Study on the role of Cathepsin B and JNK signaling pathway in the development of cerebral aneurysm
2016-07-25DongGuoYeWeiWangJiMaLeiYanTengFeiLiXinWeiHanShaoFengShuiDepartmentofRadiologyInterventiontheFirstAffiliatedHospitalofZhengzhouUniversityZhengzhouChina
Dong Guo, Ye-Wei Wang, Ji Ma, Lei Yan, Teng-Fei Li, Xin-Wei Han, Shao-Feng ShuiDepartment of Radiology Intervention, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
ABSTRACT
Objective: To investigate the correlation between JNK signal and the apoptosis of VSMC as well as the expression of Cathepsin B and to explore the role of JNK signal in the development of cerebral aneurysm. Methods: Rat models of cerebral aneurysm were established and histopathologic changes of cerebral aneurysm and the apoptosis of VSMC were analyzed. Rat models were respectively subject to subcutaneous injection of Cathepsin B siRNA and JNK inhibitor SP600125. Western blot technique was used to detect the expression of proteins like Cathepsin B, Caspase-3, and p-JNK. Spearman's rho was used to examine the correlation between p-JNK and Cathepsin B, as well as the expression of relevant proteins. Results: The success rate of modeling rats with cerebral aneurysm was 88.75%. After the respective injection of Cathepsin B siRNA, SP600125 and their combination, the cell densities of VSMC of rats with cerebral aneurysm all increased signifi cantly (P<0.05 or P<0.01), but the apoptosis rate of VSMC decreased signifi cantly (P<0.01). Compared with normal rats, the expression of Cathepsin B, Caspase-3 and p-JNK in cerebral aneurysm models increased signifi cantly. Eff ectively intervening Cathepsin B genes with Cathepsin B siRNA could signifi cantly inhibit the expression of Cathepsin B and Caspase-3, but hardly infl uence the expression of p-JNK. JNK inhibitor SP600125 had no infl uence on the expression of Cathepsin B and Caspase-3, but eff ectively inhibited the expression of p-JNK. In cerebral aneurysm tissues, positive correlation was observed between the expression of p-JNK and Cathepsin B, the correlation coeffi cient was r=0.640. Conclusion: After the attack of cerebral aneurysm, proteins like Cathepsin B, Caspase-3 and p-JNK are all involved in the apoptosis of VSMCs. This process may be realized by Cathepsin B which activates the apoptosis mechanism of Caspase-3 and mediate the apoptosis of VSMC through the JNK signaling pathway. Therefore, silencing Cathepsin B gene or inhibiting the conduction through JNK signaling pathway can mitigate the apoptosis of vascular smooth muscle cells in cerebral aneurysm.
ARTICLE INFO
Article history:
Received 15 January 2016
Received in revised form 16 February 2016
Accepted 15 March 2016
Available online 20 May 2016
Study on the role of Cathepsin B and JNK signaling pathway in the development of cerebral aneurysm
Dong Guo, Ye-Wei Wang, Ji Ma, Lei Yan, Teng-Fei Li, Xin-Wei Han, Shao-Feng Shui*
Department of Radiology Intervention, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
ABSTRACT
Objective: To investigate the correlation between JNK signal and the apoptosis of VSMC as well as the expression of Cathepsin B and to explore the role of JNK signal in the development of cerebral aneurysm. Methods: Rat models of cerebral aneurysm were established and histopathologic changes of cerebral aneurysm and the apoptosis of VSMC were analyzed. Rat models were respectively subject to subcutaneous injection of Cathepsin B siRNA and JNK inhibitor SP600125. Western blot technique was used to detect the expression of proteins like Cathepsin B, Caspase-3, and p-JNK. Spearman's rho was used to examine the correlation between p-JNK and Cathepsin B, as well as the expression of relevant proteins. Results: The success rate of modeling rats with cerebral aneurysm was 88.75%. After the respective injection of Cathepsin B siRNA, SP600125 and their combination, the cell densities of VSMC of rats with cerebral aneurysm all increased signifi cantly (P<0.05 or P<0.01), but the apoptosis rate of VSMC decreased signifi cantly (P<0.01). Compared with normal rats, the expression of Cathepsin B, Caspase-3 and p-JNK in cerebral aneurysm models increased signifi cantly. Eff ectively intervening Cathepsin B genes with Cathepsin B siRNA could signifi cantly inhibit the expression of Cathepsin B and Caspase-3, but hardly infl uence the expression of p-JNK. JNK inhibitor SP600125 had no infl uence on the expression of Cathepsin B and Caspase-3, but eff ectively inhibited the expression of p-JNK. In cerebral aneurysm tissues, positive correlation was observed between the expression of p-JNK and Cathepsin B, the correlation coeffi cient was r=0.640. Conclusion: After the attack of cerebral aneurysm, proteins like Cathepsin B, Caspase-3 and p-JNK are all involved in the apoptosis of VSMCs. This process may be realized by Cathepsin B which activates the apoptosis mechanism of Caspase-3 and mediate the apoptosis of VSMC through the JNK signaling pathway. Therefore, silencing Cathepsin B gene or inhibiting the conduction through JNK signaling pathway can mitigate the apoptosis of vascular smooth muscle cells in cerebral aneurysm.
