Influence of hydrogen sulfide on zymogen activation of homocysteineinduced matix metalloproteinase-2 in H9C2 cardiocytes
2016-07-25ZiShuWangHongJinDongMingWangDepartmentofCardiologyThirdAffiliatedHospitalofGuangzhouMedicalUniversityGuangzhouGuangdong5050ChinaDepartmentofCardiologyFirstAffiliatedHospitalofShantouUniversityMedicalCollegeShantouGuangdong
Zi-Shu Wang, Hong Jin, Dong-Ming Wang*Department of Cardiology, Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, 5050, ChinaDepartment of Cardiology, First Affiliated Hospital of Shantou University Medical College, Shantou, Guangdong 5504, China
ABSTRACT
Objective: To observe the infl uence of diff erent concentrations of homocysteine (Hcy) and hydrogen sulfi de (H2S) on the secretion and activation of matix metalloproteinase-2 (MMP-2) in cardiocytes so as to search for new ways to fight against myocardial tissue fibrosis. Methods: Cardiocytes H9C2 was cultured in vitro and diff erent concentrations of Hcy and H2S were added for 6-h and 24-h cultivation. MTT cell proliferation assay was applied to test the activation change of cardiocytes H9C2 after aff ecting by diff erent concentrations of Hcy and H2S. ELISA and MTT were employed to detect the expression and enzymatic activity of MMP-2. Results: The H9C2 cell inhibition of activity was more signifi cant with 1 000 μmol/ L of Hcy as compared with other concentrations (P<0.001). With 2.5-100.0 μmol/L Hcy and 0.1, 1.0 and 10.0 mmol/L H2S, the activity of H9C2 did not change signifi cantly (P>0.05). Hcy with concentrations of 10, 50 and 100 μmol/L could increase the quantity of MMP-2 secreted by cardiocytes H9C2, and the interaction strength was concentration-dependent (P<0.05). After interacting with 100 μmol/L of Hcy for 6 h, the zymogen activation eff ect of MMP-2 was stronger than that of the 2.5-25 μmol/L group (P<0.05). After interacting with Hcy and H2S (1.0 mmol/L) for 6 h and 24 h, the activation eff ect of MMP-2 was stronger than those interacted with 10, 25, 50 and 100 μmol/L of Hcy (P<0.05). Conclusions: Hcy can increase the production of MMP-2 secreted by H9C2 cell and improve its zymogen activation. Besides, the interaction strength is concentration-dependent; while H2S can up-regulate the activation of MMP-2 and co-promote the activation of MMP-2 with Hcy as well.
ARTICLE INFO
Influence of hydrogen sulfide on zymogen activation of homocysteineinduced matix metalloproteinase-2 in H9C2 cardiocytes
Zi-Shu Wang1, Hong Jin2, Dong-Ming Wang2*1Department of Cardiology, Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, 510150, China
2Department of Cardiology, First Affiliated Hospital of Shantou University Medical College, Shantou, Guangdong 515041, China
ABSTRACT
Objective: To observe the infl uence of diff erent concentrations of homocysteine (Hcy) and hydrogen sulfi de (H2S) on the secretion and activation of matix metalloproteinase-2 (MMP-2) in cardiocytes so as to search for new ways to fight against myocardial tissue fibrosis. Methods: Cardiocytes H9C2 was cultured in vitro and diff erent concentrations of Hcy and H2S were added for 6-h and 24-h cultivation. MTT cell proliferation assay was applied to test the activation change of cardiocytes H9C2 after aff ecting by diff erent concentrations of Hcy and H2S. ELISA and MTT were employed to detect the expression and enzymatic activity of MMP-2. Results: The H9C2 cell inhibition of activity was more signifi cant with 1 000 μmol/ L of Hcy as compared with other concentrations (P<0.001). With 2.5-100.0 μmol/L Hcy and 0.1, 1.0 and 10.0 mmol/L H2S, the activity of H9C2 did not change signifi cantly (P>0.05). Hcy with concentrations of 10, 50 and 100 μmol/L could increase the quantity of MMP-2 secreted by cardiocytes H9C2, and the interaction strength was concentration-dependent (P<0.05). After interacting with 100 μmol/L of Hcy for 6 h, the zymogen activation eff ect of MMP-2 was stronger than that of the 2.5-25 μmol/L group (P<0.05). After interacting with Hcy and H2S (1.0 mmol/L) for 6 h and 24 h, the activation eff ect of MMP-2 was stronger than those interacted with 10, 25, 50 and 100 μmol/L of Hcy (P<0.05). Conclusions: Hcy can increase the production of MMP-2 secreted by H9C2 cell and improve its zymogen activation. Besides, the interaction strength is concentration-dependent; while H2S can up-regulate the activation of MMP-2 and co-promote the activation of MMP-2 with Hcy as well.
