi F, Kong HM, Ma YP, Peng J, Wu LW. The interreaction of IL-1β and NF-κB in mesial temporal lobe epilepsy models on rats. Chin J Neuroanatomy 2013; 6: 637-643.
[17] Zhou HX, Lou N, Wei ZF, Zhang YX, Meng LL, Zhang ZF. Expressions of NF-κB and IL-6 around the hematoma and their correlations with cerebral edema after intracerebral hemorrhage in rats. J Shandong Univ (Health Sciences) 2012; 11: 30-33.
[18] Fan H, Shen L, Tang Q, Xiong PC, Shou ZX, Liao Y, et al. Effect of Wumeiwan on Cytokines TNF-α, IL-6, IL-8, IL-10 and expression of NF-κBp65 in rats with ulcerative colitis. J Huazhong University of Science and Technology (Medical Sciences) 2009; 29(5): 650-654.
[19] Ju XH, Xu HJ, Yong YH, An LL, Xu YM, Jiao PR, et al. Heat stress upregulates the expression of TLR4 and its alternative splicing variant in bama miniature pigs. J Integrative Agriculture 2014; 13(11): 2479-2487.
[20] Zhang HY, Kang J, Han WJ, Hu MM, Jia HG. The expression and signifi cance of TLR4, MyD88 and NF-κB mRNA in mouse lymph node of experimental autoimmune myositis. Chin J Cell Mol Immunol 2012; 28(3): 272-275.
[21] Yang MX, Gan H, Shen Q. Effect of LPS on the level of TLR4 and on the expression of NF-κB and Notch1 in monocytes from patients with type 2 diabetic nephropathy. J Central South Univ (Medical Sciences) 2012; 37(6): 578-585.
[22] Feng J, Guo C, Zhu Y, Pang L, Yang Z, Zou Y, et al. Baicalin down regulates the expression of TLR4 and NF-κB-p65 in colon tissue in mice with colitis induced by dextran sulfate sodium. Int J Clin Exp Med 2014; 7(11): 4063-4072.
ΔThese two authors share the co-fi rst authorship.
Tel: 13126180026
E-mail: hbch1120@163.com.
Foundation project: It was supported by Research Fund of Health Bureau of Hebei Province (Project No. 20090601).
Toll-like receptor 4
NF-κB
Myocardial ischemia reperfusion
doi:Document heading 10.1016/j.apjtm.2016.03.021
*Corresponding author:Hao Chen, Master’s Degree, Deputy Attending Physician, Department of Cardiology, Affi liated People’s Hospital of Hebei Medical University; Shijiazhuang No.1 Hospital.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Study on the role of Cathepsin B and JNK signaling pathway in the development of cerebral aneurysm
- Polarization of M1 tumor associated macrophage promoted by the activation of TLR3 signal pathway
- Influence of hydrogen sulfide on zymogen activation of homocysteineinduced matix metalloproteinase-2 in H9C2 cardiocytes
- Effect and mechanism of miR-34a on proliferation, apoptosis and invasion of laryngeal carcinoma cells
- Exploration of the regulatory effect of miR-21 on breast cancer cell line proliferation and invasion as well as the downstream target genes
- Serological survey on some pathogens in wild brown hares (Lepus europaeus) in Central Italy