ARTICLE INFO
Article history:
Received 15 January 2016
Received in revised form 16 February 2016
Accepted 15 March 2016
Available online 20 May 2016
Keywords:
Cerebral aneurysm
Cathepsin B
JNK Signaling pathway
Correlation analysis
1. Introduction
Cerebral aneurysm is a common cerebrovascular disorder and its rupture usually leads to high rate of disability and/or death [1] . Regarding the pathogenesis of cerebral aneurysm, current studies mainly focus on genetic factors, haemodynamic factors, and acquired degenerative changes in arterial walls [2-4] . However, it's also significant to explore an effective way to intervene the growth of cerebral aneurysm by studying its pathogenesis from the perspective of molecular biology. Normally, the proliferation and apoptosis of VSMC help to maintain the balance and stability in blood vessels. However, the occurrence of cerebral aneurysm breaks such balance, causing the apoptosis of a large number of VSMCs, as a result of which weakened walls of blood vessels in the brain fail to sustain the impact of blood fl ow [5] . Previous study showedthat excessive apoptosis of VSMC is an important mechanism for the formation of cerebral aneurysm [6] .
Cathepsins are enzymes in lysosomes, including Cathepsin B, C, K, L, and S and others which play a vital role in the process of apoptosis. Cathepsin B is a lysosomal cysteine protease. A wide range of diseases results in elevated levels of Cathepsin B, which causes pathological processes of numerous diseases like cancer, infl ammation, and degenerative diseases. The infl uence of Cathepsin B on apoptosis has also been widely studied [7] . Caspase-3 is the most critical protease in cell apoptosis and an activator of the execution-phase of cell apoptosis[8]. c-Jun N-terminal kinases (JNKs) belong to the mitogen-activated protein kinase family (MAPK) and JNK signaling pathways play a vital role in a variety of physiological processes like cell diff erentiation, apoptosis and stress[9]. Phosphorylated JNKs (pJNK) eventually cause a series of certain biological effects by regulating downstream signaling molecules. SP600125, an inhibitor of JNKs, can eff ectively inhibit the conduction of JNK signaling pathways [10] . By establishing the mouse model of cerebral aneurysm, this study explored the apoptosis rate of VSMC and changes in the expression of Cathepsin B, Caspase-3 and p-JNK. This study also analyzed the influence on cerebral aneurysm by effectively intervening Cathepsin B or blocking JNK signaling pathways in order to find out the role of Cathepsin B and JNK signals in the pathogenesis of cerebral aneurysm.
2. Material and methods
2.1. Establishment of the rat model of cerebral aneurysm
A total of 80 male Sprague-Dawley rats, 5-7 weeks old, provided by Laboratory Animal Center of Zhengzhou University, were anesthetized by intraperitoneal injection of 3% pentobarbital sodium (40 mg/kg). Subsequently, the rats were subjected to the ligation of left common carotid artery and the bipolar coagulation of posterior branches of bilateral renal arteries. One week after the procedures, the rats were fed with 1% saline instead of water until the 12th week. The cerebral vessel wall tissues were separated from the right ACAOA under the microscope, fi xed with 4% paraformaldehyde in 0.1 M PBS for 24-48h, subject to paraff i n embedding, sectioning, and hematoxylin-eosin staining. Finally, histopathologic changes were observed. TUNEL assay was used to detect the cell density and apoptosis rate of VSMC. This experiment has been approved by Laboratory Animal Ethics Committee of Zhengzhou University.