ARTICLE INFO
Keywords:
Article history:
Received 15 January 2016
Received in revised form 16 February 2016
Accepted 15 March 2016
Available online 20 May 2016
1. Introduction
Cardiac extracellular matrix (ECM) is mainly composed of fi brous collagens, elastin, fi bronectin and so on, which are responsible for the support and bind of myocardial cells [1] . Types Ⅱ and Ⅲ are the most important collagens for ECM which account on around 80%. For instance, the barrier of collagen synthesis and degradation pathways can lead to myocardial tissue fi brosis, aff ect the structure and function of heart and also influence the occurrence and development of heart diseases [2,3] . In addition, cardiac interstitial fi brosis will accelerate the change of artrial electrophysiological characteristic. Hence, improvement of atrial tissue fi brosis might be a new way to prevent and treat atrial fibrillation [4] . Matix metalloproteinase-2 (MMP-2) can degrade most ECM, and adjust the metabolism of ECM with endogenous inhibiting factor (TIMPs) accurately, which make it an important factor for the reconstruction of heart tissue[5-8]. It can be presumed that the regulation mechanism of MMPs/TIMPs is closely related to the prevention and treatment of heart diseases [9] . Some scholars claim that homocysteine (Hcy) can activate the in-vivo MMPs zymogen and participate in the pathogenesis of various cardiovascular andcerebrovascular diseases such as hypertension, stroke and peripheral vascularartery atherosclerosis [10] . Hydrogen sulfi de (H2S) possesses a wide physiological regulatory eff ect on cardiovascular, nervous, digestive and endocrine system and presents a crossed regulating effect with Hcy[11]. The aim of this study was to observe the infl uence of diff erent concentrations of Hcy and H2S on the secretion and activation of MMP-2 in cardiocytes so as to provide new theoretical basis to prevent and treat myocardial tissue fi brosis.
2. Materials and methods
2.1. Source of cells
The embryonic cardiac H9C2 cells of rats were purchased for the Cell Centre of Shanghai Institutes for Biological Sciences, CAS. BDIX rat embryonic cardiomyocytes offered freely the Pathogen Biology Laboratory by of Shantou University Medical College were from ATCC.
2.2. Reagents and instruments
Reagents and instruments used in this study included MMP-2 quantitative enzyme-linked detection kit, DMEM medium with high glucose (Gibco), 96-well culture plates, 25 cm2cell culture flask (Corning), UVP gel scanning system (Bio-RAD), invert microscope, XS105 electronic analytical balance and 756 ultraviolet spectrophotometer (Shanghai Medical Devices).
2.3. Experimental methods
Rat embryonic H9C2 cardiocytes were cultivated serially till 80% of them were blended. They were inoculated on a 96-well culture plates with 100 μL in each hole and an inoculation density of 2伊104/ mL. After 24 h, 0, 10, 50, 100, 500 and 1 000 μmol/L Hcy incubated cells without phenol red medium and 0.1, 1.0 and 10 mmol/L H2S were added respectively. NaHS with experimental concentrations of Hcy and H2S but without phenol red medium was used to incubate cells.