2.2. Grouping and treatment of experimental animals
A total of 50 rat models of cerebral aneurysm were equally divided into 5 groups, namely the scramble siRNA group subject to subcutaneous injection of Scramble siRNA, the Cathepsin B siRNA group subject to subcutaneous injection of Cathepsin B siRNA, the SP600125 group subject to subcutaneous injection of SP600125, the Cathepsin B siRNA+ SP600125 group subject to subcutaneous injection of Cathepsin B siRNA +SP600125, and the model control group subject to subcutaneous injection of equivalent normal saline.
2.3. TUNEL assay used to detect the cell density and apoptosis rate of VSMC
Deparaffin cerebral vessel wall tissues of all groups with dimethylbenzene and ethanol till all the paraf fi n was replaced by water. Use Proteinase K to digest the tissues at room temperature, use 3% H2O2in methanol to block the activity of endogenous horseradish peroxidase, add TdT and digoxin-labeled dUTP for mixture reaction, incubate the tissues, add horseradish peroxidase-labeled anti-digoxin antibody for incubation, use DAB-H2O2solution to stain. Cells with dark brown nuclei were in the process of apoptosis. Finally, restain, dehydrate, clear, and mount the tissues. For negative control groups, replace TdT solution with PBS buff er. Count the number of cells in certain visual fi elds under 400X microscope, calculate cell density. Given 200 cells in each visual f ield, calculate the percentage of cells in apoptosis, namely cell apoptosis rate.
2.4. Western blotting
Extract the total protein of tissues of all groups, use 50 μg protein for 15% SDS-PAGE, then transfer proteins to PVDF membrane, use 5% nonfat milk in TBST buffer as a blocking agent, incubate overnight at 4 ℃ respectively with Cathepin Brat anti-human polyclonal antibody (1:200), Caspase-3rabbit anti-human polyclonal antibody (1:300), rat anti-human p- JNK monoclonal antibody (1:400), and β-actin rat anti-human monoclonal antibody (1:200), then incubate with HRP-labeled goat anti-rat second antibody (1:500) at 37 ℃ for 1h, rinse the membrane with TBST, then use electrochemical luminescence (ECL) assay to observe the results. All antibodies were bought from Santa Cruz Biotechnology Inc. The same method was used to detect the expression of p-JNK and Cathepsin B in the other 21 cerebral aneurysm rat models.
2.5. Statistical analysis
Software SPSSl7.0 was used for One-way ANOVA of relevant data. Spearman's rho was used to analyze the correlations between the expression of p-JNK and Cathepsin B. All the data were represented with Mean±SD. A value of P<0.05 was considered statistically signifi cant.
3. Results
3.1. Establishment of the rat model of cerebral aneurysm
Under light microscope, 30 of 71 rats have aneurysm-like lesions, all of which were at the intersection of anterior cerebral arteryolfactory artery (ACA-OA) on the contralateral side of the circle of Willis with the success rate of modeling being 88.75%. HE staining showed that normal arteries had complete endothelial cells and smooth muscle f ibers and tunica adventitia were neat and compact, while in cerebral aneurysm arteries collagenous f ibers of cerebral aneurysm thickened, smooth muscle layer atrophied, tunica adventitia was loose, and there was thrombosis at the lumen of the aneurysm.
3.2. Detecting the cell density and apoptosis rate of VSMC
In Situ cell death detection with TUNEL assay showed that compared with the model control group, VSMC cell densities of the Cathepsin B siRNA group, the SP600125 group, and the Cathepsin B siRNA+ SP600125 group all increased significantly (P<0.05 or P<0.01), but decreased signifi cantly compared with that of normal rats (P<0.01). Compared with normal rats, the VSMC apoptosis rate of the cerebral aneurysm model control group increased signif icantly (P<0.01). Compared with the cerebral aneurysm model control group, VSMC apoptosis rates of the Cathepsin B siRNA group, the SP600125 group, and the Cathepsin B siRNA+ SP600125 group decreased signifi cantly (P<0.01), but were all higher than that of normal rats (P<0.01) (Table 1).
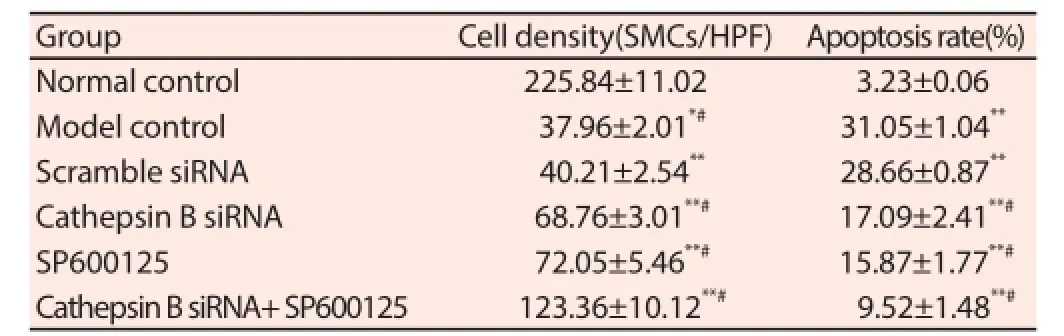
Table 1Cell density and apoptosis rate of VSMC.