2.4. Detected methods for concentrations and activity of MMP-2
After culturing for 6 h and 24 h, MTT cell proliferation assay was applied to test the activation of H9C2 cardiocytes, while ELISA and MTT were employed to detect the expression and enzymatic activity of MMP-2.
2.5. Statistical management
The experimental data were recorded and counted with SPSS13.0. Comparisons between groups were tested by t-test and comparisons among groups were analyzed by one-way ANOVA. P<0.05 indicated that the dif ferences were statistically signifi cant and 毩=0.05 was the inspection level.
3. Results
3.1. Influence of Hcy on cell activity of cardiocytes and synthesis and secretion of MMP-2
The results showed that the cell activity of H9C2 decreased with the increase of the concentrations of Hcy, the cell activity of H9C2 was inhibited signifi cantly when the concentration of Hcy reached 1 000 μmol/L (P<0.001), and the activity of H9C2 showed no statistical signifi cance when the concentration of Hcy was from 2.5-100.0 μmol/L (P>0.05) (Table 1). After co-aff ected and cultivated by 2.5-100.0 μmol/L Hcy and 0.1, 1.0 and 10.0 mmol/L H2S for 6 h or 24 h, the cell activity of H9C2 showed no signifi cant diff erence as compared with that of the control group (P>0.05).
Hcy with concentrations of 10, 50 and 100 μmol/L could increase the secretion quantity of MMP-2, and the interaction strength was concentration-dependent (P<0.05) (Table 1).
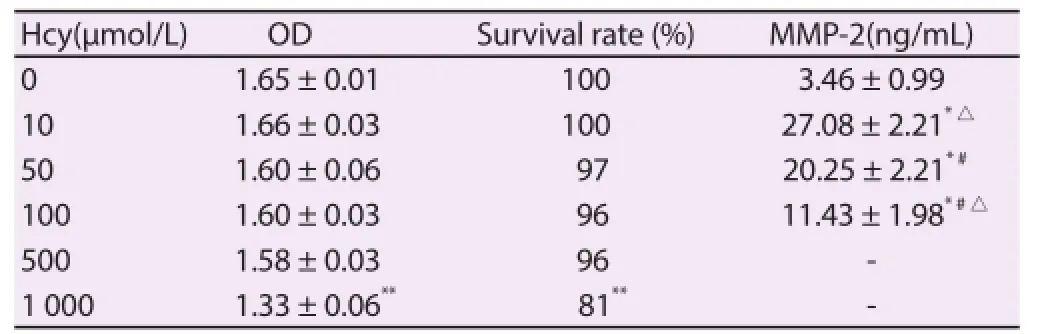
Table 1Infl uence of Hcy on cell activity of cardiocytes and total concentration of MMP-2 secreted by cardiocytes.
3.2. Dose-effect relationship of Hcy on activity of MMP-2
The study revealed that Hcy could promote the zymogen activation of MMP-2 signifi cantly (P<0.05). After interacting with 100 μmol/ L of Hcy for 6 h, the zymogen activation effect of MMP-2 was stronger than that of the 2.5-25.0 μmol/L group and after interacting with 5-100 μmol/L of Hcy for 24 h, its zymogen activation eff ect was stronger than that of the 2.5 μmol/L group (P<0.05), which indicated that the promotion ef fect of Hcy on the zymogen activation of MMP-2 was concentration-dependent (Figure 1 and 2).

Figure 1. Regulatory eff ect on activity of MMP-2 after aff ected by Hcy for 6 h. Compared with 0 μmol/L,*P<0.05; compared with 100 μmol/L,#P<0.05.
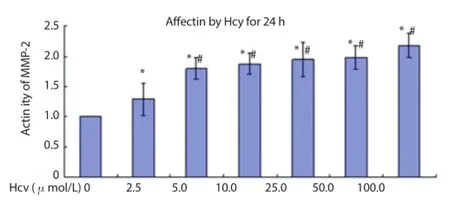
Figure 2. Regulatory eff ect on activity of MMP-2 after aff ected by Hcy for 24 h.
3.3. Time-effect relationship of Hcy on zymogen activation of MMP-2
After aff ected by 5-100 μmol/L for 24 h, the activation of MMP-2 was stronger than that after aff ected by the same number of Hcy for 6 h (P<0.05) (Figure 3).
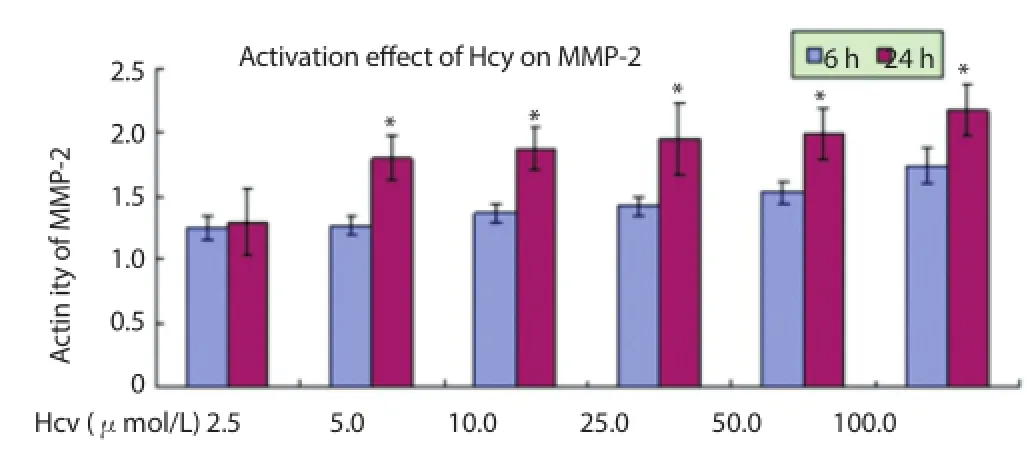
Figure 3. Regulatory eff ect on activity of MMP-2 after aff ected by Hcy for 6 h and 24 h.
3.4. Regulatory effect of H2S on activity of MMP-2
H2S with concentrations of 0.1, 1.0 and 10.0 mmol/L could signifi cantly promote the activation of MMP-2 (P<0.05). There was signifi cant diff erence between the activation eff ects of 0.1 and 10.0 mmol/L of H2S and 1.0 mmol/L of H2S (P<0.01) (Figure 4).
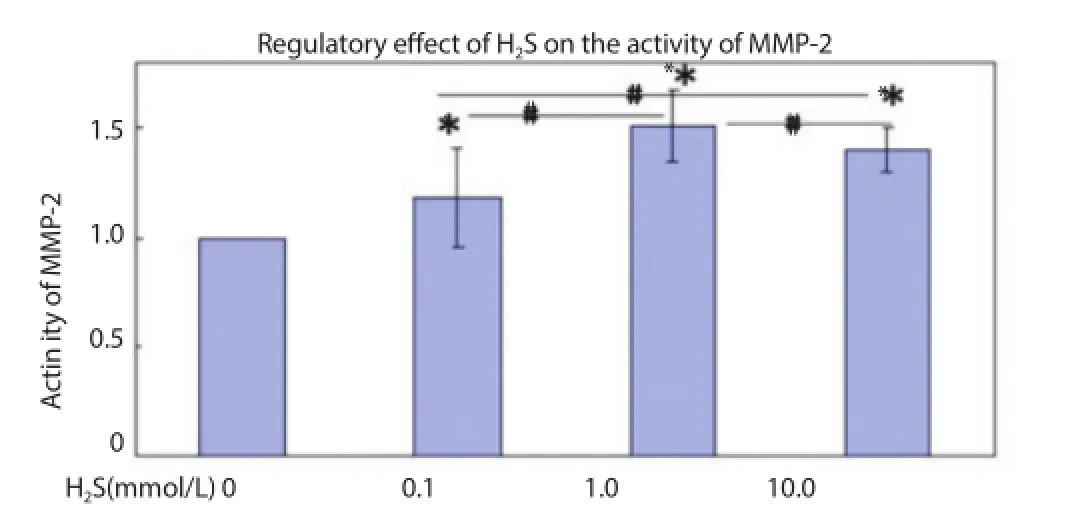
Figure 4. Regulatory eff ect on activity of MMP-2 after aff ected by H2S for 24 h.