3.3. Detecting the expression of relevant proteins
There was weak or no expression of Cathepsin B in the tissues of cerebral vessel wall of normal rats, but significantly increased expression of Cathepsin B was observed in cerebral aneurysm models. Eff ectively intervening Cathepsin B genes with Cathepsin B siRNA could obviously inhibit the expression of relevant proteins. However, JNK inhibitor SP600125 had no influence on the expression of Cathepsin B. Caspase-3 was expressed in normal tissues, but cerebral aneurysm caused signifi cantly higher expression level of Caspase-3. The expression of Caspase-3 in cerebral aneurysm models was inhibited by the injection of Cathepsin B siRNA, but not influenced by the injection of JNK inhibitor SP600125. p-JNK was expressed in normal tissues, but more signifi cantly expressed in cerebral aneurysm tissues. Cathepsin B siRNA had no signifi cant infl uence on the expression of Cathepsin B; however, JKN inhibitor SP600125 effectively inhibited the expression of p-JNK (Table 2).
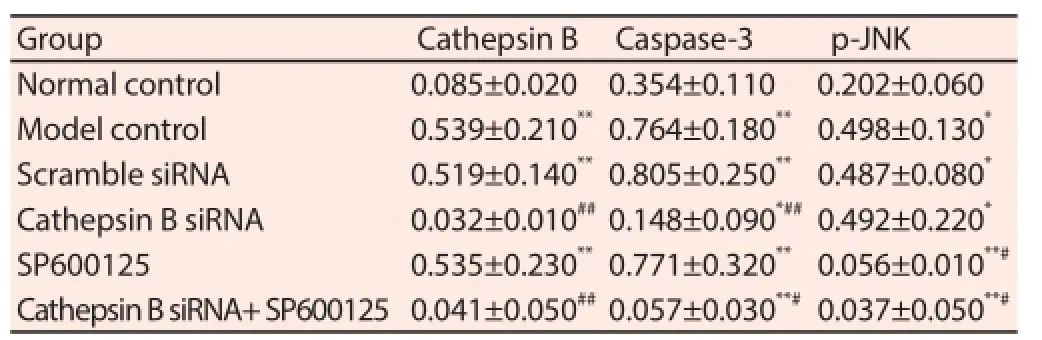
Table 2Relative expression of Cathepsin B, Caspase-3 and p-JNK.
3.4. Correlation between the expression of p-JNK and Cathepin B
The expression of p-JNK and Cathepin B in cerebral aneurysm models subject to no treatment was respectively examined. Spearman's rho was used to analyze the correlation between the expressions of them. Positive correlation was observed between the expression of p-JNK and Cathepsin B, the correlation coefficient was r=0.640, P=0.002. According to the fi tting degree of the trend line, the coeffi cient of determination (R2)=0.589.
4. Discussions
The incidence of 2%-3% of cerebral aneurysm leads to an annual incidence of 0.6‰-2‰ of subarachnoid hemorrhage (SAH) [11, 12] . Endothelial dysfunction is the initial symptom of cerebral aneurysm, followed by the phenotypic modulation of VSMCs, extracellular matrix remodeling, the apoptosis of VSMC and the degeneration, expansion and rupture of the vessel wall [13] . However, the specifi c pathogenesis remains unclear. This study investigated the role of Cathepsin B, Caspase-3 and JNK signaling pathway, which are closely related to cell apoptosis, in the attack and development of cerebral aneurysm from the perspective of molecular biology.