3.5. Corporate regulatory effect of Hcy and H2S on activity of MMP-2
The results of the co-eff ect of Hcy and H2S showed that 1.0 mmol/ L of H2S could promote the activation effect of Hcy significantly (Figure 5); 1.0 and 10.0 mmol/L of H2S could up-regulate the activation effect of Hcy significantly and P=0.002 after affected by 1.0 mmol/L H2S for 6 h, while P=0.042 after aff ecting for 24 h (Figure 6); 1.0 mmol/L of H2S could raise activation eff ect of Hcy on the activity of MMP-2 significantly (Figure 7); and 1.0 mmol/L of H2S could also strengthen the enzyme activation of Hcy (Figure 8).
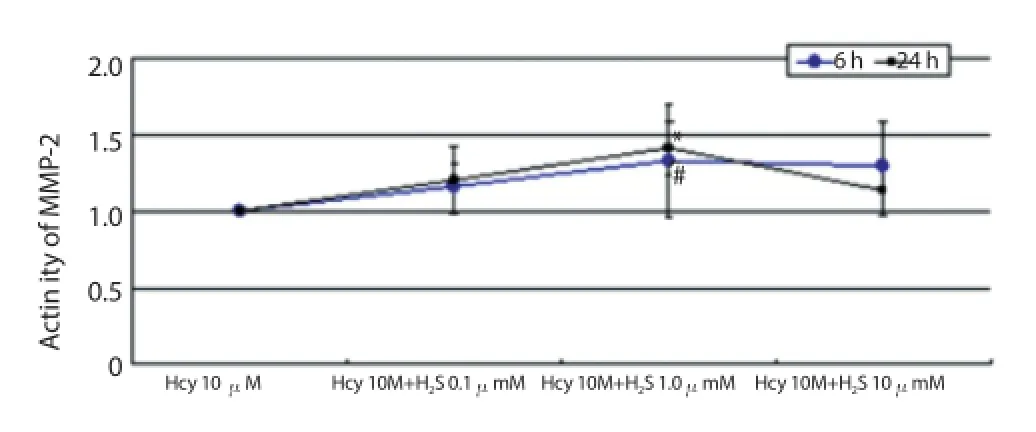
Figure 5. Activity of MMP-2 after co-affected by 10 μmol/L Hcy and H2S for 6 h and 24 h.
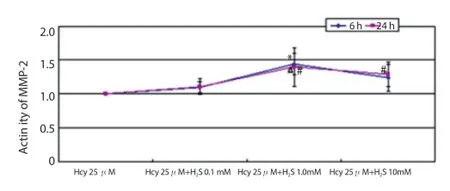
Figure 6. Activity of MMP-2 after co-affected by 25 μmol/L Hcy and H2S for 6 h and 24 h.

Figure 7. Activity of MMP-2 after co-aff ected by 50 μmol/L Hcy and H2S for 6 h and 24 h.
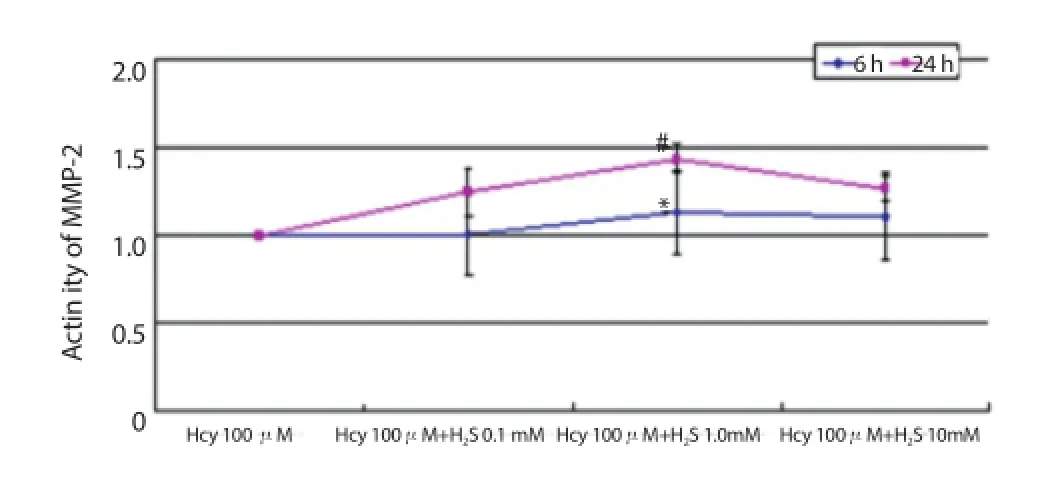
Figure 8. Activity of MMP-2 after co-aff ected by 100 μmol/L Hcy and H2S for 6 h and 24 h.
4. Discussion
Recently, epidemiological studies have shown that Hcy is closely related to the incidence and development of diseases, such as hypertension, atrial fibrillation, stoke, dementia and so on, and also the abnormal increase of blood concentration of Hcy is an independent risk factor of cardia-cerebrovascular disease [11-13] . The latest in vitro studies have demonstrated that Hcy can synthesize H2S directly by catalysis of CSE enzyme. Although the synthesized H2S only represents a small part, it can increase with the raise of the concentration of Hcy, and the synthesis of H2S can increase signifi cantly at a super high concentration of Hcy [14] . H2S has active sulfydryl. Therefore, the key point of this study is that whether the enzyme activity of MMP-2 cardiocytes has a regulatory eff ect.
The activity imbalance between MMPs and TIMPs is a factor causing atrial tissue fi brosis [15] . At present, 28 kinds of MMPs have been discovered. Among them, MMP-2 and MMP-9 all belong to gelatinase and participate in the pathogenesis of many cardiovascular diseases [16] . Some scholars hold the idea that the abnormal of the activity of MMP-2 is the main risk factor of atrial fibrillation[17-20] . Some other scholars insist that Hcy can stimulate the zymogen activation of MMP-2 and facilitate the activation of MMP-2 in the ventricular tissues of rats eff ectively [21] . There are also researches revealing that Hcy possesses a toxic eff ect on H9C2 cardiocytes [22] . For example, it can accelerate the apoptosis of H9C2 cardiocytes or inhibit their growth activity evidently. Hcy can obviously promote the apoptosis of H9C2 at a super high concentration of 2.73 mmol/ L and 100 and 1 100 μmol/L of Hcy can even lead to reversible changes of the ATP value, mitochondrial transmerabrane potential and epicyte of H9C2 cardiocytes. Hence, the eff ect of Hcy on the activity of cardiocytes was investigated in this study in the first place. The results of MTT showed that the survival rate of H9C2 cardiocytes decreased with the increase of the concentration of Hcy. When the concentration of Hcy reached 1 000 μmol/L, the activity of H9C2 cardiocytes was inhibited significantly (P<0.001), which was identical with the reported ones[23]. In this study, the activity of H9C2 cardiocytes was not infl uenced signifi cantly when aff ected by 2.5-100.0 μmol/LHcy, which implied that it could be used continuously and safely in the subsequent experiences. In this study, the eff ect of Hcy on the expression and zymogen activation of MMP-2 of H9C2 cardiocytes was also observed. The results demonstrated that being aff ected by 0-100 μmol/L of Hcy for 24 h could stimulate the synthesis and activity of MMP-2 cardiocytes in a concentrationand time-dependent manner, which implied that Hcy participate in the reconstruction of heart tissues by inducing the expression and activation of MMP-2[24]. NaHS was applied as the exogenous donor for H2S in this study and three concentration groups (0.1, 1.0 and 10 mmol/L) were established. The results showed that H2S with the above concentrations had no signifi cantly infl uence on the activity of H9C2 cardiocytes, while after acting for 24 h, it promoted the activation of MMP-2 signifi cantly and presented as a concentrationdependent manner. After that, H2S with the above concentrations were added into the Hcy group, the results showed no changes of the cell activity. Moreover, the study results also showed that Hcy+H2S synergistically facilitate the activity of MMP-2 positively after acting for 6 h and 24 h, but the time-dependent manner was not found. The results of this study manifest that Hcy could increase the production of MMP-2 secreted by H9C2 cells and improve its zymogen activation. Besides, the interaction strength is concentration-dependent; while H2S could up-regulate the activation of MMP-2 and co-promote the activation of MMP-2 with Hcy as well.