Normally, Cathepsin B lies in lysosomes and is involved in physiological processes, like the growth of the body. However, when the cellular injury leads to increased permeability of lysosome membrane or even the rupture of lysosome membrane, Cathepsin B will enter the cytoplasm or surrounding tissues, be activated and mediate infl ammatory necrosis and apoptosis of cells [14, 15] . Experimental results showed that the expression of Cathepsin B and Caspase-3 was significantly higher in cerebral aneurysm rat models than in normal rats, and the injected siRNA inhibited not only the expression of Cathepsin B but also that of Caspase -3. This suggests that during the attack of cerebral aneurysm, Cathepsin B may cause the apoptosis of cells by activating Caspase-3. However, no fi nal conclusion has yet been reached on the specifi c mechanism of Cathepsin B activating Caspase-3. Some research has shown thatCathepsin B can directly catalyze Caspase precursor to activate it thus causing cell apoptosis[16]. Some studies conclude that Cathepsin B indirectly activates Caspase, fi rstly by triggering mitochondrion through Bid to release cytochrome c, then activating Caspase family to cause apoptosis [17, 18] . However, both approaches fi nally work on Caspase-3, the effector of apoptosis, which cause apoptosis together with the apoptosis substrate. Tsubokawa et al. [19] found that in the rat model of ischemia-reperfusion injury, the expression of Cathepsin B in the ischemic cortex increased and caused an increased expression of Caspase-3 via bcl-2 family members, thus influencing the apoptosis of neurocytes. Moreover, some study found that the expression of Cathepsin B during the apoptosis of neurocytes in the rat model of ischemia-reperfusion injury increased and it infl uenced the apoptosis of neurons through signaling pathway or other ways [20] .
According to results of this study, in cerebral aneurysm tissues, the expression of p-JNK and Cathepsin B both increased signifi cantly and positive correlation was observed. However, Cathepsin B siRNA had no influence on the expression of p-JNK, neither did JNK inhibitor SP600125 on the expression of Cathepsin B. The specifi c correlation between Cathepsin B and JNK signaling pathway has not being clear yet. There is a research proving that JNK signaling pathway is an important pro-apoptotic pathway [21] . However, Cathepsin B is involved in cell apoptosis in a quite complicated way. It may mediate the apoptosis either through one protein or certain signaling pathway[22, 23]. Therefore, we conclude that in cerebral aneurysm Cathepsin B may mediate the apoptosis of vascular smooth muscle cells through JNK signaling pathway.
Conflict of interest statement
We declare that we have no confl ict of interest.
References
[1] Chalouhi N, Hoh BL, Hasan D. Review of cerebral aneurysm formation, growth, and rupture. Stroke 2013; 44(12):3613-3622.
[2] Mohan D, Munteanu V, Coman T, Ciurea AV. Genetic factors involves in intracranial aneurysms--actualities. J Med Life 2015; 8(3):336-341.
[3] Poelma C, Watton PN, Ventikos Y. Transitional flow in aneurysms and the computation of haemodynamic parameters. J R Soc Interface 2015;12(105): DOI:10.1098/rsif.2014.1394
[4] Matsukawa H, Shinoda M, Fujii M, Uemura A, Takahashi O, Niimi Y. Arterial stiff ness as a risk factor for cerebral aneurysm. Acta Neurol Scand 2014; 130(6): 394-399.
[5] Shimamura N, Ohkuma H. Phenotypic transformation of smooth muscle in vasospasm after aneurysmal subarachnoid hemorrhage. Transl Stroke Res 2014; 5(3):357-364.
[6] Starke RM, Chalouhi N, Ding D, Raper DM, Mckisic MS, Owens GK, et al. Vascular smooth muscle cells in cerebral aneurysm pathogenesis. Transl Stroke Res 2014; 5(3):338-346.
[7] Kim SH, Zhao MH, Liang S, Cui XS, Kim NH. Inhibition of cathepsin B activity reduces apoptosis by preventing cytochrome c release from mitochondria in porcine parthenotes. J Reprod Dev 2015;61(4):261-268.
[8] Boland K, Flanagan L, Prehn JH. Paracrine control of tissue regeneration and cell proliferation by Caspase-3. Cell Death Dis 2013; 4:e725.
[9] Bogoyevitch MA, Ngoei KR, Zhao TT, Yeap YY, Ng DC. c-Jun N-terminal kinase (JNK) signaling: recent advances and challenges. Biochim Biophys Acta 2010 ;1804(3):463-475.
[10] Davies C, Tournier C. Exploring the function of the JNK (c-Jun N-terminal kinase) signalling pathway in physiological and pathological processes to design novel therapeutic strategies. Biochem Soc Trans 2012; 40(1):85-89.
[11] Cohen-Gadol AA, Bohnstedt BN. Recognition and evaluation of nontraumatic subarachnoid hemorrhage and ruptured cerebral aneurysm. Am Fam Physician 2013; 88(7):451-456.