Conflict of interest statement
We declare that we have no confl ict of interest.
References
[1] Wang YJ, Suo YR, Zeng WY, Kan BH, Jiang XJ, Fan YC. The synergy between Xinfukang and Hone marrow mesenchymal stem cells increases the expression of GATA4 and Cx43 in cardiac stem cells. Tianjin J Tradit Chin Med 2015; 32(5): 291-294.
[2] Dai GH, Song XB, Ma PZ, Liu N, Yao J. Biological characteristics of angiogenesis of microvascular endothelial cells in rat with myocardial ischemia. J Tianjin Univ Tradit Chin Med 2014; 33(5): 278-282.
[3] Gao Q, Ji H, Hu XT, Wang YJ, Fan YC. 5-azacytidine combined with salvianolic acid B can promote differentiation from bone marrow mesenchymal stem cells derived from rats to cardiomyocytes. Tianjin J Tradit Chin Med 2013; 32(1): 24-27.
[4] Cui J, Fan YC, Xue L. Experimental study on isolation, culture and identification of MSCs in rats. Tianjin J Tradit Chin Med 2012; 29(5): 463-464.
[5] Gao YC, Li M, Li RY, Sun YF. Revealing nourishing kidney drugs in the culture and diff erentiation of stem cells. J Clin Rehabil Tissue Eng Res 2013; 17(4): 2609-2616.
[6] Li LJ, Wang HJ, Lv SC, Song HJ. Association of resistin and matrix metalloproteinase-2 with T2DM macroangiopathy. Chin J Diabetes 2013; 21(3): 243-245.
[7] Wang HJ, Li LJ, Song HJ. The research progress of the pathological changes of matix metalloproteinase-2 and type 2 diabetic macroangiopathy. Chin J Gerontol 2013; 27(14): 3522-3523.
[8] Li W, Zang W, Liu P, Wang Y, Du Y, Chen X, et al. MicroRNA-124 inhibits cellular proliferation and invasion by targeting Ets-1 in breast cancer. Tumour Biol 2014; 35(11):10897-10904.
[9] Hu CB, Li QL, Hu JF, Zhang Q, Xie JP, Deng L. MiR-124 inhibits growth and invasion of gastric cancer by targeting ROCK1. Asian Pac J Cancer Prev 2014; 15(16): 6543-6546.
[10] Ansari R, Mahta A, Mallack E, Luo JJ. Hyperhomocysteinemia and neurologic disorders: a review. J Clin Neurol 2014; 10(4): 281-288.
[11] Yu X, Li Z. MicroRNAs regulate vascular smooth muscle cell functions in atherosclerosis. Int J Mol Med 2014; 34(4): 923-933.
[12] Zhang D, Wen X, Wu W, Xu E, Zhang Y, Cui W. Homocysteine-related hTERT DNA demethylation contributes to shortened leukocyte telomere length in atherosclerosis. Atherosclerosis 2013; 231(1): 173-179.
[13] Yang AN, Wang L, Zhou LX, Zhao L, Wang YH, Cai X, et al. Effects of Hcy on cholesterol eff ux of THP-1monocyte-derived foam cells and mechanism of ABCA1 and ACAT1 DNA methylation regulation. Chin Pharm Bull 2014; 30(3): 340-344.