[12] Serrone JC, Maekawa H, Tjahjadi M, Hernesniemi J. Aneurysmal subarachnoid hemorrhage: pathobiology, current treatment and future directions. Expert Rev Neurother 2015; 15(4):367-380.
[13] Penn DL, Witte SR, Komotar RJ, Sander Connolly E Jr. The role of vascular remodeling and infl ammation in the pathogenesis of intracranial aneurysms. J Clin Neurosci 2014; 21(1):28-32.
[14] Chu SC, Yang SF, Tzang BS, Hsieh YS, Lue KH, Lu KH. Cathepsin B and cystatin C play an infl ammatory role in gouty arthritis of the knee. Clin Chim Acta 2010; 411(21-22):1788-1792.
[15] Matarrese P, Ascione B, Ciarlo L, Vona R, Leonetti C, Scarsella M, et al. Cathepsin B inhibition interferes with metastatic potential of human melanoma: an in vitro and in vivo study. Mol Cancer 2010; 9:207.
[16] Ishisaka R, Utsumi K, Utsumi T. Involvement of lysosomal cysteine proteases in hydrogen peroxide-induced apoptosis in HL-60 cells. Biosci Biotechnol Biochem 2002; 66(9):1865-1872.
[17] Reiners JJ Jr, Caruso JA, Mathieu P, Chelladurai B, Yin XM, Kessel D. Release of cytochrome c and activation of pro-caspase-9 following lysosomal photodamage involves Bid cleavage. Cell Death Differ 2002; 9(9):934-944.
[18] Stoka V, Turk B, Schendel SL, Kim TH, Cirman T, Snipas SJ, et al. Lysosomal protease pathways to apoptosis. Cleavage of bid, not procaspases, is the most likely route. J Biol Chem 2001; 276(5):3149-3157.
[19] Tsubokawa T, Yamaguchi-Okada M, Calvert JW, Solaroglu I, Shimamura N, Yata K, et al. Neurovascular and neuronal protection by E64d after focal cerebral ischemia in rats. J Neurosci Res 2006 ; 84(4):832-840.
[20] Zhang ZB, Li ZG. Cathepsin B and Phospo-JNK in relation to ongoing apoptosis after transient focal cerebral ischemia in the rat. Neurochem Res 2012; 37(5):948-957.
[21] Li W, Fan M, Chen Y, Zhao Q, Song C, Yan Y, et al. Melatonin induces cell apoptosis in AGS cells through the activation of JNK and P38 MAPK and the suppression of nuclear factor-Kappa B: a novel therapeutic implication for gastric cancer. Cell Physiol Biochem 2015; 37(6):2323-2338.
[22] Malla R, Gopinath S, Alapati K, Gondi CS, Gujrati M, Dinh DH, et al. Downregulation of uPAR and cathepsin B induces apoptosis via regulation of Bcl-2 and Bax and inhibition of the PI3K/Akt pathway in gliomas. PLoS One 2010; 5(10):e13731.
[23] Alapati K, Kesanakurti D, Rao JS. Abstract 2617: uPAR and cathepsin B knockdown-induced nuclear translocation of JNK inhibits migration and induces apoptosis in glioma-initiating cells. Cancer Res 2013; 73(8 Supplement):2617-2617.
Tel:+86-0371-66862161
E-mail: shaofeng_shui@163.com
Foundation project: This paper was supported by The Scientifi c and Technological Research Key Project of the Education Department of Henan Province (grant No. 14A320037).
doi:Document heading 10.1016/j.apjtm.2016.03.020
*Corresponding author:Shao-Feng Shui, Department of Radiology Intervention , the First Affi liated Hospital of Zhengzhou University, NO.1 Eastern Jianshe Road, Zhengzhou 450052, China.
杂志排行
Asian Pacific Journal of Tropical Medicine的其它文章
- Mechanism of TLR-4/NF-κB pathway in myocardial ischemia reperfusion injury of mouse
- Polarization of M1 tumor associated macrophage promoted by the activation of TLR3 signal pathway
- Influence of hydrogen sulfide on zymogen activation of homocysteineinduced matix metalloproteinase-2 in H9C2 cardiocytes
- Effect and mechanism of miR-34a on proliferation, apoptosis and invasion of laryngeal carcinoma cells
- Exploration of the regulatory effect of miR-21 on breast cancer cell line proliferation and invasion as well as the downstream target genes
- Serological survey on some pathogens in wild brown hares (Lepus europaeus) in Central Italy