[14] Yang LX, Zhang R, Li M, Wu XJ, Wang JH, Huang L, et al. A functional miR-124 binding-site polymorphism in IQGAP1 aff ects human cognitive performance. PLoS One 2014; 9(9): e107065.
[15] Luo YQ, Wu XX, Ling ZX, Cheng YW, Chen JY, Xiang C. MicroRNA-133a targets Foxl2 and promotes diff erentiation of C2C12 into myogenic progenitor cells. DNA Cell Biol 2015; 34(1): 29-36.
[16] Wang D, Yan X, Xia M, Yang Y, Li D, Li X, et al. Coenzyme Q10 promotes macrophage cholesterol effl ux by regulation of the activator protein-1/miR-378/ATP-binding cassette transporter G1-signaling pathway. Arterioscler Thromb Vasc Biol 2014; 34(9): 1860-1870.
[17] Liu YM, Xu YH, Na MH, Teng MZ, Wu DZ, Jiang MX. The effect of Kanli granule on calcium transport in cardiac muscle sarcoplasmic reticulum of rats with diastolic heart failure induced by pressure overload. J Tradit Chin Med 2015; 56(21): 1867-1870.
[18] Tian W, Gu Y, Deng SL, Zhang L. Effects of urocortin栺postconditioning on mitochondrial membrane potential during myocardial hypoxia/reoxygenation injury. J Zunyi Med Univ 2015; 38(1): 64-66, 73. [19] Wang RX, Xu JH. Genomic DNA methylation and histone methylation. Hereditas 2014; 36(3): 191-199.
[20] Han XB, Zhang HP, Cao CJ, Wang YH, Tian J, Yang XL, et al. Aberrant DNA methylation of the PDGF gene in homocysteine-mediated VSMC proliferation and its underlying mechanism. Mol Med Rep 2014; 10(2): 947-954.
[21] Zhang DH, Wen XM, Zhang L, Cui W. DNA methylation of human telomerase reverse transcriptase associated with leukocyte telomere length shortening in hyperhomocysteinemia-type hypertension in humans and in a rat model. Circ J 2014; 78(8): 1915-1923.
[22] Zhou Q, Long L, Shi GX, Zhang J, Wu T, Zhou B. Research of the methylation status of miR-124a gene promoter among rheumatoid arthritis patients. Clin Dev Immunol 2013; 2013: 524204.
[23] Zhang JY, Gao XK, Wang D, Qin HQ, Chang C, Liu YF. Protective eff ects of tanshinone栻A on daunorubicin-induced cardiomyocyte injury. Chin J Pract Med 2015; 42(5): 26-28.
[24] Li Y, Xue RC, Liu C, Dong YG. Modulation of endoplasmic reticulum stress mediated by D J-1 in neonatal cardiomyocytes. J Sun Yat-senUniv (Medical Sciences) 2015; 36(6): 816-820.
Tel: 13076371996
E-mail: gdwangdongming@sina.com
Foundation project: It is supported by the Natural Science Foundation of Guangdong Province (Grant No. 06033503).
Homocysteine
Hydrogen sulfi de
Matix metalloproteinase-2
doi:Document heading 10.1016/j.apjtm.2016.03.023
*Corresponding author:Dong-Ming Wang, First Affiliated Hospital of Shantou University Medical College, Shantou, Guangdong 515041, China.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Study on the role of Cathepsin B and JNK signaling pathway in the development of cerebral aneurysm
- Polarization of M1 tumor associated macrophage promoted by the activation of TLR3 signal pathway
- Effect and mechanism of miR-34a on proliferation, apoptosis and invasion of laryngeal carcinoma cells
- Mechanism of TLR-4/NF-κB pathway in myocardial ischemia reperfusion injury of mouse
- Exploration of the regulatory effect of miR-21 on breast cancer cell line proliferation and invasion as well as the downstream target genes
- Serological survey on some pathogens in wild brown hares (Lepus europaeus) in Central Italy
